ABSTRACT
Glaciers overrun organic matter (OM) during periods of advance, making this overrun OM available as a metabolic substrate for subglacial microbes. The biogeochemical fate of this overrun OM remains poorly understood, ultimately limiting our understanding of subglacial biogeochemical cycling, particularly for cold-based glaciers. This study presents evidence for the biogeochemical transformation of algal mat material that was overrun by a cold-based glacier (Suess Glacier, Taylor Valley, Antarctica) during its advance 4840–3570 years BP. We use a suite of stable isotope analyses to show that active nitrogen cycling has depleted N-isotope values to amongst the lowest reported in Taylor Valley (-15.59 ‰) from an initial value of ~-1.88 ‰, while potentially depleting C-isotope values by 2.46 ‰. While this study examines biogeochemical conditions beneath a single glacier, all glaciers export meltwater during the melt season that may host algae and other OM in proglacial streams and lakes that may be overridden during glacier advance. As such, subglacial nitrogen cycling detected here may represent processes that occur in cold zones beneath glaciers generally.
Introduction
Once considered to be biologically sterile, glacial and subglacial environments are now recognized as microbially active and biogeochemically relevant ecosystems (Anesio and Laybourn-Parry Citation2012; Fountain and Tranter Citation2008; Hodson et al. Citation2008; Stibal, Sabacka, and Zarsky Citation2012). Glaciers cover approximately 10 percent of the Earth’s land surface and have often overrun substrata with high organic matter (OM) content, such as soils and limnic sediments (Barker et al. Citation2006; Willerslev et al. Citation2007). The biogeochemical fate of this legacy OM in glacial environments remains poorly constrained, ultimately limiting our understanding of the global significance of glacial environments in global biogeochemical cycling. This is particularly true of cold-based glaciers (those that are frozen to the glacier bed), tidewater glaciers, and ice sheets where subglacial meltwater cannot necessarily be used to monitor biogeochemical processes occurring subglacially. Thus, subglacial environments beneath large ice sheets represent an unknown in our understanding of the global nutrient cycling, where the potential exists for biogeochemically altered substrates to produce potentially important metabolic byproducts such as methane, carbon dioxide, or NOx species.
Here, we present evidence for the biogeochemical transformation of overrun OM beneath Suess Glacier, a cold-based alpine glacier in Taylor Valley, Antarctica. We use a suite of isotopic analyses to show that subglacial heterotrophy affects nitrogen cycling using an ancient organic substratum.
Methods
Field site and sample collections
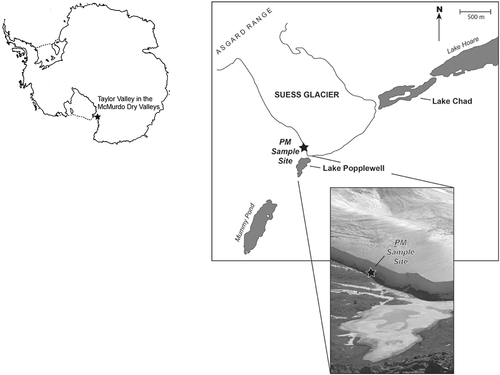
Suess Glacier (77° 38.70′S, 162° 42.61′E) is a 5 km long alpine glacier that descends from the Asgard Range and crosses the floor of Taylor Valley, Antarctica (). It currently forms a physical barrier that separates Lake Popplewell (Fitzsimons Citation1996; also referred to as Mummy Pond by Hendy Citation2000) to the west and Lake Chad to the east. Suess Glacier is cold-based and perennially frozen to the underlying bed. As is typical of other Dry Valley glaciers, Suess Glacier displays thick sequences (~10 m) of layered debris incorporated into its base. Stable isotopic evidence indicates that this ice formed by the accretionary freezing of wet sediment onto the glacier sole (Lorrain et al. Citation1999) during its advance across Taylor Valley.
Figure 1. The location of the PM sampling site at Suess Glacier, Taylor Valley, Antarctica (77° 38.70′S, 162° 42.61′E). Antarctica base map courtesy of the National Science Foundation (Citation2017)
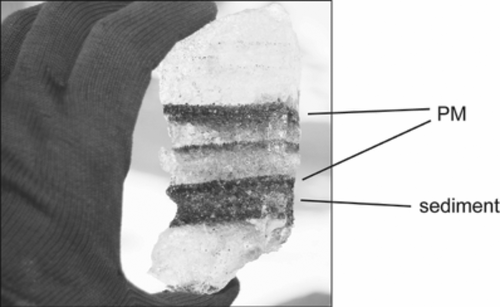
Thin stringers of organic mud, likely of algal origin, are found within the parallel laminae in the basal ice (Lorrain et al. Citation1999). This organic mud appears as dark, fine-grained, or fissile material in distinct layers in contact with the coarser-grained sedimentary matrix (). The organic mud is readily identifiable by its pungent odor, and is referred to here as pungent mud (PM) to differentiate it from other, nonpungent OM.
We sampled PM from a site located approximately 5 m above Lake Popplewell and approximately 25 cm beneath the upper surface of accreted basal ice on Suess Glacier’s the west margin. Two PM samples were excavated by digging a small trench with an ethanol-bathed and flame-sterilized chisel to a depth of 10 cm into the basal ice. Samples were placed in sterile Whirlpack bags and were transported frozen and in the dark to the Ohio State University. The samples were thawed in the dark, dried at 60°C, and combined into a single PM sample. They were then homogenized and subsampled for radiocarbon, stable isotopic analyses, and an analysis of siliceous microfossil content. All subsamples were stored frozen in sterile Whirlpack bags prior to analysis.
Dried algae was collected from the surface of the permanent ice cover on Lake Chad and was used as a reference material against which the PM was compared. Lake Chad is a shallow lake (maximum depth of ~2 m) on the east side of Suess Glacier’s lateral moraine, with intermittent hydrological connection to Lake Hoare. This type of entombed algal deposit has been observed in the permanent ice cover of other Dry Valley lakes and is thought to have been entrained into the lower surface of the ice cover and advected up through the ice as the upper surface sublimates and new ice is added to the lower surface (Parker et al. Citation1982). Advected algae was not found in the Lake Popplewell ice cover, and there was not an open moat from which algae could be directly sampled from the lake bed at the time of sampling from Suess Glacier basal ice. We assume that the Lake Chad algae is reasonably representative of algal material from shallow lakes in the Dry Valleys that has not undergone any subglacial biogeochemical alteration. Lake Chad algae was sampled with an ethanol-bathed and flame-sterilized stainless-steel blade and was stored frozen in sterile Whirlpack bags prior to analysis.
Siliceous microfossils
Pungent mud subsamples were prepared for siliceous microfossil analysis by oxidation in 30 percent H2O2 followed by repeated rinsing with deionized water. Subsamples of the slurry were air dried onto aluminum stubs, sputter-coated with Au, and examined with a JEOL-6301F field-emission scanning electron microscope (SEM) operating at 25 kV.
Radiocarbon dating
Two frozen PM subsamples were thawed, ground to a powder, transferred into sterile aluminum dishes, and acid fumigated with trace metal grade HCl for 12 h to remove carbonate (Lorrain et al. Citation2003). After fumigation, they were placed in sterile amber glass vials that had been combusted at 500°C for 8 h. 14C age was determined by accelerator mass spectrometry (AMS) at the NSF-Arizona AMS Facility (University of Arizona). Subsequent calibration of the 14C age to age before present (BP) was performed at the NSF-Arizona AMS Facility using the SHCal13 atmospheric curve (Hogg, Hua, and Blackwell Citation2013).
Stable isotopic analysis
Two frozen PM subsamples were thawed and fumigated as described earlier. These subsamples were ground to a dry powder and analyzed in duplicate for stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) in the Stable Isotope Biogeochemistry Laboratory at the Ohio State University. Each subsample was combusted in a Costech Elemental Analyzer and the resultant CO2 and N2 gases were analyzed for their C and N stable isotope composition, respectively, using a Finnigan Delta IV Plus isotope ratio mass spectrometer via a Finnigan ConFlow III open split interface. Acetanilide was used as a standard for determining the percent C and N in each sample. The δ13C (the permil deviation of 13C:12C relative to Vienna Peedee Belemnite Limestone Standard [V-PDB; Coplen Citation1994]) and δ15N (permil deviation of 15N:14N relative to atmospheric nitrogen [Mariotti Citation1983]) were measured in triplicate on the two PM subsamples. Analytical precision for δ13C and δ15N were ±0.12‰ and 0.22‰, respectively.
Results
The radiocarbon age of PM is 4,211 (±40 years), corresponding to a calibrated age of 4840–3570 years BP. Perhaps the most noticeable feature of the PM is its pungent odor (rotting vegetation, septic, putrid) and dark color relative to surrounding sediment (). This odor was not detected in any streams or ice-free moats around the perimeter of lakes Fryxell, Hoare, or Bonney, and was not detected in buried algae that was exposed along stream-cut banks in Taylor Valley. Further, the odor disappeared when PM was exposed to air for more than approximately 72 h.
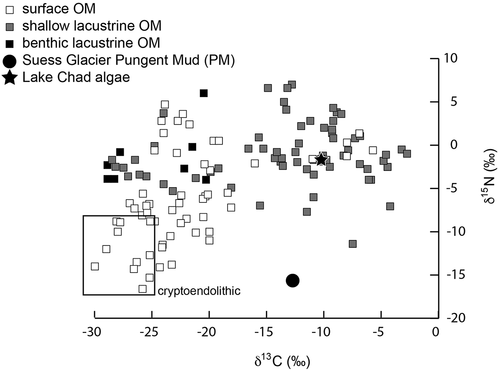
illustrates the δ13C and δ15N values of PM, Lake Chad algae, and previously reported values for OM in the McMurdo Dry Valleys. Previously reported δ13C values for lacustrine OM (mostly algal mat material) show less depleted δ13C than benthic lacustrine OM, ranging from −2.76‰ (cyanobacteria in a shallow moat, Lake Fryxell; Lawson et al. Citation2004) to −38.9‰ (OM from −35 m depth, Lake Bonney; data not shown because no corresponding δ15N is given by Lawson et al. Citation2004). Lacustrine OM δ15N values do not show a clear relationship with water depth but instead show strong δ15N depletion for cryptoendolithic OM relative to lacustrine-derived OM (inset in ). The δ15N values range from 6‰ (surface mat, Lake Hoare; Wharton, Lyons, and DesMarais Citation1993) to −16.6‰ (Battleship Promontory soil OM 137 m above Lake Hoare; Burkins et al. Citation2000). The Battleship Promontory data point is from endolith-derived OM (Burkins et al. Citation2000) sampled from sandstone in Alatna Valley in the McMurdo Dry Valleys. The soil data points surrounding the endolith-derived OM are generally from samples collected higher above Taylor Valley lakes and, presumably, are derived from OM remaining from previous high-lake stands (Burkins et al. Citation2000). The Lake Chad algae has a δ13C of −10.29‰ ±0.05 (n = 2) and a δ15N of −1.88‰ ±0.02 (n = 2), suggesting that it is of shallow lacustrine or surface origin, which is in accordance with the environment from which it was sampled. The mean PM δ13C value is −12.75‰ ±0.7 (n = 2), suggesting a shallow lacustrine or surface origin; however, the mean δ15N value of −15.59‰ ±0.4 (n = 2) is strongly depleted and suggestive of a cryptoendolithic origin.
Figure 3. The ratio of δ13C to δ15N for the PM sample and Lake Chad algae sample relative to previously reported values for OM in the McMurdo Dry Valleys (Burkins et al. Citation2000; Lawson et al. Citation2004; Wharton, Lyons, and DesMarais Citation1993). The most strongly depleted δ15N values are associated with OM that is associated with terrestrial cryptoendolithic communities (Burkins et al. Citation2000)
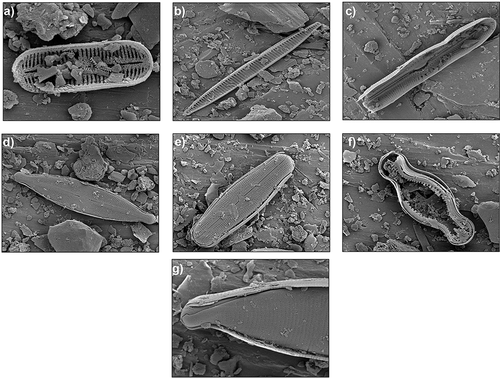
The siliceous microfossil assemblage present in the PM contains benthic freshwater diatom species of the genera Chamaepinnularia, Diadesmis, Hantzschia, Muelleria, Nitzschia, and Stauroneis, which were moderately well preserved with frequent fragmentation (). These taxa are representative of the littoral flora of lakes in the McMurdo Dry Valleys (Spaulding et al. Citation2017). No extinct freshwater marine taxa, such as those reported in Miocene sediments from the region (Lewis et al. Citation2008), were present.
Figure 4. Siliceous microfossils associated with the PM deposit. (A) Diadesmis sp. (×11,000 magnification), (B) Nitzschia westii (×3,000), (C) Muelleria varipunctata (×2,700), (D) Stauroneis anceps (×2,500), (E) Muelleria sabbei (×2,500), (F) Chamaepinnularia sp. (×4,000), (G) Hantzchia amphioxys (×6,500)
Discussion
Pungent mud source
The PM collected from the west margin of Suess Glacier is between 4,840 and 3,570 years old (~4,211 14C years), providing an estimate of (1) when algae grew in a lacustrine environment on the Taylor Valley floor and (2) a maximum age for Suess Glacier advance to the Taylor Valley floor and incorporation into Suess Glacier basal ice. This date agrees with the conclusion by Denton et al. (Citation1989) that Taylor Valley alpine glaciers advanced during this time. The age of the PM deposit and its current position of approximately 5 m above the current lake level suggests either that Lake Popplewell was larger when Suess Glacier overran it or that a low-energy stream flowing into Lake Popplewell existed at this location.
Several lines of evidence indicate that the subglacial PM originated as algal mat material from a shallow freshwater aquatic environment along the Taylor Valley floor prior to being buried and overrun by an advancing Suess Glacier. First, the preservation of laminated sediment in basal ice along Suess Glacier’s west margin indicates that unfrozen lacustrine sediment from Lake Popplewell froze onto the bottom of Suess Glacier as it descended from the mountains of the Asgard Range (Lorrain et al. Citation1999). The overriding Suess Glacier acted as a cold source that froze water and entrapped sediment, and any OM (e.g., algae), onto its base during basal ice formation (Lorrain et al. Citation1999). Second, stable isotopic analyses of the basal ice (δ18O and δD) do not follow the meteoric water line, indicating that lake-water freezing makes a significant contribution to the basal ice sequence (Lorrain et al. Citation1999; Sleewaegen et al. Citation2003). Finally, diatoms identified in the PM sample represent a typical Holocene assemblage found in shallow freshwater environments in the Dry Valleys (Spaulding et al. Citation2017), suggesting that the PM originated as a perennially subaqueous algal mat formed in a shallow freshwater environment.
Biogeochemical alteration since burial
Despite the biologically hostile environment implied by accretion onto the base of a cold-based glacier, the PM’s odor suggests that it has been biogeochemically degraded by microorganisms since its burial. The putrid odor can be generated by a variety of compounds that are associated with the microbial degradation of algae and diatoms under anoxic conditions. For example, the rotting vegetation odor may be associated with cleavage products of unsaturated fatty acids, while the septic odor may be associated with dimethyl disulfide and dimethyl trisulfide produced during the microbial degradation of algae (Costaris et al. Citation1995). Rancid and/or putrid odors can be generated by the bacterial oxidation of the amino acid leucine (Hrudey, Gac, and Daignault Citation1988). While we did not attempt to determine the presence or absence of these compounds during this study, the odors associated with PM have not been reported for living algal communities in Dry Valley streams and open moats in Dry Valley lakes or in dried algae (Lake Chad algae) or buried algae that are exposed in stream-cut banks. Further, the disappearance of the odor on exposure to oxic conditions suggests that anaerobic processes predominate the OM degradation, further suggesting that the PM’s odor results from anaerobic subglacial processes.
Similar to PM, the algae analyzed from Lake Chad originated from a shallow lacustrine environment. Lake Chad algae δ15N signatures lie within values cited for live lacustrine algal mat material (), supporting our hypothesis that it can be used as a reference material for shallow lacustrine algae in Taylor Valley. The difference in stable isotope ratios between Lake Chad algae and PM indicates subglacial microbial activity and suggests that OM has been biogeochemically modified in the 4,840–3,570 years since its burial.
The main factor influencing the δ13C of algal mat OM is the limit imposed by the cell membrane as CO2 diffuses across it, resulting in a depleted δ13C in the order of −3 to −20‰. Additional 13C depletion in live algae can result from increases in pCO2 of the surrounding water (Burkins et al. Citation2000) and/or the selective preservation of 13C-depleted compounds during the degradation of algae by heterotrophic microorganisms (Lehmann et al. Citation2002). While the PM and the Lake Chad algae fall within the range reported for Dry Valley algal material, the PM is depleted by 2.4‰ relative to the Lake Chad algae (). Whether this depletion results from subglacial degradation or is the result of limnic conditions during algal mat growth is unknown.
The PM δ15N value is more depleted than those reported for lacustrine algal mat material in the Dry Valleys and is 13.7‰ more depleted than Lake Chad algae. However, the PM δ15N value falls within the range of those reported for endolithic microbial communities and OM found on older surfaces in Taylor Valley (; Burkins et al. Citation2000). This may not be surprising, because the subglacial environment beneath cold-based glaciers is similar in many respects to more familiar endolithic environments. Similar to other endolithic habitats (e.g., cryptoendolithic and hypoendolithic habitats), the subglacial environment is shielded from incoming solar radiation, in this case by the light-attenuating effect of the surrounding ice (e.g., Zhao et al. Citation2010) or sediment. However, oxygen depletion or anoxia may be more prevalent in subglacial environments than in other crypto/hypoendolithic environments, particularly beneath cold-based glaciers where replenishment by oxygenated supraglacial meltwater does not occur. The only potentially significant subglacial source of oxygen at Suess Glacier is from a limited input from trapped bubbles in the ice.
Although endolithic communities are capable of nitrogen fixation (Cowan et al. Citation2011), the endolith-mediated nitrogen cycle appears to use predominantly abiotically fixed nitrogen and assimilated ammonia as nitrogen sources (Friedmann and Kibler Citation1980). Large fractionations associated with the incorporation of both NO3− and NH3 into microbial biomass results in their depleted bulk δ15N. δ15N depletion is also reported for bacterially mediated anaerobic ammonium oxidiation (anammox) reactions. The anammox catalyzed reduction of NO2− to NO3− is associated with a N isotope effect of approximately −13.1‰ (Brunner et al. Citation2013), and while these reactions would deplete δ15N values in the original OM (represented by Lake Chad algae) to δ15N levels reported for the PM, we did not measure NO2−, NO3−, or N2, and so we cannot determine if anammox reactions are occurring. While mechanisms that anaerobically deplete δ15N in OM to levels reported here for the subglacial PM are known in other environments (e.g., Brunner et al. Citation2013; Clement et al. Citation2005), we lack the data to definitively isolate the biogeochemical process responsible here.
Summary and implications
We have identified a 4,840–3,570 year old lacustrine algal material beneath Suess Glacier, a cold-based valley glacier in the McMurdo Dry Valleys. This material was buried and incorporated into Suess Glacier basal ice during the Holocene advance of alpine glaciers from the Asgard Mountains to the Taylor Valley floor. The location of the PM deposit suggests that the area was a shallow freshwater environment, approximately 10 m above the current Lake Popplewell level. The PM’s strong odor indicates that OM has been degraded under anaerobic conditions beneath Suess Glacier.
Stable isotopic analyses of the bulk PM material is consistent with the hypothesis that metabolized OM led to pronounced depletion of 15N. The extent of nitrogen isotopic depletion observed here is exceptional and is among the most depleted δ15N values reported for the McMurdo Dry Valleys, suggesting biological fractionation within the subglacial nitrogen cycle that is comparable to that observed in endolithic microbial communities. The depleted δ15N in endolithic communities results from the use of isotopically depleted allochthonous NO3− as the proximate N source, or depleted autochthonous NO3− resulting from anammox reactions. In contrast, the biogeochemical processes operating under Suess Glacier exploit autochthonous N associated with legacy OM, resulting in its δ15N depletion. The depleted PM δ15N and strong odor indicate that biologically mediated OM degradation is occurring beneath Suess Glacier, leading to preferential accumulation of 14N. While the process responsible for this is unknown, it appears that active nitrogen cycling using an ancient organic substrate is occurring.
This is the first documentation of biogeochemical transformations involving a defined organic substratum in a polar subglacial environment. However, the biogeochemical transformation of glacially overrun organic-rich lake sediments is potentially a widespread glacial phenomenon, because most glaciers export meltwater during the melt season and potentially create downstream algal-rich aquatic environments. These OM-rich sediments can subsequently be overridden during glacier advance, and consequently, subglacial nitrogen cycling and the synthesis of biogeochemical byproducts, as observed beneath Suess Glacier, may be occurring beneath glaciers elsewhere.
Acknowledgments
The authors would like to thank Alex Wolfe for imaging and identifying the diatoms, as well as the McMurdo Dry Valleys Long Term Ecological Research (LTER) program for supporting the fieldwork component of this project. We are thankful to Martyn Tranter and two anonymous reviewers who greatly improved the quality of this manuscript.
References
- Anesio, A. M., and J. Laybourn-Parry. 2012. Glaciers and ice sheets as a biome. Trends in Ecology and Evolution 27:1–7.
- Barker, J. D., M. J. Sharp, S. J. Fitzsimons, and R. J. Turner. 2006. Abundance and dynamics of dissolved organic carbon in glacier systems. Arctic, Antarctic, and Alpine Research 38:163–72.
- Brunner, B., S. Contreras, M. F. Lehmann, O. Matantseva, M. Rollog, T. Kalvelage, G. Klockgether, G. Lavik, M. S. M. Jetten, B. Kartal, et al. 2013. Nitrogen isotope effects induced by anammox bacteria. Proceedings of the National Academy of Science 110:18994–99.
- Burkins, M. B., R. A. Virginia, C. P. Chamberlain, and D. H. Wall. 2000. Origin and distribution of soil organic matter in Taylor Valley, Antarctica. Ecology 81:2377–91.
- Clement, J.-C., J. Shrestha, J. G. Ehrenfeld, and P. R. Jaffe. 2005. Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biology and Biochemistry 37:2323–28.
- Coplen, T. B. 1994. Reporting of stable hydrogen, carbon, and oxygen isotopic abundances. Pure and Applied Chemistry 66:273–76.
- Costaris, E., A. Bruchet, J. Mallevialle, and D. B. Bursill. 1995. The identification of odorous metabolites produced from algal monocultures. Water Science and Technology 31:251–58.
- Cowan, D. A., J. A. Sohm, T. P. Makhalanyane, D. G. Capone, T. G. A. Green, S. C. Cary, and I. M. Tuffin. 2011. Hypolithic communities: Important nitrogen sources in Antarctic desert soils. Environmental Microbiology Reports 3:581–86.
- Denton, G. H., J. G. Bockheim, S. C. Wilson, and M. Stuiver. 1989. Lake Wisconsin and early Holocene glacial history, inner Ross Embayment, Antarctica. Quaternary Research 31:151–82.
- Fitzsimons, S. J. 1996. Formation of thrust-block moraines at the margins of dry-based glaciers, south Victoria Land, Antarctica. Annals of Glaciology 22:68–74.
- Fountain, A. G., and M. Tranter. 2008. Introduction to a special section on microcosms in ice: Biogeochemistry of Cryoconite Holes. Journal of Geophysical Research 113:G02S91.
- Friedmann, E. I., and A. P. Kibler. 1980. Nitrogen economy of endolithic microbial communities in hot and cold deserts. Microbial Ecology 6:95–108.
- Hendy, C. H. 2000. Late Quaternary lakes in the McMurdo Sound region of Antarctica. Geografiska Annaler 82:A: 411–432.
- Hodson, A., A. M. Anesio, M. Tranter, A. Fountain, M. Osborn, J. Priscu, J. Laybourn-Parry, and B. Sattler. 2008. Glacial ecosystems. Ecological Monographs 78:41–67.
- Hogg, A. G., Q. Hua, and P. G. Blackwell. 2013. SHCal13 southern hemisphere calibration, 0–50,000 years cal BP. Radiocarbon 55:1889–903.
- Hrudey, S. E., A. Gac, and S. A. Daignault. 1988. Potent odour-causing chemicals arising from drinking water disinfection. Water Science and Technology 20:55–61.
- Lawson, J., P. T. Doran, F. Kenig, D. J. Des Marais, and J. C. Priscu. 2004. Stable carbon and nitrogen isotopic composition of benthic and pelagic organic matter in lakes of the McMurdo Dry Valleys, Antarctica. Aquatic Geochemistry 10:269–301.
- Lehmann, M. F., S. M. Bernasconi, A. Barbieri, and J. A. McKenzie. 2002. Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochimica Et Cosmochimica Acta 66:3573–84.
- Lewis, A. R., D. R. Marchant, A. C. Ashworth, L. Hedenas, S. R. Hemming, J. V. Johnson, M. J. Leng, M. L. Machlus, A. E. Newton, J. I. Raine, et al. 2008. Mid-Miocene cooling and the extinction of tundra in continental Antarctica. Proceedings of the National Academy of Science 105:10676–80.
- Lorrain, A., N. Savoye, L. Chauvaud, Y. M. Paulet, and N. Naulet. 2003. Decarbonation and preservation method for the analysis of organic C and N contents and stable isotope ratios of low-carbonated suspended particulate material. Analytica Chimica Acta 491:125–33.
- Lorrain, R. D., S. J. Fitzsimons, M. J. Vandergoes, and M. Stievenard. 1999. Ice composition evidence for the formation of basal ice from lake water beneath a cold-based Antarctic glacier. Annals of Glaciology 28:277–81.
- Mariotti, A. 1983. Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303:685–87.
- National Science Foundation. 2017. U.S. Permanent Stations in Antarctica. https://www.usap.gov/USAPgov/sciencesupport/GIS/images/AntarcticPermStations.gif.
- Parker, B. C., G. M. Simmons Jr., R. A. Wharton Jr., and K. G. Seaburg. 1982. Removal of organic and inorganic matter from Antarctic lakes by aerial escape of bluegreen algal mats. Journal of Phycology 18:72–78.
- Sleewaegen, S., D. Samyn, S. J. Fitzsimons, and R. D. Lorrain. 2003. Equifinality of basal ice facies from an Antarctic cold-based glacier. Annals of Glaciology 37:257–62.
- Spaulding, S., R. Esposito, D. Lubinski, S. Horn, M. Cox, D. McKnight, A. Alger, B. Hall, M. Mayernick, T. Whittaker, etal. 2017. Antarctic Freshwater Diatoms web site, McMurdo Dry Valleys LTER. http://huey.colorado.edu/diatoms/.
- Stibal, M., M. Sabacka, and J. Zarsky. 2012. Biological processes on glacier and ice sheet surfaces. Nature Geoscience 5:771–74.
- Wharton, R. A., Jr., W. B. Lyons, and D. J. DesMarais. 1993. Stable isotopic geochemistry of carbon and nitrogen in a perennially ice-covered Antarctic lake. Chemical Geology 1–2:159–72.
- Willerslev, E., E. Cappelline, W. K. Boomsma, R. Nielsen, M. B. Hebsgaard, T. B. Brand, M. Hofreiter, M. Bunce, H. N. Poinar, D. Dahl-Jensen, et al. 2007. Ancient biomolecules from deep ice cores reveal a forested southern Greenland. Science 317:111–14.
- Zhao, J.-P., T. Li, D. Barber, J.-P. Ren, M. Pucko, S. J. Li, and X. Li. 2010. Attenuation of lateral propagating light in sea ice measured with an artificial lamp in winter Arctic. Cold Regions Science and Technology 61:6–12.