Abstract
The petroleum industry is often faced with accidental spills and discharges that pollute valuable natural resources such as soil. The purpose of this study was to assess bioremediation potential of an on-site landfarming unit (LU), a highly economical solution that complies with the zero-waste policy, for bioremediation of the contaminated soil after an actual diesel fuel leakage in a fuel depot. The first aim was to evaluate the effects of different climates on hydrocarbon bioremediation. For this reason, a part of the contaminated soil was moved from the initial location with a sub-Mediterranean climate to an LU at another location with a temperate continental climate. Our results demonstrated that remediation in sub-Mediterranean climate is less effective than the remediation in a temperate continental climate. The second aim of this study was to evaluate the effect of different plant species on the microbial population during bioremediation. For that purpose, 365-day monitoring of phospholipid fatty acids (PLFA) was performed. Our results support the hypothesis that plant-assisted bioremediation can diminish toxic effects of diesel-polluted soil and that the changes in plant species during bioremediation cause changes in the microbial population.
NOVELTY STATEMENT
The main objective of this study was to implement a landfarming bioremediation technique after an actual diesel fuel pollution in the sub-Mediterranean climate and diminish toxic effects of pollutants in soil. Since soil bioremediation is performed by soil microorganisms, their communities are primarily affected by the growing vegetation and climatic conditions. For future bioremediation strategies or ex situ approaches, it is crucial to assess the influence of a specific climate on the degradation rate of hydrocarbons in soil and select the most efficient plant species for this purpose.
Introduction
Owing to modern society’s dependence on petroleum, petroleum-derived hydrocarbon contaminants are the most ubiquitous pollutants found in the soil. They are represented by an array of organic compounds and by-products that pose a substantial hazard to all life forms (Ossai et al. Citation2020).
The petroleum industry is often faced with accidental spills and discharges owing to leakage from oil pipelines or storage tanks, blowout accidents during oilfield development, and equipment maintenance (Xu et al. Citation2018). Petroleum hydrocarbon pollutants have impacted nearly 80,000 sites in Australia and have caused the contamination of at least 342,000 sites in Western Europe (Hoang, Lamb, Seshadri, Sarkar, Choppala, et al. Citation2021). Furthermore, approximately 7% of the crude oil and petroleum products produced annually in the Russian Federation ended up being spilled, which, for example, caused the contamination of more than 6,000 hectares of land in 2017 (Vasilyeva et al. Citation2020).
Hydrocarbon-polluted soils can be chemically or physically treated by incineration, chemical washing, thermal desorption, or solvent extraction (Stegmann et al. Citation2001). The disadvantages associated with these technologies include high cost, undesirable environmental impacts, and technical demands (Tran et al. Citation2021). Moreover, chemical and physical treatments may destroy the biological structure of soil (Suding et al. Citation2004).
Biological remediation (bioremediation) is considered a simpler, greener, and more economical alternative to physical and chemical remediation of soil. During bioremediation, petroleum hydrocarbon contaminants serve as organic carbon sources for indigenous soil microorganisms that convert them into carbon dioxide, water, humus, and biomass (Suding et al. Citation2004), which leads to the enrichment of oil-degrading microbial populations (Li et al. Citation2018).
Different types of biological remediation methods have been used for the treatment of hydrocarbon-polluted soils. For example, Wu et al. demonstrated that biostimulation with nutrients enhanced hydrocarbon degradation (Wu et al. Citation2017). Moreover, bioremediation in combination with earthworms and microbes results in a high hydrocarbon removal efficiency (Rodriguez-Campos et al. Citation2019). Another approach to the degradation of hydrocarbons is phytoremediation based on rhizosphere degradation (Tischer and Hübner Citation2002), which describes a process involving the mutual relationship between the rhizosphere and its microorganisms (Saravanan et al. Citation2020) and has been recognized as an efficient and highly economical method, especially at field sites with low to moderate hydrocarbon content (Hoang, Lamb, Seshadri, Sarkar, Choppala, et al. Citation2021).
It needs to be emphasized that utilization of plants in hydrocarbon remediation on a large-scale, especially under actual weather conditions, has some weaknesses. Plants can be affected by heat, drought, and soil type. Moreover, the selected plant species must be easy to cultivate, tolerant to the toxic-effect of hydrocarbons, and be unattractive for herbivores (Kafle et al. Citation2022).
This study presents 365-day monitoring of the biological structure and microbial biomass in a landfarming unit (LU) constructed after an actual diesel fuel reservoir leakage in a fuel depot in Sermin, Slovenia. Landfarming is an above-ground remediation technology for soils that reduces petroleum contamination through biodegradation and is considered the simplest bioremediation technique due to its low cost and requirement of little equipment (Azubuike et al. Citation2016). Aeration of soil in our study was achieved by mechanical turn overs (similar to tillage) and landfarming was combined with plant-assisted bioremediation technology.
Diesel contamination of soil occurred in a sub-Mediterranean region of Slovenia (Location 1), in an area with the highest annual duration of solar radiation. To evaluate the impact of different climatic conditions on hydrocarbon remediation, a part of contaminated soil was relocated to a secondary LU at a location with a temperate continental climate (Location 2). Sub-Mediterranean climate is highly influenced by the Adriatic sea with mean annual precipitation between 1000 and 1200 mm (Darko Citation1996), an almost non existing snow cover (Kozjek et al. Citation2017), the average temperature of the coldest month above 4 °C, and the average temperature of the warmest month above 22 °C. Temperate continental climate is present in lowlands on the East part of the country, which is open to the Pannonian plain, with annual rainfall from 800 to 1,000 mm and a subcontinental rainfall regime. The average temperatures in April are higher than or similar to those in October, while the summer months are on the verge of drought due to the relatively low amount of precipitation (Darko Citation1996).
The first aim of the presented study was to evaluate the effects of different climatic conditions namely, different temperature ranges, rain regimes, and the daily amount of solar radiation on hydrocarbon remediation and the time needed for the contaminated soil to become suitable for reuse or restoration. It is important to evaluate climatic effects on bioremediation, since LUs are usually established at locations predetermined by the waste-management companies or companies providing petroleum products (spill sites). Because most studies on the topic of bioremediation are conducted as laboratory tests, the impact of solar radiation or natural temperature fluctuations cannot be considered (Margesin et al. Citation2007; Wu et al. Citation2017; Rodriguez-Campos et al. Citation2019).
The second aim of this study was to evaluate effect of different plant species on microbial population during plant-assisted bioremediation by performing phospholipid fatty acid (PLFA) analysis. At the beginning of soil remediation process, diesel-contaminated soil was mixed with compost to diminish the toxic effects of diesel, which is known to reduce species richness and phylogenetic diversity (Xu et al. Citation2018). Compost was also added to enhance remediation by the addition of organic substances, due to its positive effect on the physical properties of soil (mainly water retention and availability) (Kästner and Miltner Citation2016).
Materials and methods
Establishment of a landfarming unit (LU)
Soil pollution originated from an underground leakage of reservoir R19 (see description and Figures S1-S, S2-S, and S3-S in the Supporting Information). According to the Slovenian soil classification, the excavated soil belongs to the class of automorphic soils, 1st class: poorly developed soils; soil type: Regosol, which is commonly found in flysch and marl formations. Such soil is distinguished by the rapid weathering of the parent material and the production of fine particles (Vrščaj et al. Citation2017). The soil texture (loam – silt loam) was determined according to ISO 17892-4:2016 using a hydrometer method (ASTM 151H Soil hydrometer), the >2 mm fraction represented 51.4% of soil and was determined by sieving. The pH of soil was determined with CaCl2 extraction according to EN 15933:2012 (7.73 ± 0.01) and specific electrical conductivity was determined according to SIST-TS CEN/TS 15937:2013 (1,063 ± 266 uS/cm).
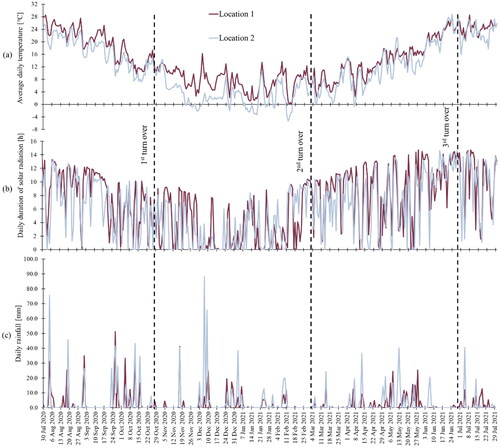
Figure 1. (a) The average daily temperature, (b) the daily duration of solar radiation, (c) and the daily rainfall at Location 1 (dark purple) and Location 2 (light blue) during the study. The dashed lines mark the day of the specific turn over.
For remediation, all (a total of 45 m3 or approximately 30 tons) of diesel-contaminated soil was excavated and moved into an LU in the industrial zone of a fuel depot in Sermin (Location 1: (WGS84) Lat: 45.561029° (N) Lon: 13.768573°(E)). To improve the conditions for soil remediation, 4 m3 of 1st-grade quality compost (according to the Slovenian Decree on the Treatment of Biodegradable Waste and the Use of Compost or Digestate (MOP Citation2013)) provided by: JP VOKA SNAGA d.o.o was added. The LU was constructed on an impermeable asphalt surface with a wooden frame built around it to prevent soil scattering (Figure S4-S in the Supporting Information)
After the establishment of the LU at Location 1, all of the contaminated soil was mixed with compost, and 0.5 m3 (approximately 300 kg) of that soil was transported to Location 2: (WGS84) Lat: 46.373494° (N) Lon: 15.778347° (E) and placed into a similar but smaller LU.
LU at Location 1 was treated as a full-scale on-site study and Location 2 as a small-scale off-site study. Soil sampling was conducted as it would have been for a contaminated site area. Replication in this study was provided by sampling soil in triplicates (n = 3) for the PLFA analysis. Monitoring of hydrocarbon content was conducted in accordance with Slovenian Rules on Soil Quality Monitoring for Contaminated Sites (MOP Citation2019).
Both LUs were placed outdoors and exposed to actual weather conditions. At Location 1, the LU was exposed to a sub-Mediterranean climate, whereas the LU at Location 2 was exposed to a temperate continental climate.
All soil samples were collected in accordance with ISO 18400-203: Investigation of potentially contaminated sites (Annex to Accreditation Certificate LP-O48 IKEMA d.o.o.). Students t-test was used to determine statistically significant differences in total hydrocarbon content between the two locations (Miller and Miller Citation2018).
Weather conditions
To assess the influence of the weather-related parameters, the following information was obtained: average daily temperatures, daily duration of solar radiation, and daily rainfall (). The data were obtained from the Slovenian Environment Agency at the Ministry of the Environment and Spatial Planning and from automatic weather stations close to the LUs – Portorož Airport for Location 1 and Edvard Rusjan Airport for Location 2.
Plant species selection during bioremediation
In this study, the LUs were first seeded with Trifolium repens (zero state). This plant species was chosen for its ability to fixate nitrogen via a symbiotic relationship with Rhizobium species, which is also a common agricultural practice in natural soil fertilization. Fabaceae species are known for their ability to enhance the remediation of hydrocarbon contaminants, to improve soil nutrient and oxygen status, and to increase the bioavailability of contaminants (Tischer and Hübner Citation2002). Wang and Oyaizu studied the remediation potential of four plant species to evaluate their impact on the remediation of dibenzofuran-contaminated soils (Yanxu and Oyaizu Citation2009). Their findings revealed that the white clover Trifolium repens was the most promising plant species for this purpose. Trifolium repens is also widespread in the sub-Mediterranean (Location 1) and temperate continental climates in the sub-Pannonian region (Location 2). The vegetation growing on both LUs was used as a natural fertilizer after the turn overs, thus adding nutrients to the polluted soil.
After the construction of the LUs, a soil moisture protocol was established. The moisture protocol for Location 1 included an automatic irrigation system (2 h per day) which was skipped in the event of rainfall. The moisture protocol for Location 1 included regular checking of soil moisture and the addition of water in case of drought. The soil in both LUs was turned over 3-times during the 365-day study, with vegetation mixed within.
To improve rhizoremediation after the 2nd and the 3rd turn over, both LUs were seeded with mixed vegetation, namely Trifolium repens (40%), Fagopyrum esculentum (20%), Sinapis alba L. (20%), and Medicago sativa (20%). All seeds were obtained from Semenarna Ljubljana (plant species are represented in wt.% seeds).
Microbial biomass composition
For the determination of the microbial biomass composition, PLFA analysis was performed. PLFA analysis provides quantitative data on the living microbial community by giving information on the content of fatty acids in the living microorganisms’ membranes (Orwin et al. Citation2018). Advantages of this method include quantification of living biomass and specific taxon identification without restriction to a specific type of soil or environmental samples (Bloem et al. Citation2006) and were shown to be sensitive and reproducible (Agnihotri et al. Citation2023). For the PLFA analysis, composite soil samples were collected using a soil auger. At Location 1, the sample was collected in 15 increments, and a simple systematic pattern was used. Due to the much smaller size of the LTU at Location 2, only 3 increments were taken. Soil samples for Location 1 and Location 2 were taken immediately after the establishment of the LU (zero state), after every turnover and at a 1-year milestone. All samples were transported to a laboratory in dark and cool storage (4 ± 2 °C), with free access to air. Prior to the extraction of PLFAs, soil samples were sieved through a 2 mm sieve, and 2.5 g of the sample was used to fill a 40 mL glass vial (ALP-LAB, napredne tehnologije, d.o.o., Ptuj, Slovenia). PLFAs were extracted using a modified method by Frostegård et al. (Frostegård et al. Citation1993), which is based on the method of Bligh and Dyer (Bligh and Dyer Citation1959). Before adding a mixture of chloroform, methanol, and phosphate buffer (1:2:0.8; V:V:V, V = volume), the water content of the samples was determined (on a split sample), and the V of the added phosphate buffer was adjusted accordingly.
The lipids were separated into natural lipids, glycolipids, and phospholipids on a solid-phase extraction (SPE) column (2 g; 6 mL, Silicycle, Quebec City, Quebec, Canada). The phospholipids were eluted with 15 mL of methanol and reduced to near dryness in a water bath (T < 30 °C) under a stream of nitrogen. The compositions of the FAMEs were identified using an Agilent 6890 gas chromatograph (GC, Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with an Agilent 5973 mass selective (MS) detector (Agilent Technologies, Inc., Santa Clara, CA, USA). An internal standard, 1-methyl naphthalene (TraceCERT®), was used, and 37 Component FAME Mix (CRM47885) was employed as a certified reference material (both supplied by Supelco Bellefonte, PA, USA).
The PLFA nomenclature used in this study was adopted after Willers et al. (Citation2015a).
All PLFA analyses were conducted in triplicate and were calculated as the mean ± standard deviation. The biomarkers used for the interpretation of the PLFAs were used to evaluate the soil microbiological communities (Hedrick et al. Citation2005; Willers et al. Citation2015b). The principal component analysis (PCA) was used to evaluate changes between the microbial communities at Location 1 and Location 2. PCA was performed within Microsoft Excel using the XLSTAT package.
Results
Hydrocarbon bioremediation and total PLFA extracted
The hydrocarbon concentration in soil was monitored in both LUs to obtain information on the efficiency of bioremediation (). Hydrocarbon content was determined in accordance with EN ISO 16703 – determination of extractable compounds in the range of hydrocarbons C10–C40. Their fractions were calculated from measured values by the gas chromatography (GC) method with FID detection (all analyses were performed under ISO 17025 accreditation).
Figure 2. The hydrocarbon content and the total PLFA content at five different sampling dates in the LU at Location 1 and in the LU at Location 2 (n = 2).
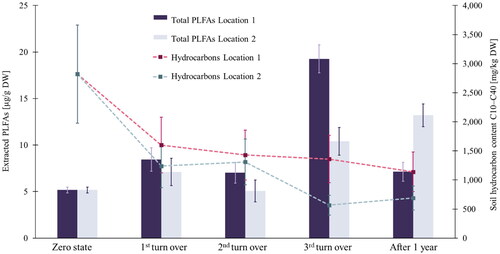
shows the total content of hydrocarbons in soil for both locations. To test significant differences between the samples at individual sampling dates, an independent t-test (p < 0.05) was performed. Statistically significant differences were determined only between the samples obtained at the 3rd turn over and overall point, whereas the t-test did not confirm any statistically significant differences between the samples obtained at the 1st and at the 2nd turn over. Furthermore, a statistically significant difference (p < 0.05) between the results obtained at Location 1 and Location 2 was confirmed with a paired difference test (paired by time point; n = number of pairs), with a 60% reduction of total hydrocarbons for Location 1 and a 75% reduction of total hydrocarbons for Location 2.
The total PLFAs extracted in our study () represent all the identified FAMEs. Results in show a general increase in the microbial biomass at Location 2, with a slight drop at the 2nd turn over. At Location 1, a drop in total PLFAs extracted can also be observed at the 2nd turn over and after 1 year. During hydrocarbon biodegradation, enzymatic and metabolic processes dictate degradation pathways, producing intermediate metabolites, which can be toxic to the associated organisms (Shen et al. Citation2016). We hypothesize that the latter could affect the total living microbial population as well. Moreover, between the 1st and the 2nd turn over, the soil was influenced by winter weather conditions, while between the 3rd turn over and the 1-year milestone, the soil in the LUs was exposed to the hottest air temperatures, typical for summer months (see ).
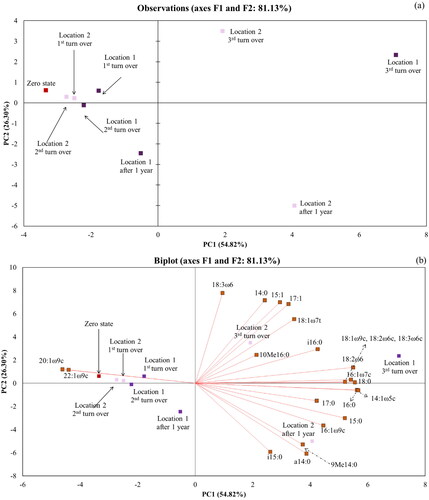
Microbiological profile interpretation using PCA
PCA revealed that the two principal components, PC1 and PC2, explained 54.82% and 26.30% of the total variance, respectively (). The two-dimensional plot in shows the clustering of soil samples analyzed at the zero state, the 1st turn over, and the 2nd turn over, regardless of location. Samples at the 3rd turnover and at the 1-year milestone were displaced along PC1 and PC2, indicating significant differences in the biological profile between the two samples. PC1 and PC2 separated the samples obtained at Location 1 after 1 year and those obtained at Location 1 at the 3rd turn over, and the samples obtained at Location 2 after 1 year and those obtained at Location 2 at the 3rd turn over from the cluster on the leftmost side of the PCA graph. PC1 is mainly attributable to PLFAs associated with bacteria (i.e., 22:1 ω9c and 20:1ω9c), which seem to be strongly negatively correlated with another bacterial biomarker, 17:0. PCA revealed how the biological profile from Location 1 changed between the last two sampling dates. Considering the significant drop in the total amount of extracted PLFAs at Location 1 after one year () and PCA, fungi seem to be the predominant species in this bioprofile. It is likely that, following the consumption of available nutrients, fungi have a competitive advantage over bacteria because of their capacity to use more recalcitrant carbon sources (Langarica-Fuentes et al. Citation2014; Covino et al. Citation2016) or are not as affected by heat. A similar displacement can be observed for Location 2, (along the PC2 axis), but without a drop in the content of extracted PLFAs (). A common characteristic of the samples collected at the 1st turn over and at the 2nd turnover is the presence of Trifolium repens. After the 1st turn over, this plant species was reseeded, but after the 2nd turn over, a mixture of Trifolium repens, Fagopyrum esculentum, Sinapis alba L., and Medicago sativa was grown on both LUs. However, the effect of vegetation on the microbiological profile of soil cannot be seen before the 3rd turn over.
Discussion
Our results showed a significant reduction in hydrocarbon pollution. Nonetheless, according to the Slovenian legislation, that is, the decree on burning soil by spreading waste (Decree on burdening of soil with waste spreading Uradni list RS, št. 34/08 in 61/11 Citation2008) (which is relevant for the use of inert waste as fillers), the criterion of 500 mg/kg DW was not reached. Approximately 60% of the total hydrocarbons were removed within a year at Location 1, whereas the removal rate at Location 2 was approximately 75%. The results are similar to those published by Johnsen et al., who studied the capacity of an experimental land farm to biodegrade diesel-polluted soil under high-Arctic tundra conditions in northeast Greenland and reported 64% removal of the total petroleum hydrocarbons within a year (Johnsen et al. Citation2021). It should be emphasized that the two sets of results are not directly comparable because the studies were conducted in two very different climates and with two very different types of soil.
To determine the effect of different climates on polluted soil in this study, part of the polluted soil was relocated from sub-Mediterranean climate (Location 1) to a temperate continental climate (Location 2). Soil in the LU at Location 1 was exposed to a greater amount of solar radiation and had a lower hydrocarbon degradation rate than the soil in the LU at Location 2, which has seen fewer solar radiation. Furthermore, when analyzing the data obtained by the PLFA analysis, one can notice a decrease in the amount of total PLFAs extracted for the data at the 3rd turn over in comparison to the data at the end of the study (after 1 year) for sub-Mediterranean climate. The reason for this probably lies in the fact that only 23 days had passed from one sampling to another, and the LU was exposed to the highest temperatures and the highest amount of solar radiation. The vegetation was also scarcer because of the limited time frame and unfavorable environmental conditions (the hottest part of the summer). A lack of vegetation coverage seemed to have a lesser effect on the living biomass in the LU at Location 2, between the 3rd turn over and the end of the study. Therefore, the milder weather conditions typical for moderate continental climates seemed to be favorable for the microbial community in soil at Location 2. Our results are similar to the ones published by Nakase and Eguchi who studied microbial mineralization activity in sediments. Their findings showed that an increase in solar UV radiation decreased mineralization activities. Moreover, solar UV radiation had a considerable seasonal effect on microbial mineralization activities in tidal flats (Nakase and Eguchi Citation2015).
Several researchers studied the effects of different plant species on rhizoremediation (Ridl et al. Citation2016; Lopez-Echartea et al. Citation2020; Hoang, Lamb, Seshadri, Sarkar, Cheng, et al. Citation2021). It was previously reported that the selection of plant species has a greater effect on the rhizosphere microbial community than the choice of fertilizer (Ridl et al. Citation2016). Therefore, the plant species seeded after each turn over could have influenced changes in the microbial biomass in this study. Slower degradation rates after the 1st turn over could indicate an inadequate nutrient supply for hydrocarbon-degrading microorganisms in both LUs. At the 2nd turn over, the decrease in the hydrocarbon content in both LUs was minimal, and no significant changes in the living biomass were observed. This might be explained by the low fertility of the soil, as only vegetation was mixed in, and no additional compost was added. The mixing of fresh vegetation supplied nutrients that differed from those provided by the addition of compost at the beginning of the remediation process. Compost might have supplied an excess of amended nutrients, which could have stimulated the activity of non-hydrocarbon-degrading microbes (Li et al. Citation2018). Unfortunately, soil’s nutrient content was not constantly monitored, so a lack of direct insight into nutrient changes, is a weakness of this study. The reason for the drop in the rate of degraded hydrocarbons after the 1st turn over, regardless of location (), might also have been the rhizosphere effect. Decay products of roots and other plant biomass can become an important source of carbon for the microorganisms found in soil, which can stimulate hydrocarbon degradation or, on the contrary, become a readily available carbon source and hinder hydrocarbon degradation (Balseiro-Romero et al. Citation2018).
PLFA analysis is widely used in soil ecology because of its ability to estimate microbial structure. Zheng et al. confirmed that the PLFAs extracted from soil are only representative of the fatty acids of living biomass because phospholipids released into soil after microbial death are rapidly hydrolyzed (Zhang et al. Citation2019). Data on the total amount of extracted PLFAs () did not reveal the actual differences in the biological profiles of the soil samples taken at the 3rd turnover and the 1-year milestone. Similar findings were published by Li et al., who studied biosurfactant-enhanced soil bioremediation of petroleum hydrocarbons (Li et al. Citation2018). Their results did not show any changes in the total microbial biomass at two different sampling dates 36 days apart; however, PCA revealed a significant difference between soil profiles. PCA shows that significantly different microbiological profiles can be represented by similar values in the total extracted PLFAs. Because both LUs were regularly watered, drought was prevented, but the effect of extreme temperatures, typical of both summer and winter months, was unavoidable.
Finally, establishing a good remediation practice can be challenging, as actual petroleum spills do not occur on ideal soils or in ideal climates. Moreover, when conducting an ex-situ approach in a hotter climate, the availability of an appropriate water source for the maintenance of the LU needs to be considered. This study demonstrated that relocating the polluted soil to a location with a milder climatic condition might increase the efficiency of bioremediation, so one needs to evaluate whether transportation costs outweigh the prolonged time required for soil remediation. Another important finding was that the choice of plant species influences the degrading microbial population. According to our results of the PCA, significant shifts in the microbial community occurred when Trifolium repens was replaced with a mixture of different species, namely Trifolium repens, Fagopyrum esculentum, Sinapis alba L., and Medicago sativa. Nonetheless, the question remains, how can we stimulate microbiological population to achieve quicker hydrocarbon degradation rates through the selection of plant species. We must not overlook the fact that plant species must be selected with care, since they need to thrive under the climatic conditions of the LU and in the type of soil that was polluted. The amount of the PLFAs extracted at the end of this study at Location 1 revealed a drop in the total living microbial biomass, which suggests that plants might have served as a buffer for solar exposure and heat. Lastly, the LU provided an unexpectedly appealing visual effect (especially with vegetation in bloom) on a predominantly asphalt-covered ground (Figure S5-S) and became a source of food for pollinating organisms.
Conclusions
Our results showed that a temperate continental climate with milder summer temperatures and less solar radiation provides more favorable conditions for hydrocarbon-degrading microorganisms to thrive in the soil. The presented results support the assumption that climatic conditions, especially extreme temperatures, have an influence on the natural processes in soil. The differences between the two climates were reflected in the different biological structures of soil despite the use of identical plant species. The ex situ bioremediation has shown to be an applicable approach for treating diesel-fuel-contaminated soil on site. Although the removal of hydrocarbons was not complete, diesel pollution was significantly reduced. This study’s future endeavors include the implementation of earthworm-assisted remediation to further reduce the hydrocarbon content and make the soil suitable for reuse.
Supplemental Material
Download MS Word (8.4 MB)Acknowledgments
We are grateful to Petrol for their participation and support in this study. The authors acknowledge the financial support from the Slovenian Research Agency (research core funding No. P2-0118).
Disclosure statement
No potential conflict of interest was reported by the authors. The authors herby confirm that this work is original and has not been published elsewhere nor is it currently under consideration for publication elsewhere. All the authors have seen and approved the manuscript for submission.
Additional information
Funding
References
- Agnihotri R, Gujre N, Mitra S, Sharma MP. 2023. Decoding the PLFA profiling of microbial community structure in soils contaminated with municipal solid wastes. Environ Res. 219:114993. doi:10.1016/j.envres.2022.114993.
- Azubuike CC, Chikere CB, Okpokwasili GC. 2016. Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol. 32(11):180. doi:10.1007/s11274-016-2137-x.
- Balseiro-Romero M, Monterroso C, Casares JJ. 2018. Environmental fate of petroleum hydrocarbons in soil: review of multiphase transport, mass transfer, and natural attenuation processes. Pedosphere. 28(6):833–847. doi:10.1016/S1002-0160(18)60046-3.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37(8):911–917. doi:10.1139/o59-099.
- Bloem J, Hopkins D, Benedetti A. 2006. Microbiological methods for assessing soil quality. Wallingford: CABI.
- Covino S, Fabianová T, Křesinová Z, Čvančarová M, Burianová E, Filipová A, Vořísková J, Baldrian P, Cajthaml T. 2016. Polycyclic aromatic hydrocarbons degradation and microbial community shifts during co-composting of creosote-treated wood. J Hazard Mater. 301:17–26. doi:10.1016/j.jhazmat.2015.08.023.
- Darko O. 1996. Podnebni tipi v Sloveniji. Geografski vestnik. 68:39–56.
- Decree on burdening of soil with waste spreading Uradni list RS, št. 34/08 in 61/11. 2008. vol Uradni list RS, št. 34/08 in 61/11. Uradni list Republike Slovenije.
- Frostegård Å, Bååth E, Tunlio A. 1993. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biology and Biochemistry. 25(6):723–730. doi:10.1016/0038-0717(93)90113-P.
- Hedrick DB, Peacock A, White DC. 2005. Interpretation of fatty acid profiles of soil microorganisms. In: Margesin R, Schinner F, editors. Monitoring and assessing soil bioremediation. Berlin, Heidelberg: Springer Berlin Heidelberg; p. 251–259. doi:10.1007/3-540-28904-6_12.
- Hoang SA, Lamb D, Seshadri B, Sarkar B, Cheng Y, Wang L, Bolan NS. 2021. Petroleum hydrocarbon rhizoremediation and soil microbial activity improvement via cluster root formation by wild proteaceae plant species. Chemosphere. 275:130135. doi:10.1016/j.chemosphere.2021.130135.
- Hoang SA, Lamb D, Seshadri B, Sarkar B, Choppala G, Kirkham MB, Bolan NS. 2021. Rhizoremediation as a green technology for the remediation of petroleum hydrocarbon-contaminated soils. J Hazard Mater. 401:123282. doi:10.1016/j.jhazmat.2020.123282.
- Johnsen AR, Boe US, Henriksen P, Malmquist LMV, Christensen JH. 2021. Full-scale bioremediation of diesel-polluted soil in an Arctic landfarm. Environ Pollut. 280:116946. doi:10.1016/j.envpol.2021.116946.
- Kafle A, Timilsina A, Gautam A, Adhikari K, Bhattarai A, Aryal N. 2022. Phytoremediation: mechanisms, plant selection and enhancement by natural and synthetic agents. Environ Adv. 8:100203. doi:10.1016/j.envadv.2022.100203.
- Kästner M, Miltner A. 2016. Application of compost for effective bioremediation of organic contaminants and pollutants in soil. Appl Microbiol Biotechnol. 100(8):3433–3449. doi:10.1007/s00253-016-7378-y.
- Kozjek K, Dolinar M, Skok G. 2017. Objective climate classification of Slovenia. Int J Climatol. 37(S1):848–860. doi:10.1002/joc.5042.
- Langarica-Fuentes A, Zafar U, Heyworth A, Brown T, Fox G, Robson GD. 2014. Fungal succession in an in-vessel composting system characterized using 454 pyrosequencing. FEMS Microbiol Ecol. 88(2):296–308. doi:10.1111/1574-6941.12293.
- Li X, Fan F, Zhang B, Zhang K, Chen B. 2018. Biosurfactant enhanced soil bioremediation of petroleum hydrocarbons: design of experiments (DOE) based system optimization and phospholipid fatty acid (PLFA) based microbial community analysis. Int Biodeterior Biodegrad. 132:216–225. doi:10.1016/j.ibiod.2018.04.009.
- Lopez-Echartea E, Strejcek M, Mukherjee S, Uhlik O, Yrjälä K. 2020. Bacterial succession in oil-contaminated soil under phytoremediation with poplars. Chemosphere. 243:125242. doi:10.1016/j.chemosphere.2019.125242.
- Margesin R, Hämmerle M, Tscherko D. 2007. Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: effects of hydrocarbon concentration, fertilizers, and incubation time. Microb Ecol. 53(2):259–269. doi:10.1007/s00248-006-9136-7.
- Miller JN, Miller JC. 2018. Statistics and chemometrics for analytical chemistry. 7th ed. Harlow: Pearson.
- MOP. 2013. Decree on the treatment of biodegradable waste and the use of compost or digestate. (Uradni list RS, št. 99/13, 56/15, 56/18 in 44/22 – ZVO-2). doi:http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED6281.
- MOP. 2019. Rules on soil quality monitoring. (Uradni list RS, št. 68/19 in 44/22 – ZVO-2) doi:http://www.pisrs.si/Pis.web/pregledPredpisa?id=PRAV12716.
- Nakase G, Eguchi M. 2015. Influence of seasonal solar ultraviolet radiation on microbial mineralization activity in tidal flats in Osaka Bay, Japan. Fish Sci. 81(6):1099–1104. doi:10.1007/s12562-015-0927-y.
- Orwin KH, Dickie IA, Holdaway R, Wood JR. 2018. A comparison of the ability of PLFA and 16S rRNA gene metabarcoding to resolve soil community change and predict ecosystem functions. Soil Biology and Biochemistry. 117:27–35. doi:10.1016/j.soilbio.2017.10.036.
- Ossai IC, Ahmed A, Hassan A, Hamid FS. 2020. Remediation of soil and water contaminated with petroleum hydrocarbon: a review. Environ Technol Innov. 17:100526. doi:10.1016/j.eti.2019.100526.
- Ridl J, Kolar M, Strejcek M, Strnad H, Stursa P, Paces J, Macek T, Uhlik O. 2016. Plants rather than mineral fertilization shape microbial community structure and functional potential in legacy contaminated soil. Front Microbiol. 7:995–995. doi:10.3389/fmicb.2016.00995.
- Rodriguez-Campos J, Perales-Garcia A, Hernandez-Carballo J, Martinez-Rabelo F, Hernández-Castellanos B, Barois I, Contreras-Ramos SM. 2019. Bioremediation of soil contaminated by hydrocarbons with the combination of three technologies: bioaugmentation, phytoremediation, and vermiremediation. J Soils Sediments. 19(4):1981–1994. doi:10.1007/s11368-018-2213-y.
- Saravanan A, Jeevanantham S, Narayanan VA, Kumar PS, Yaashikaa PR, Muthu CMM. 2020. Rhizoremediation – a promising tool for the removal of soil contaminants: a review. J Environ Chem Eng. 8(2):103543. doi:10.1016/j.jece.2019.103543.
- Shen W, Zhu N, Cui J, Wang H, Dang Z, Wu P, Luo Y, Shi C. 2016. Ecotoxicity monitoring and bioindicator screening of oil-contaminated soil during bioremediation. Ecotoxicol Environ Saf. 124:120–128. doi:10.1016/j.ecoenv.2015.10.005.
- Stegmann R, Brunner G, Calmano W, Matz G. 2001. Treatment of contaminated soil: fundamentals, analysis, applications. Cham: Springer. doi:10.1007/978-3-662-04643-2.
- Suding KN, Gross KL, Houseman GR. 2004. Alternative states and positive feedbacks in restoration ecology. Trends Ecol Evol. 19(1):46–53. doi:10.1016/j.tree.2003.10.005.
- Tischer S, Hübner T. 2002. Model trials for phytoremediation of hydrocarbon-contaminated sites by the use of different plant species. Int J Phytoremediation. 4(3):187–203. doi:10.1080/15226510208500082.
- Tran H-T, Lin C, Bui X-T, Ngo H-H, Cheruiyot NK, Hoang H-G, Vu C-T. 2021. Aerobic composting remediation of petroleum hydrocarbon-contaminated soil. Current and future perspectives. Sci Total Environ. 753:142250. doi:10.1016/j.scitotenv.2020.142250.
- Vasilyeva G, Kondrashina V, Strijakova E, Ortega-Calvo J-J. 2020. Adsorptive bioremediation of soil highly contaminated with crude oil. Sci Total Environ. 706:135739. doi:10.1016/j.scitotenv.2019.135739.
- Vrščaj B, Repe B, Simončič P. 2017. The soils of Slovenia. Cham: Springer. doi:10.1007/978-94-017-8585-3.
- Willers C, Jansen van Rensburg PJ, Claassens S. 2015a. Microbial signature lipid biomarker analysis - an approach that is still preferred, even amid various method modifications. J Appl Microbiol. 118(6):1251–1263. doi:10.1111/jam.12798.
- Willers C, Jansen van Rensburg PJ, Claassens S. 2015b. Phospholipid fatty acid profiling of microbial communities–a review of interpretations and recent applications. J Appl Microbiol. 119(5):1207–1218. doi:10.1111/jam.12902.
- Wu M, Li W, Dick WA, Ye X, Chen K, Kost D, Chen L. 2017. Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere. 169:124–130. doi:10.1016/j.chemosphere.2016.11.059.
- Xu X, Liu W, Tian S, Wang W, Qi Q, Jiang P, Gao X, Li F, Li H, Yu H. 2018. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Front Microbiol. 9(:2885. doi:10.3389/fmicb.2018.02885.
- Yanxu W, Oyaizu H. 2009. Evaluation of the phytoremediation potential of four plant species for dibenzofuran-contaminated soil. J Hazard Mater. 168(2–3):760–764. doi:10.1016/j.jhazmat.2009.02.082.
- Zhang Y, Zheng N, Wang J, Yao H, Qiu Q, Chapman SJ. 2019. High turnover rate of free phospholipids in soil confirms the classic hypothesis of PLFA methodology. Soil Biol Biochem. 135:323–330. doi:10.1016/j.soilbio.2019.05.023.