ABSTRACT
Temperature is commonly assumed to act as the primary constraint on the timing of plant growth, and strong advances in plant phenology have been seen with recent atmospheric warming. The influence of temperature on the timing of root growth, however, is less clear, and controls on root phenology are not well understood. The influence of temperature on above- and belowground phenology is particularly important in the Arctic, where most plant biomass is belowground and warming is occurring at a higher rate than in other ecosystems. We examined the influence of experimental warming on graminoid and shrub communities in the Arctic in southern west Greenland. We found that warming since 2012 did not advance the timing of aboveground seasonal dynamics during two years or belowground seasonal dynamics during three years. We suggest that growing-season temperature may no longer be the primary constraint on plant phenology at this site, and plant phenological responses to future warming at the site may consequently be weaker.
Globally, climate change has significantly advanced the timing of seasonal events, or phenology (Parmesan and Yohe Citation2003), and plant phenology is one of the strongest indicators of global warming (IPCC Citation2014). Changes in plant phenology can have strong effects on ecosystem processes, greenhouse gas emissions, and species interactions (Cleland et al. Citation2007; Ernakovich et al. Citation2014; Post and Forchhammer Citation2008; Richardson et al. Citation2013; Visser and Both Citation2005). Temperature is often cited as a primary factor controlling the timing of aboveground growth (Wielgolaski Citation1999), and it is credited with an advanced spring phenology of 2.5 days per decade in Europe (Menzel et al. Citation2006). These impacts may be particularly strong in the Arctic, where temperatures are increasing at twice the global rate (Anisimov et al. Citation2007; McBean et al. Citation2005; Post et al. Citation2009).
Current models predict that arctic warming will continue to be strong, especially given positive feedbacks between sea-ice melt and local air temperature (Flanner et al. Citation2011; Vihma Citation2014). If warming advances phenology, earlier plant growth may increase yearly carbon uptake in the Arctic and elsewhere (Cahoon, Sullivan, and Post Citation2016). It is commonly assumed that warmer arctic temperatures will continue to cause earlier green-up and an increase in vegetation types that grow earlier in the year, such as shrubs (Myers-Smith et al. Citation2011). The International Tundra Experiment (ITEX), which seeks to examine the influence of warming across the Arctic by using passive open-top warming chambers, found that short-term experimental warming advanced aboveground plant phenology (Arft et al. Citation1999). Long-term ITEX results, however, did not show universal advances in phenology with warming. They also found that as temperatures continue to increase, it takes a greater cumulative amount of warming to advance phenology (Oberbauer et al. Citation2013). The effect of warming on individual species may be nonlinear, where initial changes in temperature elicit much stronger effects than later changes (Fu et al. Citation2015).
Current estimates of shifts in plant phenology in response to climate change are based solely on aboveground phenology and do not account for potential changes in root phenology. Because roots can account for as much as 70 percent of total plant biomass and may have a 50 percent longer growing season in the Arctic (Blume-Werry et al. Citation2015; van Wijk et al. Citation2003), it is essential to account for both above- and belowground phenological responses to warming. Additionally, root traits can drive ecosystem processes, such as carbon and nutrient cycling (Bardgett, Mommer, and De Vries Citation2014; Iversen et al. Citation2015). Most terrestrial biosphere models assume that above- and belowground phenology are synchronous and roots will respond to warming in the same way as shoots (Abramoff and Finzi Citation2015). Experimental evidence does not support these assumptions, because root and shoot can be asynchronous (Abramoff and Finzi Citation2015; Sloan, Fletcher, and Phoenix Citation2016), and belowground phenology may not respond to warming in the same way as aboveground phenology (Blume-Werry, Jansson, and Milbau Citation2017; Radville, Post, and Eissenstat Citation2016c). Additionally, shrub expansion across the Arctic may be linked to shrubs having earlier and more shallow rooting than graminoids (Wang et al. Citation2016). If above- and belowground phenology respond differently to warming, estimates of future carbon exchange driven by presumed phenological dynamics may be inaccurate.
In this study we examined the influence of experimental warming with open-top chambers on both above- and belowground growth in southern west Greenland. We recorded the timing of root production, root standing crop, and leaf cover of graminoid (Poa spp.; Carex spp.) and shrub species (Betula nana) throughout three growing seasons. We hypothesized that (1) warmer air temperatures would cause both leaves and roots to grow earlier in the year and (2) shrubs would be more responsive to warming than grasses.
Methods
Study site and design
This experiment was conducted near Kangerlussuaq in southern west Greenland (67.11°N, 50.30°W). The study was set up on dry acidic tundra on noncarbonated bedrock in Arctic shrub-tundra (Elvebakk Citation1999). In this permafrost ecosystem the average active layer depth was 63 cm between May and August 2014 (Cahoon, Sullivan, and Post Citation2016). The mean annual air temperature was −4.4°C in 2014 and −7.7°C in 2015. In 2016, from January 1 to June 26, the average temperature was −4.13°C. We were unable to continue to collect air temperature data past June 2016 because we no longer had personnel at the field site. Vegetation types occur in easily distinguished patches at this site, primarily of Betula nana, Salix glauca, and mixed graminoid species, including Poa pratensis and Carex spp.
In 2012 we selected forty-eight plots, including sixteen plots that were 100 percent Betula nana, sixteen plots that were 100 percent graminoid species, and sixteen plots that were 50 percent of each vegetation type, which we refer to as “mixed” plots. Half of these plots were on a slightly south-facing slope while the others were on a west-facing slope. The two slope types were approximately 100 m apart. Of each vegetation type (shrub, graminoid, and mixed), half were warmed (n = 8 per vegetation by warming treatment). Warming was achieved with open-top chambers (OTCs) May–August from 2012 to 2016, and through September in 2014. OTCs were placed after snowmelt in spring and before snowfall in fall. The passive warming chambers were 1.5 m in diameter and were constructed according to ITEX protocols (Henry and Molau Citation1997). Matching ambient, unwarmed plots were also 1.5 m in diameter.
Root seasonal dynamics
Two minirhizotron tubes were installed in each plot in 2012. Minirhizotron tubes were constructed of clear acrylic cylinders buried at a thirty-degree angle to the vertical and anchored to the ground with steel rods. To seal the tubes from weather and light and to prevent solar radiative heating, all tubes were sealed at the surface end with a plumbing plug, wrapped with electrical tape, painted white, and shielded with a white, aluminum cover. The inside of the tube was filled with removable tubular insulation during nonmeasurement periods to prevent temperature changes inside the tube.
To monitor root growth a minirhizotron camera (Bartz Technology Corporation, CA, USA) was lowered into the tube and root images were captured at depth intervals of 1.3 cm along the tube. Each tube was photographed once per week from 2014 to 2016, with all tubes imaged throughout the course of each week. Because we could not photograph all minirhizotron tubes in one day each week, values of root growth for all tubes were grouped by week in order to statistically analyze plot replicates and to visually represent data. Ice obscured the view of roots in some images, so these were excluded from all analyses. Seasonal root production and root standing crop were quantified by tracing images of roots with Rootfly software (Clemson University, Clemson, SC, USA). We determined the length of roots visible on tubes on each date to obtain standing crop (cm roots · cm−2 viewing surface). To calculate root production, the length of new root initiation and elongation occurring between two consecutive dates (cm roots · cm−2 viewing surface) was divided by the number of days since the previous measurement (cm roots · cm−2 viewing surface · day−1). New roots were reliably identified by their bright white appearance. Root standing crop was recorded as the length of all roots present in each tube on each date (cm roots · cm−2 viewing surface).
Given high variation in root dynamics among plots and years, we had only modest power to determine a true difference in the means of root production between warmed and ambient plots. The power to determine a 25 percent difference in the means was 0.29 (29 percent of the time we would correctly reject the null hypothesis if we reran the study many times with random samples); the power to detect a 50 percent difference in the means was 0.80; and the power to detect a 75 percent difference in the means was 0.99.
Abiotic conditions
In order to measure soil temperature at different depths, thermocouples were buried at 10, 20, 30, and 40 cm from the bottom of the organic layer in all plots. To measure soil moisture at these depths, time domain reflectometry (TDR) wave guides were buried at 10–20, 20–30, and 30–40 cm, where 0 cm is the top of the mineral soil (the bottom of the organic layer) in all plots. To measure temperature and moisture at 0–10 cm, where 0 cm is the top of the mineral soil layer, a thermocouple and a TDR probe were manually inserted into the soil. Measurements were taken at mid-day on each date that root images were obtained from 2014 to 2016. The organic layer depth (approximately 5 cm) and mineral soil physical and chemical properties were similar among plots. In addition, automated measurements of air temperature, soil temperature, soil moisture, and humidity were obtained in twelve plots (n = 2 per warming by vegetation type). In these plots, Campbell CR-1000 dataloggers scanned sensors every 30 seconds and stored hourly averages of air temperature (°C, 10 cm above soil surface), soil temperature (°C, 10 cm below soil organic layer), and volumetric soil water content (10 cm below soil organic layer) beginning in June 2015. Two additional CR-1000 dataloggers were used to record meteorological conditions: one at the subset of plots in the south-facing slope and one at the subset of plots with a west-facing slope. These dataloggers recorded hourly air temperature (°C, 2 m above soil surface), soil temperature (°C, 10 cm below soil surface), and soil water content (10 cm below soil surface).
Aboveground seasonal dynamics
To estimate the timing of seasonal leaf cover expansion, canopy NDVI (normalized difference vegetation index) was recorded in each plot once per week from 2014 to 2015; NDVI = (R800 − R660) / (R800 + R660), where R800 is the reflectance at 800 nm, representing a near-infrared wavelength, and R660 is reflectance at 660 nm, representing a photosynthetically active wavelength (as in Boelman et al. Citation2003). NDVI was recorded with a Unispec-DC (PP Systems, Haverhill, MA, USA), and was determined from comparisons of incident and reflected light in each plot. To account for light conditions each day, we calibrated the Unispec-DC with a white standard. Each measurement was taken with the Unispec-DC placed 2 m above each plot to yield a measurement footprint of 0.39 m2. Three measurements were averaged across each plot. We did not directly calibrate NDVI to changes in leaf cover, but this was done in 2013 at the same site and on the same study plants (Cahoon, Sullivan, and Post Citation2016). Cahoon, Sullivan, and Post (2016) found that NDVI was significantly correlated with leaf area index (m · m−2; R2 = 0.84). Street et al. (Citation2007) also found a significant correlation between NDVI and leaf area at another arctic site (R2 = 0.75).
We had large statistical power to determine a true difference in the means of NDVI between warmed and ambient plots. Our power to determine a 5 percent difference in the means was 0.78, and our power to detect a 10 percent difference in the means was 0.99.
Statistical analyses
Because we were interested in the timing of root growth, we wanted to remove the normal high spatial variation in the amount of roots from minirhizotron tube to tube and plot to plot. Thus, we standardized data to the maximum value for that plot, summed across the two minirhizotron tubes, in that year. The proportion of maximum root production on a given date was computed by root production on that date divided by maximum cumulative root production occurring in that plot during that year. We obtained the proportion of maximum NDVI and the proportion of maximum root standing crop for each plot in the same way.
The influence of warming treatment on measured variables was examined in the R environment version 2.0–33 with linear mixed models using the ‘lmer’ function in the “lme4” package (Bates et al. Citation2015; RCoreTeam Citation2015). The model was run using the measured variable (e.g., “proportion of maximum root production”) as the dependent variable; date, treatment, vegetation type, date*treatment, and date*vegetation type as fixed effects; and plot nested within date as the random effect. The plot within date was allowed to have a random slope and intercept because of the nonindependence of plots measured repeatedly across dates. The date variable was calculated as numerical days since January 1, 2014 (e.g., day 366 was January 1, 2015), so year was included in the variable. Years were not analyzed separately. This model was run separately for each of the following dependent variables: volumetric soil water content, soil temperature, proportion of maximum root production, proportion of maximum root standing crop, and proportion of maximum NDVI. The ‘lmer’ function for linear mixed models cannot give exact p values and degrees of freedom. To aid in interpreting the results, we estimated these values with the ‘lmerTest’ package (Kuznetsova, Brockhoff, and Christensen Citation2016). These p values were calculated with Satterthwaite (Citation1946) approximations.
Results
Effects of open-top chambers
Across all dates and vegetation types, OTCs increased the average plot-surface daily air temperature by 0.9 ± 0.2°C (model estimate ± SE; ; t value = 4.3, P < 0.001). When vegetation types were analyzed separately, graminoids were seen to experience the largest amount of warming. Average graminoid plot temperatures were increased by 1.5 ± 0.3°C (model estimate ± SE; t value = 4.7, P < 0.001), and average mixed plots were increased by 0.2 ± 0.09°C (model estimate ± SE; t value = 2.4, P = 0.02). Average temperature in shrub plots was only marginally increased (temperature increase of 0.6 ± 0.3°C [model estimate ± SE]; t value = 1.8, P = 0.07). Slope position (south-facing versus west-facing) did not significantly affect average temperature in OTCs (t value = 1.1, P = 0.3). Between June and August 2015, warming advanced cumulative air temperatures by forty growing degree–days.
Figure 1. (A) The difference between daily maximum air temperature in warmed plots and daily maximum temperature in ambient plots (°C). (B) The difference between daily minimum air temperature in warmed plots and daily minimum temperature in ambient plots (°C). (C) The difference between daily mean air temperature in warmed plots and daily mean temperature in ambient plots (°C). In all panels, values are averaged across all vegetation types. On average across all years and vegetation types, open-top chambers warmed maximum daily temperatures by 2.2 ± 0.6°C (model estimate ± SE) and mean daily temperatures by 0.9 ± 0.2°C (model estimate ± SE). Warming had little effect on minimum daily temperature. Error bars represent standard error of the mean. N = 6 per warming treatment
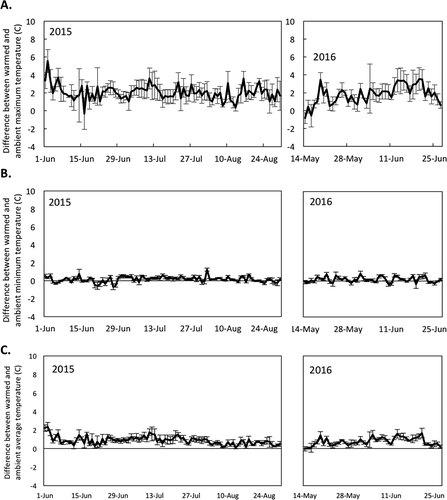
Across all dates and vegetation types, OTCs increased the average plot-surface maximum daily air temperature by 2.2 ± 0.6°C (model estimate ± SE; ; t value = 3.4, P = 0.01). This difference was driven by graminoid plots (). The maximum daily air temperature in graminoid plots was significantly increased by 4.0 ± 0.4°C (model estimate ± SE; t value = 9.5, P < 0.001). Mixed-plot maximum temperatures increased modestly, but nonsignificantly, by 1.6 ± 0.6°C (; model estimate ± SE; t value = 2.7, P = 0.11), and the maximum temperature in shrub plots was not significantly increased (; temperature increase of 0.93 ± 1.4°C [model estimate ± SE]; t value = 0.68, P = 0.57). Slope position (south-facing versus west-facing) did not significantly affect maximum temperature in OTCs (t value = 0.44, P = 0.67). OTCs did not significantly affect minimum daily air temperature (; increase of 0.05 ± 0.06°C; t value = 0.78, P = 0.44). OTCs also did not significantly affect mean daily air temperature (increase of 0.9 ± 1.3°C; t value = 0.71, P = 0.49).
Measurements taken from 0 cm to 40 cm on each sampling date show that open-top chambers did not significantly affect soil water content or soil temperature (; soil water content: t value = −0.69, P = 0.50; soil temperature: t value = −0.88, P = 0.38). Findings from the continuous dataloggers in the top 10 cm support this, because measures of soil temperature and moisture were nearly indistinguishable between plots with and without OTCs ().
Table 1. Continuous abiotic measurements recorded hourly from CR-1000 dataloggers in twelve plots (n = 2 per warming by vegetation type), separated by warming treatment. Values represent mean ± standard error of the mean of raw data
Figure 2. (A) Mean daily air temperature in graminoid (Poa spp.) plots from 2015 to 2016 in warmed and ambient treatments. Values are separated by warming treatment. Temperatures in warmed plots were 1.5 ± 0.3°C higher than in ambient plots (model estimate ± SE). (B) Mean daily air temperature in mixed plots (half Poa spp. and half Betula nana) from 2015 to 2016 in warmed and ambient treatments. Temperatures in warmed plots were 0.2 ± 0.09°C higher than in ambient plots (model estimate ± SE). (C) Mean daily air temperature in shrub plots (Betula nana) from 2015 to 2016 in warmed and ambient treatments. Temperatures in warmed plots were only marginally increased (temperature increase of 0.6 ± 0.3°C [model estimate ± SE]). In all panels, the gray line represents ambient plots, and the black line represents warmed plots. Error bars represent standard error of the mean. N = 2 per vegetation type by treatment
![Figure 2. (A) Mean daily air temperature in graminoid (Poa spp.) plots from 2015 to 2016 in warmed and ambient treatments. Values are separated by warming treatment. Temperatures in warmed plots were 1.5 ± 0.3°C higher than in ambient plots (model estimate ± SE). (B) Mean daily air temperature in mixed plots (half Poa spp. and half Betula nana) from 2015 to 2016 in warmed and ambient treatments. Temperatures in warmed plots were 0.2 ± 0.09°C higher than in ambient plots (model estimate ± SE). (C) Mean daily air temperature in shrub plots (Betula nana) from 2015 to 2016 in warmed and ambient treatments. Temperatures in warmed plots were only marginally increased (temperature increase of 0.6 ± 0.3°C [model estimate ± SE]). In all panels, the gray line represents ambient plots, and the black line represents warmed plots. Error bars represent standard error of the mean. N = 2 per vegetation type by treatment](/cms/asset/87376da8-56a1-4999-942e-c660d8700bf7/uaar_a_1414457_f0002_b.gif)
Figure 3. (A) Mean daily soil temperature in all plots from 2015 to 2016, as measured from continuous dataloggers. (B) Mean daily volumetric soil water content in all plots from 2015 to 2016, as measured from continuous dataloggers. In both panels, values are averaged across all treatments and vegetation types. Warmed and ambient plots were not significantly different. Error bars represent standard error of the mean (N = 12)
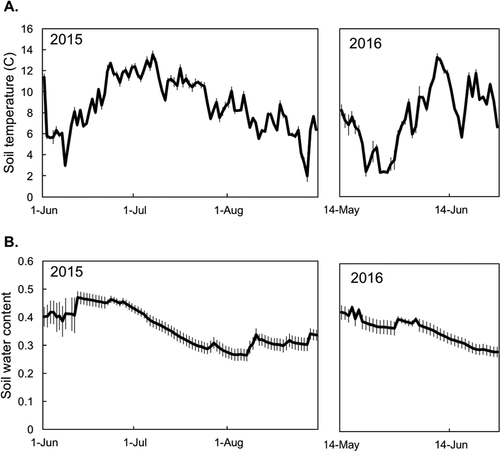
Soil temperature did not differ significantly among the three vegetation types. Graminoid plots tended to be slightly cooler than mixed plots (; 1.1 ± 0.7°C cooler [model estimate ± SE]; t value = 1.6, P < 0.11), and graminoid and shrub plots did not differ (t value = 0.38, P < 0.71). Graminoid plots were wetter than shrub plots (0.12 ± 0.03°C higher volumetric water content [model estimate ± SE]; t value = 3.5, P = 0.001), and graminoid and mixed plots did not differ significantly (t value = 0.45, P < 0.69).
Table 2. Continuous abiotic measurements recorded hourly from CR-1000 dataloggers in twelve plots (n = 2 per warming by vegetation type), separated by vegetation type. Values represent mean ± standard error of the mean of raw data
Aboveground seasonal dynamics
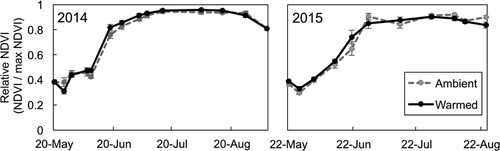
Although open-top chambers warmed maximum air temperatures by an average of 2.2°C, warming did not significantly alter the timing of aboveground productivity, measured as relative NDVI (; warming*date: t value = 0.66, P = 0.51), although warmed plots peaked slightly (and nonsignificantly) earlier in the year in 2014. Vegetation types did not significantly differ in the timing of aboveground leaf cover (vegetation type*date: graminoid plots compared to mixed plots, t value = 0.044, P = 0.96; graminoid plots compared to shrub plots, t value = 1.3, P = 0.21). Because the warming treatment was only significantly effective in the graminoid plots, we reran statistical analyses with only graminoid plots. Warming did not significantly alter the timing of NDVI in graminoid plots (warming*date: t value = 0.17, P = 0.86).
Figure 4. Relative leaf cover (NDVI on a given date/maximum NDVI in that year) averaged for ambient and warmed plots in 2015 and 2016. The dashed gray line and gray points are the mean of ambient plots, and the solid black line and points are the mean of warmed plots. There was no significant effect of the warming treatment. Error bars represent standard error of the mean. N = 24 per warming treatment
Root seasonal dynamics
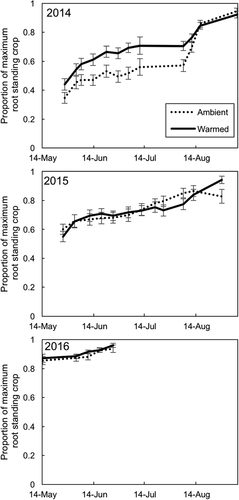
New root production was highest in spring (May and June) and in fall (late August to early September), and in 2014 there was a third peak in early July (). The timing of root production did not differ by vegetation type or by warming treatment (; warming*date: t value = 0.28, P = 0.78; vegetation type*date: graminoid plots compared to mixed plots, t value = 0.64, P = 0.52; graminoid plots compared to shrub plots, t value = 0.38, P = 0.70). Again, we examined graminoid plots alone. Warming did not significantly alter the timing of root production in graminoid plots (warming*date: t value = 0.47, P = 0.65). In all years, relative root standing crop was highest late in the growing season. The timing of relative root standing crop differed by warming treatment (; warming*date: t value = 2.83, P = 0.007), but these differences were driven primarily by responses in 2014. In 2014, ambient plots had 21 ± 0.03 percent higher root standing crop than warmed plots. In all years, standing crop was highest at the last sampling date of the season (except for ambient plots in 2014, which peaked on the second-to-last sampling date). The timing of root standing crop did not differ significantly by vegetation type (vegetation type*date: graminoid plots compared to mixed plots, t value = 0.15, P = 0.88; graminoid plots compared to shrub plots, t value = 1.3, P = 0.21). When analyzing graminoid plots alone, the warming treatment significantly altered the timing of root standing crop (warming*date: estimate ± SE = −0.0119 ± 0.004, t value = −2.8, P = 0.015). Ambient graminoid plots had a higher root standing crop early in the season than warmed plots.
Figure 5. Timing of relative new root production (root production on a given date/maximum root production in that year) in warmed and ambient plots for three growing seasons. The warming treatment did not have a significant effect on the timing of root production. The gray line and points are the mean of ambient plots, and the black line and points are the mean of warmed plots. Error bars represent standard error of the mean. N = 24 per warming treatment
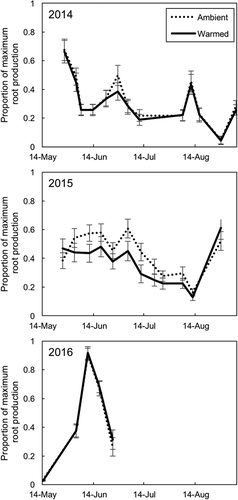
Figure 6. Timing of relative root standing crop (roots present on a given date/maximum root standing crop in that year) in warmed and ambient plots for three growing seasons. The gray line and points are the mean of ambient plots, and the black line and points are the mean of warmed plots. Error bars represent standard error of the mean. N = 24 per warming treatment
Discussion
Experimental warming of 0.9°C on average and 2°C at midday did not generally alter above- or belowground seasonal productivity of graminoids or a shrub species in an Arctic ecosystem during two years aboveground and three years belowground ( and ). Many studies suggest that temperature is the primary control on aboveground plant phenology and associated carbon fluxes (Peñuelas and Filella Citation2001; Wielgolaski Citation1999). We suggest, however, that advances in phenology may not be constrained primarily by temperature in all ecosystems, and terrestrial biosphere models with high temperature sensitivities may overestimate future carbon uptake in some ecosystems. Other factors, such as soil water content, soil nutrient availability, and timing of snowmelt may be at least as important as temperature, if not more important, as drivers of above- and belowground phenology in high-latitude systems (Chapin et al. Citation1995; Iler et al. Citation2013a; Reyes-Fox et al. Citation2014; Sharp et al. Citation2013). Since collection of uninterrupted temperature records began at Kangerlussuaq in 1974, mean annual temperature and mean growing season (May–August) temperature have increased by 2.05°C and 1.85°C, respectively (Danish Meteorological Institute, unpublished data). Primary control of plant phenology at our site may, as a consequence of this transition to a warmer state, be in the process of shifting to factors other than temperature, as suggested for other sites (Iler et al. Citation2013b).
Plants in this ecosystem may rely on several cues concurrently in order to begin aboveground growth. These responses may differ among species, and responses to interactions among factors, such as snowmelt, temperature, and precipitation, may be complex (Bjorkman et al. Citation2015). For example, earlier snowmelt may advance early phases of aboveground phenology, but temperature may be a stronger control later in the season (Wipf Citation2010). Evidence also suggests that individual species and vegetation types will respond differently to changes in snowmelt and temperature (Post et al. Citation2016; Rumpf et al. Citation2014; Wipf and Rixen Citation2010; Wipf, Stoeckli, and Bebi Citation2009). Late-season phenology may be fixed in some Arctic species, occurring a set amount of time after snowmelt (Semenchuk et al. Citation2016). Because warming was the only variable manipulated in this study, other factors may be stronger drivers of aboveground productivity at this site.
Drivers of phenology belowground may be equally complex, and it is unclear how global warming will impact root phenology (Radville et al. Citation2016b). Some studies suggest that exogenous factors, such as soil temperature, are the strongest controls on root phenology (Burke and Raynal Citation1994), while others suggest that endogenous factors, such as the timing of carbon allocation to roots, are also important (Joslin, Wolfe, and Hanson Citation2001; Tierney et al. Citation2003). These factors will be affected differently by global warming. Exogenous soil factors may be buffered from warming temperatures, but the timing of carbon allocation may shift as leaf production advances with warming. Soil temperature and carbon availability may not be the primary drivers of Arctic and subarctic root phenology—a study in northern Sweden found that increased early season soil temperature and advanced aboveground phenology did not shift root phenology (Blume-Werry, Jansson, and Milbau Citation2017). Root productivity may not have changed in this study because air temperature alone may not drive root growth, and other endogenous and exogenous factors were unchanged by the OTCs. More studies are needed that focus on other potential drivers of root phenology, such as water and nutrient availability.
The open-top chambers advanced growing degree days by forty between June and August 2015 (assuming a growth threshold of 10°C), and we expected an associated advancement in leaf and root productivity. We had strong statistical power to detect differences in the timing of leaf cover (power of 0.8 to detect a 5 percent difference in the means), although our power to detect differences in the timing of root production was more modest, given the inherent variation in root production (McCormack et al. Citation2014; Radville et al. Citation2016a; power of 0.8 to detect a 50 percent difference in the means). Shifts in root phenology may be difficult to track with minirhizotrons because different root orders cannot be tracked separately from each other. Each root order may have different seasonal dynamics (Chen et al. Citation2017), and by pooling all roots we may have introduced variation that masked small changes in the timing of growth of some root orders.
It is possible that the average treatment effect of 1°C was not strong enough to elicit a phenological response, particularly for shrub and mixed plots, which experienced warming of less than 1°C. Seasonal dynamics of shrub and mixed plots may shift with stronger amounts of warming. OTCs may be less effective in warming tall, dense vegetation types because solar radiation is shielded from reaching the soil (Wahren, Walker, and Bret-Harte Citation2005). Although warming was modest for shrub and mixed plots, graminoid plots experienced an average warming of 1.5°C and a maximum daily warming of 4°C (). Despite this amount of warming, we did not see shifts in graminoid productivity, suggesting that warming was not the primary control on leaf and root production. Additionally, OTCs in this experiment only increased the midday temperatures (), when solar radiation is highest, but midday temperatures are likely to have a strong influence on phenology. For example, in a study of temperate trees, Fu et al. (Citation2016) found that the impact of daytime temperatures on leaf unfolding was three times stronger than the impact of nighttime temperatures.
Previous work near this study site reported advanced aboveground phenology with warming by OTCs (Post et al. Citation2008; Radville, Post, and Eissenstat Citation2016c), but we did not find comparable advances in growth in this study () . In Post et al. (Citation2008), distinct phases of phenology, such as leaf opening, flower set, and bloom, were recorded at one- to two-day intervals, rather than total leaf cover over the entire plot, as in this study. Because our metric of seasonal dynamics was based on leaf cover and our temporal resolution was coarser (weekly), we may have missed fine-scale changes in the timing in vegetation and of other events, such as the timing of reproductive growth. Warming effects in both Post et al. (Citation2008) and Radville et al. (Citation2016c) may have been the result of a community shift to species that emerge earlier, as warmed plots moved from a graminoid-dominated to a shrub-dominated community after five years (Post and Pedersen Citation2008). Also, a recent analysis of long-term observational data from this site indicated no advance in the timing of leaf-out by Salix glauca shrubs, and only a very modest advance in the timing of leaf-out by Betula nana shrubs, in response to spring warming since 2002 (Post et al. Citation2016). These results are in line with other Arctic studies that suggest that relatively short-term experiments do not predict long-term responses (Blume-Werry et al. Citation2016; Wolkovich et al. Citation2012). Wolkovich et al. (Citation2012) suggest that relatively short-term experiments may represent plastic responses to climate change, whereas long-term observational experiments also include shifts in species composition, ecosystem dynamics, and genotype.
Although there has been rapid warming of the Arctic in recent decades, the influence of warming on phenology may decrease with time (Kremers, Hollister, and Oberbauer Citation2015; Oberbauer et al. Citation2013). Nonlinear responses to warming with time were found in European tree species, as advances in leaf phenology were reduced by 40 percent during thirty-three years (Fu et al. Citation2015). It is possible that we observed a saturation effect of warming, wherein individual species do not continue to advance with additional warming (sensu Kremers, Hollister, and Oberbauer Citation2015).
The influence of warming may differ by latitude, as some evidence suggests a stronger influence of temperature at high latitudes (IPCC Citation2014; Prevéy et al. Citation2017). A meta-analysis found that, on average, passive warming from one to four years advanced phenology in Arctic and alpine ecosystems (Arft et al. Citation1999), but leaf phenology was advanced in three out of four years in the high Arctic, whereas leaf phenology was only advanced in one out of four years in the low Arctic. Experimental studies comparing the high and low Arctic suggest that temperature may be a stronger constraint at more northern sites, and other factors, such as nutrient availability, may be stronger controls at more southern sites (Havström, Callaghan, and Jonasson Citation1993; Wookey et al. Citation1993). Because our site is in southern west Greenland at a relatively warm, nutrient-poor site, temperature may not be the primary limiting factor. The lack of a treatment effect may also have been caused by a relatively short period of warming (five years), although other studies describe advanced phenology after fewer years (Arft et al. Citation1999).
In order to predict the future carbon budget, it is important to understand constraints on plant phenology. In southern west Greenland, observed advances in phenology have increased ecosystem carbon sink strength by an estimated 1.3 g C m−2y−1 in Betula nana and 2.1 g C m−2y−1 in graminoid tundra (Cahoon, Sullivan, and Post Citation2016). If warming is not the primary control on seasonal dynamics, increased temperatures may not cause such strong carbon sinks. In conclusion, we suggest that temperature may not be the primary control on above- or belowground phenology in all Arctic ecosystems, and factors such as snowmelt and water and nutrient availability should also be considered when making predictions about future phenological shifts.
Acknowledgments
We are grateful for feedback from two anonymous reviewers and the associate editor, whose comments improved the manuscript. This work would not have been possible without the work of Thomas Adams, Lissette Bencosme, Thomas Bentley, Eva Beyen, Sean Cahoon, Elizabeth Elmstrom, Chénira Smith, Savannah Putnam, Tyler Tran, and Casey Yarbrough.
Additional information
Funding
References
- Abramoff, R. Z., and A. C. Finzi. 2015. Are above- and below-ground phenology in sync? New Phytologist 205 (3):1–13. doi:https://doi.org/10.1111/nph.13111.
- Anisimov, O. A., D. G. Vaughan, T. V. Callaghan, C. Furgal, H. Marchant, T. D. Prowse, H. Vilhjálmsson, and J. E. Walsh. 2007. Polar regions (Arctic and Antarctic). In Climate change 2007: Impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change, eds. M. L. Parry, O. F. Canziani, J. P. Palutikof, P. J. van Der Linden, and C. E. Hanson, 653–85. Cambridge: Cambridge University Press.
- Arft, A. M., M. D. Walker, J. Gurevitch, J. M. Alatalo, M. S. Bret-Harte, M. Dale, M. Diemer, F. Gugerli, G. H. R. Henry, M. H. Jones, et al. 1999. Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecological Monographs 69 (4):491–511.
- Bardgett, R. D., L. Mommer, and F. T. De Vries. 2014. Going underground: Root traits as drivers of ecosystem processes. Trends in Ecology & Evolution 29 (12):692–99. doi:https://doi.org/10.1016/j.tree.2014.10.006.
- Bates, D., M. Maechler, B. Bolker, and S. Walker. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67 (1):1–48. doi:https://doi.org/10.18637/jss.v067.i01.
- Bjorkman, A. D., S. C. Elmendorf, A. L. Beamish, M. Vellend, and G. H. R. Henry. 2015. Contrasting effects of warming and increased snowfall on arctic tundra plant phenology over the past two decades. Global Change Biology 21 (12):4651–61. doi:https://doi.org/10.1111/gcb.13051.
- Blume-Werry, G., R. Jansson, and A. Milbau. 2017. Root phenology unresponsive to earlier snowmelt despite advanced above-ground phenology in two subarctic plant communities. Functional Ecology 31 (7):1493–1502. doi:https://doi.org/10.1111/1365-2435.12853.
- Blume-Werry, G., J. Kreyling, H. Laudon, and A. Milbau. 2016. Short-term climate change manipulation effects do not scale up to long-term legacies: Effects of an absent snow cover on boreal forest plants. Journal of Ecology 104 (6):1638–48. doi:https://doi.org/10.1111/1365-2745.12636.
- Blume-Werry, G., S. D. Wilson, J. Kreyling, and A. Milbau. 2015. The hidden season: Growing season is 50% longer below than above ground along an arctic elevation gradient. New Phytologist 209 (3):978–86. doi:https://doi.org/10.1111/nph.13655.
- Boelman, N. T., M. Stieglitz, H. M. Rueth, M. Sommerkorn, K. L. Griffin, G. R. Shaver, and J. A. Gamon. 2003. Response of NDVI, biomass, and ecosystem gas exchange to long-term warming and fertilization in wet sedge tundra. Oecologia 135 (3):414–21. doi:https://doi.org/10.1007/s00442-003-1198-3.
- Burke, M. K., and D. J. Raynal. 1994. Fine-root growth phenology, production, and turnover in a northern hardwood forest ecosystem. Plant and Soil 162 (1):135–46. doi:https://doi.org/10.1007/BF01416099.
- Cahoon, S. M. P., P. F. Sullivan, and E. Post. 2016. Greater abundance of Betula nana and early onset of the growing season increase ecosystem CO2 uptake in west Greenland. Ecosystems 19 (7):1149–63. doi:https://doi.org/10.1007/s10021-016-9997-7.
- Chapin, F. S., G. R. Shaver, A. E. Giblin, K. J. Nadelhoffer, and J. A. Laundre. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76 (3):694–711. doi:https://doi.org/10.2307/1939337.
- Chen, H., Y. Dong, T. Xu, Y. Wang, H. Wang, and B. Duan. 2017. Root order-dependent seasonal dynamics in the carbon and nitrogen chemistry of poplar fine roots. New Forests 48:1–21.
- Cleland, E. E., I. Chuine, A. Menzel, H. A. Mooney, and M. D. Schwartz. 2007. Shifting plant phenology in response to global change. Trends in Ecology & Evolution 22 (7):357–65. doi:https://doi.org/10.1016/j.tree.2007.04.003.
- Elvebakk, A. 1999. Bioclimatic delimitation and subdivision of the arctic. In The species concept in the high north: A panarctic Flora initiative, conference proceedings, eds. I. Nordal and V. Y. Razzhivin, 81–112. Oslo: The Norwegian Academy of Science and Letters.
- Ernakovich, J. G., K. A. Hopping, A. B. Berdanier, R. T. Simpson, E. J. Kachergis, H. Steltzer, and M. D. Wallenstein. 2014. Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Global Change Biology 20 (10):3256–69. doi:https://doi.org/10.1111/gcb.12568.
- Flanner, M. G., K. M. Shell, M. Barlage, D. K. Perovich, and M. A. Tschudi. 2011. Radiative forcing and albedo feedback from the Northern hemisphere cryosphere between 1979 and 2008. Nature Geoscience 4 (3):151–55. doi:https://doi.org/10.1038/ngeo1062.
- Fu, Y. H., Y. Liu, H. J. De Boeck, A. Menzel, I. Nijs, M. Peaucelle, J. Peñuelas, S. Piao, and I. A. Janssens. 2016. Three times greater weight of daytime than of night-time temperature on leaf unfolding phenology in temperate trees. New Phytologist 212 (3):590–97. doi:https://doi.org/10.1111/nph.14073.
- Fu, Y. S. H., H. F. Zhao, S. L. Piao, M. Peaucelle, S. S. Peng, G. Y. Zhou, P. Ciais, M. T. Huang, A. Menzel, J. P. Uelas, et al. 2015. Declining global warming effects on the phenology of spring leaf unfolding. Nature 526 (7571):104–7. doi:https://doi.org/10.1038/nature15402.
- Havström, M., T. V. Callaghan, and S. Jonasson. 1993. Differential growth responses of cassiope tetragona, an arctic dwarf-shrub, to environmental perturbations among three contrasting high- and subarctic sites. Oikos 66 (3):389–402. doi:https://doi.org/10.2307/3544933.
- Henry, G. H. R., and U. Molau. 1997. Tundra plants and climate change: The international tundra experiment (ITEX). Global Change Biology 3 (S1):31–39.
- Iler, A. M., T. T. Hoye, D. W. Inouye, and N. M. Schmidt. 2013a. Long-term trends mask variation in the direction and magnitude of short-term phenological shifts. American Journal of Botany 100 (7):1398–406. doi:https://doi.org/10.3732/ajb.1200490.
- Iler, A. M., T. T. Høye, D. W. Inouye, and N. M. Schmidt. 2013b. Nonlinear flowering responses to climate: Are species approaching their limits of phenological change? Philosophical Transactions of the Royal Society B: Biological Sciences 368:1624. doi:https://doi.org/10.1098/rstb.2012.0489.
- IPCC. 2014. Climate change 2014: Impacts, adaptation, and vulnerability, part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom, and New York, NY, USA. 1,132 pp.
- Iversen, C. M., V. L. Sloan, P. F. Sullivan, E. S. Euskirchen, A. D. McGuire, R. J. Norby, A. P. Walker, J. M. Warren, and S. D. Wullschleger. 2015. The unseen iceberg: Plant roots in arctic tundra. New Phytologist 205 (1):34–58. doi:https://doi.org/10.1111/nph.13003.
- Joslin, J. D., M. H. Wolfe, and P. J. Hanson. 2001. Factors controlling the timing of root elongation intensity in a mature upland oak stand. Plant and Soil 228 (2):201–12. doi:https://doi.org/10.1023/A:1004866705021.
- Kremers, K. S., R. D. Hollister, and S. F. Oberbauer. 2015. Diminished response of arctic plants to warming over time. PLoS One 10 (3):e0116586. doi:https://doi.org/10.1371/journal.pone.0116586.
- Kuznetsova, A., P. B. Brockhoff, and R. H. B. Christensen. 2016. lmerTest: Tests in linear mixed effects models. Available at https://cran.r-project.org/web/packages/lmerTest/index.html.
- McBean, G., G. Alekseev, D. Chen, E. Førland, J. Fyfe, P. Y. Groisman, R. King, H. Melling, R. Vose, and P. H. Whitfield. 2005. Arctic climate: Past and present. In Arctic climate impacts assessment (ACIA), eds. C. Symon, L. Arris, and B. Heal, 20–60. Cambridge: Cambridge University Press.
- McCormack, M. L., T. S. Adams, E. A. H. Smithwick, and D. M. Eissenstat. 2014. Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 95 (8):2224–35. doi:https://doi.org/10.1890/13-1942.1.
- Menzel, A., T. H. Sparks, N. Estrella, E. Koch, A. Aasa, R. Ahas, K. Alm-Kübler, P. Bissolli, O. G. Braslavská, A. Briede, et al. 2006. European phenological response to climate change matches the warming pattern. Global Change Biology 12 (10):1969–76. doi:https://doi.org/10.1111/j.1365-2486.2006.01193.x.
- Myers-Smith, I. H., B. C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K. D. Tape, M. Macias-Fauria, U. Sass-Klaassen, E. Levesque, et al. 2011. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters 6:4. doi:https://doi.org/10.1088/1748-9326/6/4/045509.
- Oberbauer, S. F., S. C. Elmendorf, T. G. Troxler, R. D. Hollister, A. V. Rocha, M. S. Bret-Harte, M. A. Dawes, A. M. Fosaa, G. H. R. Henry, T. T. Hoye, et al. 2013. Phenological response of tundra plants to background climate variation tested using the international tundra experiment. Philosophical Transactions of the Royal Society B-Biological Sciences 368:1624. doi:https://doi.org/10.1098/rstb.2012.0481.
- Parmesan, C., and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421 (6918):37–42. doi:https://doi.org/10.1038/nature01286.
- Peñuelas, J., and I. Filella. 2001. Responses to a warming world. Science 294 (5543):793–95. doi:https://doi.org/10.1126/science.1066860.
- Post, E., and M. C. Forchhammer. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Transactions of the Royal Society B-Biological Sciences 363 (1501):2369–75. doi:https://doi.org/10.1098/rstb.2007.2207.
- Post, E., M. C. Forchhammer, M. S. Bret-Harte, T. V. Callaghan, T. R. Christensen, B. Elberling, A. D. Fox, O. Gilg, D. S. Hik, T. T. Høye, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325 (5946):1355–58. doi:https://doi.org/10.1126/science.1173113.
- Post, E., J. Kerby, C. Pedersen, and H. Steltzer. 2016. Highly individualistic rates of plant phenological advance associated with arctic sea ice dynamics. Biology Letters 12:12. doi:https://doi.org/10.1098/rsbl.2016.0332.
- Post, E., and C. Pedersen. 2008. Opposing plant community responses to warming with and without herbivores. Proceedings of the National Academy of Sciences of the United States of America 105 (34):12353–58. doi:https://doi.org/10.1073/pnas.0802421105.
- Post, E. S., C. Pedersen, C. C. Wilmers, and M. C. Forchhammer. 2008. Phenological sequences reveal aggregate life history response to climatic warming. Ecology 89 (2):363–70. doi:https://doi.org/10.1890/06-2138.1.
- Prevéy, J., M. Vellend, N. Rüger, R. D. Hollister, A. D. Bjorkman, I. H. Myers-Smith, S. C. Elmendorf, K. Clark, E. J. Cooper, B. Elberling, et al. 2017. Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Global Change Biology 23 (7):2660–71. doi:https://doi.org/10.1111/gcb.13619.
- Radville, L., T. L. Bauerle, L. H. Comas, K. A. Marchetto, A. N. Lakso, D. R. Smart, R. M. Dunst, and D. M. Eissenstat. 2016a. Limited linkages of aboveground and belowground phenology: A study in grape. American Journal of Botany 103 (11):1897–911. doi:https://doi.org/10.3732/ajb.1600212.
- Radville, L., M. L. McCormack, E. Post, and D. M. Eissenstat. 2016b. Root phenology in a changing climate. Journal of Experimental Botany 67 (12):3617–28. doi:https://doi.org/10.1093/jxb/erw062.
- Radville, L., E. Post, and D. M. Eissenstat. 2016c. Root phenology in an Arctic shrub-graminoid community: The effects of long-term warming and herbivore exclusion. Climate Change Responses 3 (1):1–9. doi:https://doi.org/10.1186/s40665-016-0017-0.
- RCoreTeam. 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Reyes-Fox, M., H. Steltzer, M. J. Trlica, G. S. McMaster, A. A. Andales, D. R. LeCain, and J. A. Morgan. 2014. Elevated CO2 further lengthens growing season under warming conditions. Nature 510 (7504):259–62. doi:https://doi.org/10.1038/nature13207.
- Richardson, A. D., T. F. Keenan, M. Migliavacca, Y. Ryu, O. Sonnentag, and M. Toomey. 2013. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agricultural and Forest Meteorology 169:156–73.
- Rumpf, S. B., P. R. Semenchuk, S. Dullinger, and E. J. Cooper. 2014. Idiosyncratic responses of high arctic plants to changing snow regimes. PLoS One 9 (2):e86281. doi:https://doi.org/10.1371/journal.pone.0086281.
- Satterthwaite, F. E. 1946. An approximate distribution of estimates of variance components. Biometrics Bulletin 2:110–14.
- Semenchuk, P. R., M. A. K. Gillespie, S. B. Rumpf, N. Baggesen, B. Elberling, and E. J. Cooper. 2016. High arctic plant phenology is determined by snowmelt patterns but duration of phenological periods is fixed: An example of periodicity. Environmental Research Letters 11 (12):125006. doi:https://doi.org/10.1088/1748-9326/11/12/125006.
- Sharp, E. D., P. F. Sullivan, H. Steltzer, A. Z. Csank, and J. M. Welker. 2013. Complex carbon cycle responses to multi-level warming and supplemental summer rain in the high arctic. Global Change Biology 19 (6):1780–92. doi:https://doi.org/10.1111/gcb.12149.
- Sloan, V. L., B. J. Fletcher, and G. K. Phoenix. 2016. Contrasting synchrony in root and leaf phenology across multiple sub-Arctic plant communities. Journal of Ecology 104 (1):239–48. doi:https://doi.org/10.1111/1365-2745.12506.
- Street, L. E., G. R. Shaver, M. Williams, and M. T. van Wijk. 2007. What is the relationship between changes in canopy leaf area and changes in photosynthetic CO2 flux in arctic ecosystems? Journal of Ecology 95 (1):139–50. doi:https://doi.org/10.1111/jec.2007.95.issue-1.
- Tierney, G. L., T. J. Fahey, P. M. Groffman, J. P. Hardy, R. D. Fitzhugh, C. T. Driscoll, and J. B. Yavitt. 2003. Environmental control of fine root dynamics in a northern hardwood forest. Global Change Biology 9 (5):670–79. doi:https://doi.org/10.1046/j.1365-2486.2003.00622.x.
- van Wijk, M. T., M. Williams, L. Gough, S. E. Hobbie, and G. R. Shaver. 2003. Luxury consumption of soil nutrients: A possible competitive strategy in above-ground and below-ground biomass allocation and root morphology for slow-growing arctic vegetation? Journal of Ecology 91 (4):664–76. doi:https://doi.org/10.1046/j.1365-2745.2003.00788.x.
- Vihma, T. 2014. Effects of arctic sea ice decline on weather and climate: A review. Surveys in Geophysics 35 (5):1175–214. doi:https://doi.org/10.1007/s10712-014-9284-0.
- Visser, M. E., and C. Both. 2005. Shifts in phenology due to global climate change: The need for a yardstick. Proceedings of the Royal Society B-Biological Sciences 272 (1581):2561–69. doi:https://doi.org/10.1098/rspb.2005.3356.
- Wahren, C. H. A., M. D. Walker, and M. S. Bret-Harte. 2005. Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Global Change Biology 11 (4):537–52. doi:https://doi.org/10.1111/j.1365-2486.2005.00927.x.
- Wang, P., L. Mommer, J. van Ruijven, F. Berendse, T. C. Maximov, and M. Heijmans. 2016. Seasonal changes and vertical distribution of root standing biomass of graminoids and shrubs at a Siberian tundra site. Plant and Soil 407 (1–2):55–65. doi:https://doi.org/10.1007/s11104-016-2858-5.
- Wielgolaski, F. E. 1999. Starting dates and basic temperatures in phenological observations of plants. International Journal of Biometeorology 42 (3):158–68. doi:https://doi.org/10.1007/s004840050100.
- Wipf, S. 2010. Phenology, growth, and fecundity of eight subarctic tundra species in response to snowmelt manipulations. Plant Ecology 207 (1):53–66. doi:https://doi.org/10.1007/s11258-009-9653-9.
- Wipf, S., and C. Rixen. 2010. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Research 29 (1):95–109. doi:https://doi.org/10.1111/j.1751-8369.2010.00153.x.
- Wipf, S., V. Stoeckli, and P. Bebi. 2009. Winter climate change in alpine tundra: Plant responses to changes in snow depth and snowmelt timing. Climatic Change 94 (1):105–21. doi:https://doi.org/10.1007/s10584-009-9546-x.
- Wolkovich, E. M., B. I. Cook, J. M. Allen, T. M. Crimmins, J. L. Betancourt, S. E. Travers, S. Pau, J. Regetz, T. J. Davies, N. J. B. Kraft, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485 (7399):494–97.
- Wookey, P. A., A. N. Parsons, J. M. Welker, J. A. Potter, T. V. Callaghan, J. A. Lee, and M. C. Press. 1993. Comparative responses of phenology and reproductive development to simulated environmental change in sub-arctic and high arctic plants. Oikos 67 (3):490–502. doi:https://doi.org/10.2307/3545361.
