?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Dwarf bamboos are evergreen woody grasses that produce large clonal patches and dominate the understories of the montane to subalpine zones of northern Japan. Recently, dwarf bamboos have expanded their distribution to above the treeline and into alpine meadows. To clarify the mechanism of rapid invasion into the alpine, we compared the morphological performance, biomass allocation, photosynthetic activity, CO2 fixation ability, and sensitivity to temperature of dwarf bamboos in their native montane and expanding alpine sites in the Taisetsu Mountains. Alpine bamboo produced shorter but denser aboveground structures, where leaves were smaller and branching was more frequent. The total biomass of alpine bamboo was nearly half of that produced by montane bamboo. Montane bamboo produced more stems, while alpine bamboo invested more carbon in belowground structures. CO2 fixation per land area by alpine bamboo was 1.3 times higher than rates observed in montane bamboo. Optimal temperatures for photosynthesis were lower in alpine bamboo (15–20°C) than in montane bamboo (20–25°C), probably because of the rapid decrease in stomatal conductance at higher temperatures (>20°C) observed in the alpine site. Overall, leaf transpiration rates were higher in alpine bamboo, but water-use efficiency was similar between sites. A high flexibility in both morphological and physiological characteristics enabled dwarf bamboos to expand into alpine environments in response to recent climate change.
Introduction
The rapid invasion and expansion of shrubs into tundra ecosystems has been reported at the global scale in response to climate change (Frost and Epstein Citation2014; Kullman Citation2002; Myers-Smith et al. Citation2011; Strum, Racine, and Tape Citation2001). In alpine systems, shrub encroachment is most apparent in alpine meadows, where herbaceous species dominate (Brandt et al. Citation2013; Cannone, Sgorbati, and Guglielmin Citation2007; Dullinger, Dirnbock, and Grabherr Citation2003; Formica et al. Citation2014). Given their relatively high levels of productivity, nutrient and water uptake, and litter accumulation, the expansion of shrubs above the treeline will significantly change the functioning of alpine ecosystems. Low temperatures combined with strong winds and high levels of solar radiation have historically restricted the growth and survival of plants migrating from lower elevations (Körner Citation2003). This is no longer the case as alpine systems experience increased temperatures and changes in seasonal precipitation. How this has influenced the ability of lower-elevation species to colonize new habitats above the treeline remains poorly understood but may reveal the biological mechanisms by which species are able to respond to novel conditions.
Shrubs facilitate diversity in alpine plant communities by alleviating the negative effects of harsh abiotic conditions (Ballantyne and Pickering Citation2015; Pistón et al. Citation2016). However, rapid changes in light, nutrient, and water availability may negatively impact species diversity and lead to changes in the species composition of alpine communities (Brandt et al. Citation2013; Bülmann, Hiltbrunner, and Körner Citation2014). Thus, clarifying the ecological impacts of shrub expansion into alpine systems is crucial to predicting future vegetation dynamics in response to climate change. That said, the possibility of shrub expansion into tundra systems may depend on the flexibility of morphological and physiological traits of shrub species (Bret-Harte et al. Citation2001; Richards et al. 2006; Colautii and Barrett Citation2013). Therefore, a careful examination of the plasticity of invasive species experiencing new environments is paramount to predict the rates and ecological impacts of shrub expansion driven by climate change.
Which shrub species will expand their distributions above the treeline is specific to geographic regions (Hallinger, Manthey, and Wilmking Citation2010; Myers-Smith et al. Citation2011; Sanz-Elorza et al. Citation2003). In the montane regions of eastern Asia, dwarf bamboos (shrub-like, woody graminoids) are the most dominant species in the understories of subalpine forests and have expanded their distributions upward in elevation into alpine meadows above the treeline (Kudo et al. Citation2011; Winkler et al. Citation2016a). Dwarf bamboos are clonal, evergreen shrubs that spread rhizomatously, creating large and dense clone patches in forest zones. Dwarf bamboos occupy 89 percent of forested area and account for 28 percent of the woody biomass in Hokkaido, northern Japan (Toyooka, Satoh, and Ishizuka Citation1983). Dwarf bamboos account for 25 percent of annual productivity in Japanese temperate forests (Sakai et al. Citation2006). Rapid expansion rates, shading effects, and high levels of evapotranspiration are common in dwarf bamboos and restrict regeneration of forests (Konno Citation2002; Nakashizuka and Numata Citation1982; Narukawa and Yamamoto Citation2002; Takahashi Citation1997). This intensive environmental modification by dwarf bamboos can lead to serious impacts on the alpine communities into which dwarf bamboos are expanding.
Dwarf bamboos have large distributions, ranging from coastal to subalpine regions in northern Japan. Among dwarf bamboo species, Sasa kurilensis Makino et Shibata has the most widespread distribution, ranging up to subalpine zones. Dense clonal patches of S. kurilensis are often taller than 2 m (with a leaf area index of 4–5) and produce up to 11 kg m−2 of biomass in montane forests (Oshima Citation1961a, Citation1961b). Further, S. kurilensis has recently expanded its distribution to above the treeline (Kudo et al. Citation2011), owing to changes in snowmelt, available soil moisture, and seasonal soil dry-down rates coupled with changes in allocation and performance (Winkler et al. Citation2016a). It is likely that the rapid expansion of S. kurilensis into alpine communities reflects the high flexibility of the species’ morphological and physiological traits. However, the biological mechanisms that have enabled S. kurilensis to expand its range to above the treeline and, in particular, the degree to which the species alters its performance remain unclear.
The negative impacts of S. kurilensis on alpine species’ richness and soil hydrology (Kudo et al. Citation2011), the relative speed with which it is advancing above the treeline (Winkler et al. Citation2016a), and the challenge of restoring communities once it has established (Kudo et al. Citation2017) warrant labeling S. kurilensis a major species of concern. Thus, predicting future alpine ecosystem dynamics will not be possible until the biology of S. kurilensis and similar shrub species is understood. We aim to clarify the degree to which S. kurilensis exhibits morphological and physiological flexibility. We also sought to quantify the species’ carbon capture and allocation strategies in its native montane and invaded alpine communities. Finally, we examined potential differences in the optimal temperature for photosynthesis and transpiration rates between the communities. Overall, we aimed to answer the following questions:
Does S. kurilensis exhibit distinct performance and allocation strategies in both its native range below the treeline and its invaded range above the treeline?
Does S. kurilensis exhibit physiological trait sensitivities to temperature and water use in its invaded alpine range?
Is photosynthetic activity or carbon fixation limited in either environment?
Materials and methods
Research sites
Our study sites were located in the Taisetsu Mountains in the central part of Hokkaido, northern Japan. The mountain summits in this region range from 1,900 m to 2,100 m a.s.l., and the treeline is located at approximately 1,500 m a.s.l. The Taisetsu Mountains are characterized by cold, snowy winters and warm, wet summers. The annual mean temperature at 1,700 m a.s.l. was −1.8°C, ranging from −16.1°C in January to 12.7°C in August, and precipitation during the summer season was 136 mm in June, 250 mm in July, 368 mm in August, and 244 mm in September (mean values for 2002–2016; G. Kudo, unpublished data).
We selected two sites for the comparisons of morphological and physiological traits: a montane site (997 m elevation) and an alpine site (1,610 m) on the western slope of Mt. Asahi (2,290 m a.s.l.; 43°16ʹN, 142°28ʹE). The montane site is located in a mixed forest of Picea glehnii and Betula ermanii, where the understory is dominated by our target species, S. kurilensis. At the treeline, S. kurilensis mainly grows in areas sheltered by alpine dwarf pine (Pinus pumila). The alpine site is a snow meadow, where herbaceous species dominate but dense patches of S. kurilensis have appeared in recent decades (Winkler et al. Citation2016a). Snowmelt typically concludes by late May at the montane site and by mid-June at the alpine site. All measurements were carried out at both sites, except those that involved destructive sampling (i.e., biomass harvests). Biomass allocation and vertical distribution of S. kurilensis leaves were measured at our montane site. We then selected another alpine site for biomass measurements, because extensive plant sampling is prohibited by law in the alpine area of Mt. Asahi. This second alpine site is located at a snow meadow at 1,760 m a.s.l. (named Goshikigahara) and is located 12 km south of Mt. Asahi. Goshikigahara meadow has a similar community composition with the meadow on Mt. Asahi, and invasion patterns of dwarf bamboos are similar at both sites (Kudo et al. Citation2011).
Aboveground performance
Measurements of morphological traits were conducted during the middle of the growing season (early August) in 2011 at the montane and alpine sites on Mt. Asahi. We randomly selected five 1 × 1 m plots at each site, and counted the number of culms (i.e., stems) that emerged from the ground early in the growing season. We then randomly selected ten culms within each plot and measured height, the number of leaves, and the number of branches per culm. Furthermore, we sampled ten leaves per culm, immediately transported them back to the laboratory, and measured leaf area using a free software (ImageJ ver.1.45, NIH, Bethesda, MD, USA). Mean leaf area per culm was used for analyses.
Biomass allocation and leafing pattern
We measured the vertical distribution of leaves in mid-September 2013 at the montane site of Mt. Asahi and the alpine site of Goshikigahara. We again randomly established five 1 × 1 m plots within bamboo patches at each site, and measured photosynthetic active photon flux density (PPFD) from the canopy layer to the ground level at 20 cm intervals using a LI-250A logger (Li-Cor, Lincoln, NE, USA). We then harvested aboveground parts at 20 cm intervals, divided the harvest into leaves and stems (including culms and branches), oven dried the plant materials at 60°C for 48 h, and obtained dry weights. The vertical distribution of leaf mass at each site was converted to an area-based foliage distribution using the mean values of specific leaf area (SLA = 0.012 m2 g−1 in both sites), and leaf area index (LAI) was calculated for each site.
To compare biomass allocation between sites, we used a subset of data from our previous study (Winkler et al. Citation2016a), conducted at the same sites in 2014, in which we selected data obtained from typical light conditions under forest canopy at the montane site. We harvested aboveground biomass from five plots (1.0 × 0.5 m at montane and 0.5 × 0.5 m at alpine), reflecting the difference in culm density between the elevations (see “Results,” further on), and sorted tissues were separated into leaves and stems (culms and branches). Belowground soil cores (0.3 × 0.3 × 0.3 m) were sampled at five montane and three alpine plots. Soil cores were transported to the laboratory and soils were sieved, separating roots and rhizomes from each core. All samples were dried at 60°C for 48 h, or until dry weights were consistent. Finally, we scaled all biomass measurements to a 1 × 1 m area.
Photosynthetic traits and CO2 fixation
We measured photosynthetic rate responses to light per unit area at our montane and alpine sites of Mt. Asahi in the early (late June), middle (early August), and late season (late September) of 2012 using a portable closed gas-exchange system (LI-6400; Li-Cor, Lincoln, NE, USA). Photosynthetic rates were measured on three patches at upper and lower foliar layers of dwarf bamboo at each site. We exposed leaves to ten light levels (2000, 1500, 1000, 800, 500, 300, 100, 50, 10, and 0 μmol m−2 s−1) of photosynthetic active radiation (PAR) using a red-blue LED light source at constant leaf temperatures (15–20°C). Ambient CO2 concentration and humidity during each measurement were maintained at 380 μl l−1 and 1.0 vapor pressure deficit (kPa), respectively. Net photosynthetic rate per area (Parea) can be described as a nonrectangular hyperbola of photon irradiance (I, μmol m−2 s−1), using four photosynthetic parameters as follows:
where Pmax is the light-saturated photosynthetic rate per unit area (μmol m−2 s−1), f is the initial slope (μmol m−2 s−1), q is the degree of curvature (dimensionless), and R is the dark respiration rate (μmol m−2 s−1; Marshall and Biscoe Citation1980). Data obtained from plants during each campaign during the season were fitted to this equation by nonlinear least-squares estimates for each parameter to estimate photosynthetic parameters.
We calculated the CO2 fixation ability of individuals at each site using the photosynthetic parameters obtained. We used mean values of solar radiation measured at one-hour intervals during July and August at our alpine site to estimate daily CO2 fixation. To do this, we calculated mean solar radiation during the middle of summer every hour, fit the values to EquationEquation 1(1)
(1) with mean photosynthetic parameters during the season (i.e., early, middle, and late measurements), and cumulated CO2 fixation daily. To assess the effects of shading on CO2 fixation, we also calculated CO2 fixation under a 50 percent shading scenario. Finally, we estimated daily CO2 fixation per area base (1 × 1 m) at each site using LAI values.
Physiological sensitivity to ambient temperature
Physiological responses of leaves to ambient temperature were measured in late September 2012 at both the montane and alpine sites of Mt. Asahi using an LI-6400. Under bright conditions (1,500 μmol m−2 s−1), we changed the temperature of the leaf chamber from 7°C to 25°C and measured the photosynthetic rate (equivalent to Pmax), stomatal conductance (gs), and transpiration rate (E). We then calculated water-use efficiency (WUE) as the ratio of photosynthetic rate to transpiration rate. Measurements were conducted in three bamboo patches at each site.
Statistical analyses
Aboveground performance was compared between the montane and alpine sites using generalized linear mixed-effect models (GLMM) with site as an explanatory variable and plot as a random effect. We used GLMMs fitted with Gamma error distributions to test for differences in culm height and leaf size between sites. We also used GLMMs fitted with Poisson error distributions to test for differences in culm density, frequency of branching, and leaf number per culm between sites.
Physiological sensitivities to ambient temperature were analyzed using generalized linear models (GLMs) fitted with a Gamma error distribution, with individual physiological traits (Pmax, gs, E, and WUE) as response variables and site, temperature, and quadratic temperature values as explanatory variables. We included quadratic temperature values because some traits showed optimal responses to temperature within the thermal range examined. Interactions between site and temperature were also included in GLMMs to evaluate habitat-specific temperature responses. The best-fit model was determined using the Akaike’s Information Criterion (AIC). All statistical analyses were conducted in R 3.3.1 (R Development Core Team Citation2016).
Results
Performance and biomass allocation
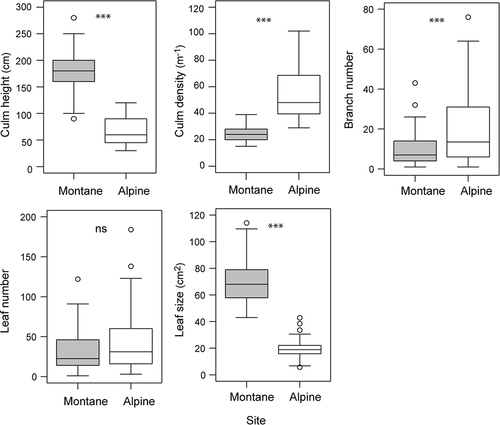
The mean culm height and individual leaf size was lower at the alpine site compared to the montane site. In contrast, branching number and culm density were higher at the alpine site, while the number of leaves per culm did not vary between sites ().
Figure 1. Comparisons of dwarf bamboo shoot performance between montane and alpine sites. Box and whisker plots represent the seventy-fifth, fiftieth, and twenty-fifth percentiles (boxes), with whiskers forming the tenth to ninetieth percentiles. Open circles represent outliers. *** P < 0.001; ns P > 0.10
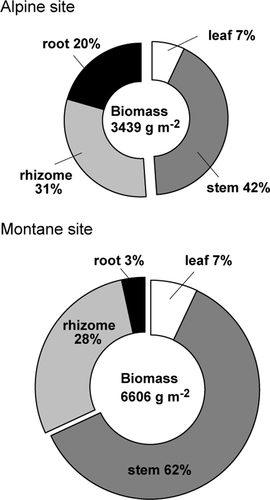
The total biomass of dwarf bamboo at our montane site was 6.6 kg m–2, in which 69 percent was allocated to aboveground parts, mostly as stems (). A majority of belowground biomass was allocated to rhizomes, with roots accounting for only 3 percent of the total biomass. In contrast, total biomass at our alpine site was 3.4 kg m–2, of which 51 percent was allocated to belowground parts, with roots accounting for 20 percent of the total biomass. Allocation to leaves (7%) and rhizomes (28–31%) were similar between sites. Overall, montane bamboo was characterized by large, heavy stems, while alpine bamboo was primarily characterized by a higher investment in roots.
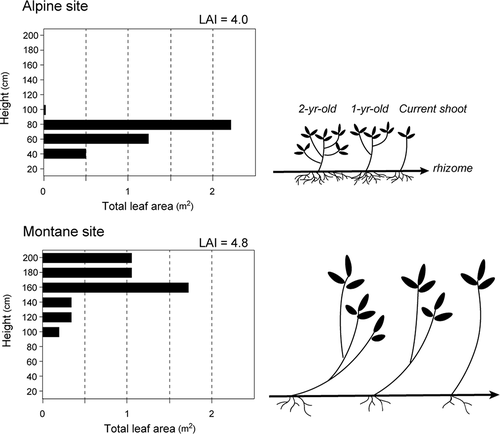
Bamboo at our montane site had leaves distributed from 100 cm to 200 cm in height with a peak at 160 cm (). Leaves at the alpine site were distributed between 40 cm and 80 cm in height and were concentrated in the upper layer (). Mean LAI was 4.8 at the montane site and 4.0 at the alpine site.
Photosynthetic activity and CO2 fixation
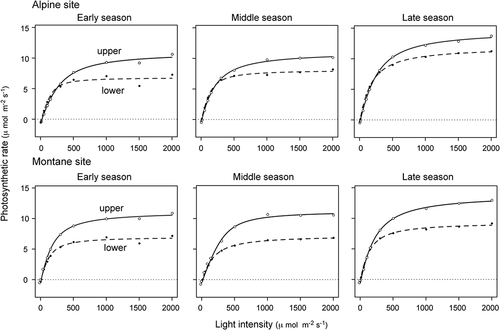
Dwarf bamboo showed similar photosynthetic patterns between sites (). Photosynthetic activity (Pmax) was higher in upper foliage layers than in lower layers, and was generally higher later in the season when new leaves matured (). These results suggest that dwarf bamboo maintains high photosynthetic activity throughout the summer growing season.
Table 1. Photosynthetic parameters of dwarf bamboo in the early, middle, and late growing season at montane and alpine sites. Measurements were conducted on upper and lower foliage layers. f = initial slope; Pmax = maximum photosynthetic rate; R = respiration rate; q = curvature
Figure 4. Seasonal dynamics of light-response curves of photosynthetic rates at montane and alpine sites. Photosynthetic curves for upper (solid line) and lower canopy (dashed line) are indicated. See for information on photosynthetic parameters
Estimated CO2 fixation by dwarf bamboo was 44.5 g m−2 day−1 at our montane site and 56.9 g m−2 day−1 at our alpine site under full light conditions (i.e., no shading effect). Although the total biomass of alpine bamboo was approximately 50 percent less than montane bamboo, and the leaf biomass of the alpine bamboo (241 ± 21 SE g m−2) was lower than that of the montane bamboo (463 ± 92 g m−2), alpine bamboo exhibited CO2 fixation patterns that were 1.3 times larger than those observed at the montane site. Under 50 percent shade, CO2 fixation was estimated to be 27.4 g m−2 day−1 at the montane site and 40.9 g m−2 day−1 at the alpine site, indicating that more than 62–72 percent of photosynthetic ability was retained under shaded conditions.
Physiological sensitivity to ambient temperature
The Pmax indicated strong temperature dependence, with a peak Pmax under moderate temperatures (). However, a significant interaction between site and temperature (t = 3.91, P < 0.001) indicated a difference in the optimum temperature between sites (); 15–20°C was the optimum in alpine bamboo, while 18–25°C was the optimum in the montane population.
Table 2. GLM results for the responses of physiological traits to ambient temperature: (a) maximum photosynthetic rate, (b) stomatal conductance, (c) transpiration rate, and (d) water-use efficiency
Figure 5. Physiological responses of dwarf bamboo to ambient temperatures at montane and alpine study sites. Patterns of maximum photosynthetic rates (Pmax), stomatal conductance (gs), leaf transpiration rates (E), and water-use efficiency (WUE) are indicated with curve fitting. See for statistical results
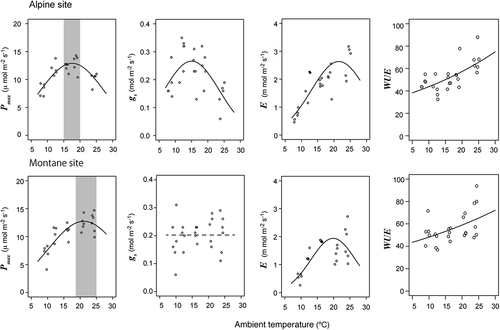
Stomatal conductance also showed temperature dependence at the alpine site, where gs values were highest at approximately 15°C and decreased abruptly at higher temperatures (). However, there was no clear trend in stomata responses to temperature changes at the montane site. Leaf transpiration rates showed clear temperature dependence and were highest at approximately 20–25°C at both sites. Overall, alpine bamboo showed higher E values than montane bamboo. Estimated water loss by leaf transpiration at 20°C based on the LAI at each site was 697 ml h−1 at the alpine site and 588 ml h−1 at the montane site. Finally, WUE increased with temperature at an accelerated rate at both sites (, ).
Discussion
Our study reveals that dwarf bamboo exhibits distinct performance and allocation patterns both above and below the treeline. Dwarf bamboo grow as short, dense plants with small leaves in response to their alpine environment, where strong winds and high levels of irradiance accelerate water deficit as evaporative demand increases (Bliss Citation1971; Körner et al. Citation1989; Körner and Renhardt Citation1987). The plant height of dwarf bamboo varied from 20 cm in the alpine to more than 3 m in their native habitat (Oshima Citation1961a), and leaf size varied from 10 cm2 to more than 100 cm2. These results suggest a large flexibility in the morphological performance of dwarf bamboo. However, it is unclear whether the differences in performance between habitats are the result of genetic variation or plasticity. That said, our previous studies report large variation in plant height from the central to marginal parts within a patch of bamboo at our alpine site (Kudo et al. Citation2011, Citation2017). This suggests that a high level of plasticity in morphological traits may explain dwarf bamboo success and its ability to invade alpine communities.
In addition to morphological differences, physiological traits also varied between sites in which dwarf bamboo above the treeline exhibit photosynthetic traits to match typical alpine environmental conditions. Plants in the alpine decreased gs when ambient temperatures were higher than 15°C, preventing water loss as evaporative demand increased. However, a decrease in gs was accompanied by a decrease in the photosynthetic rate, resulting in a maximum photosynthetic ability at approximately 15–20°C in alpine bamboo. In contrast, stomatal responses to temperature were not consistent at the montane site, indicating lower drought stress even under high temperatures. Thus, our study confirms that dwarf bamboo exhibit high levels of drought tolerance in the alpine (e.g., Winkler et al. Citation2016a) and further reveals the physiological mechanisms by which the species is able to respond to stress as it experiences relatively harsher conditions.
Regardless of lower gs values at higher temperatures, high E values were maintained throughout the range of temperatures examined in the alpine. High transpiration rates at the alpine site are likely a result of larger root biomass as well as increased evaporative demand at the site. Higher investment in belowground structures is common in alpine species because water and nutrient uptake needs are increasingly crucial for growth and survival in alpine environments (Körner et al. Citation1989; Ma et al. Citation2010). Biomass allocation to root was 20 percent at the alpine site, while it was only 3 percent at the montane site. Root mass of alpine plants (707 ± 54 g m−2) was three times larger than that of montane plants (225 ± 62 g m−2). This large difference in biomass allocation between the sites strongly suggests the importance of being able to tolerate varied water availability in the alpine. Increasing allocation to belowground parts to prevent desiccation as soils dry has been reported in another bamboo species (Qing, Yunxiang, and Zhangcheng Citation2004). In contrast, belowground allocation to rhizomes was larger at the montane site, where 90 percent of total biomass was allocated to stems and rhizomes (compared to 73 percent at the alpine site). The biomass of spacer (rhizomes or stolons) is strongly related to ramet biomass in clonal plants with horizontal rhizomes (de Kroon and Schieving Citation1991). Thus, a larger investment of resources in rhizomes may be required for the production of large culms during longer time periods.
Additionally, high transpiration rates by dwarf bamboo changes soil moisture availability and influences the growth of native alpine species (Ishii et al. Citation2008; Takahashi et al. Citation2003). Our previous study conducted at our alpine site revealed that soil moisture fluctuated dramatically as transpiration rates of dwarf bamboo increased in abundance and soil moisture was higher and stable throughout the summer when dwarf bamboo were clipped (Kudo et al. Citation2017). Because alpine plant community composition is primarily determined by soil water availability and is sensitive to changes in soil moisture (Berdanier and Klein Citation2011; Isard Citation1986; Winkler, Chapin, and Kueppers Citation2016b), the invasion of dwarf bamboo has serious implications for alpine communities because of both its possible shading effects and its major modification of the edaphic environment (Kudo et al. Citation2011).
Surprisingly, estimated CO2 fixation of dwarf bamboo per land area was higher at the alpine site than at the montane site irrespective of lower leaf biomass at the alpine site. Lower photosynthetic production at the montane site is likely related to the structure of branching and leaf patterns. Although leaves at the alpine site were distributed at 40–80 cm in height, leaves in the montane population were distributed at 100–200 cm. Longevity of culms is approximately nine years, and leaf longevity is two years in a montane environment of 500–800 m a.s.l. (Oshima Citation1961a). First-year shoots (main culm) are single stems without branches, and leaves are produced on the top of the stem. New leaves are produced on branches the following year, and branching is repeated for several years. Because the length of branches is larger at the montane site, the overall distribution of leaves increases (see the illustration in ). A larger distribution of leaves throughout the canopy coupled with leaves that are larger themselves enhances self-shading (van der Bijl, Sand-Jensen, and Hjermind Citation1989; Falster and Westby Citation2003; Poorter et al. Citation2003), resulting in lower rates of photosynthesis at the whole-plant level. In the montane environment, competition for light among plant species is intense in the understory. High levels of investment in stems enable plants to deploy leaves higher up in an attempt to capture more light in the understory. Therefore, any increase in competitive ability for light capture (i.e., producing taller stems) at the cost of leaf production and a more efficient arrangement of leaves is critical to bamboo at lower elevations.
In alpine meadows, most herbaceous species are less than 30 cm tall (Kawai and Kudo Citation2014), while the height of dwarf bamboo reaches 50–100 cm and forms dense patches. The aboveground biomass of native alpine meadow species typically ranges from 100 g m−2 to 400 g m−2 (Körner Citation2003). We estimated the aboveground biomass of dwarf bamboo to be 2.1–2.4 kg m−2 at our alpine site, nearly six times greater than the entire alpine community. Because of their relatively large aboveground structures, dwarf bamboo carry out photosynthesis under full light conditions in the alpine. Therefore, bamboo may regulate performance and biomass allocation in these newly invaded meadows to maximize photosynthetic production under the relatively harsh environmental conditions experienced in alpine ecosystems. The high photosynthetic ability of shrubs regulates carbon dynamics in alpine meadows because of their large foliage biomass (Yashiro et al. Citation2010). The expansion of dwarf bamboos in alpine vegetation may modify the carbon balance of alpine ecosystems.
In conclusion, our results indicate that alpine environments are suitable for the growth of dwarf bamboo because of their high levels of morphological and physiological flexibility in traits related to performance and drought avoidance. Phenotypic plasticity is a crucial trait for rapid range expansion under climate change (Bret-Harte, Shaver, and Zoerner et al. Citation2001). Dwarf bamboo show potential to continue their expansion above the treeline and into alpine environments. However, the actual distribution of dwarf bamboo at most alpine sites is generally limited to areas sheltered by alpine dwarf pines. This distributional trend reflects snow patterns in alpine regions, given the amount of protection snow cover provides for overwintering dwarf bamboo (Oshima Citation1962). At the same time, however, snowmelt should restrict photosynthetic gain depending on the length of the summer growing season. Additionally, optimal temperatures for photosynthesis (15–20°C) are higher than any actual thermal conditions plants would experience (the maximum temperature at our alpine site was 13–14°C). That said, recent climate change has seen higher temperatures, leading to accelerated snowmelt in the Taisetsu Mountains (Kudo Citation2014). These changes have likely contributed to the ability of dwarf bamboo to fix carbon at sites where it was previously not possible, resulting in a range expansion into the snow meadow sites in this study. As mentioned before, shrub expansion into alpine ecosystems is a worldwide trend largely driven by global change, but key species differ regionally(Brandt et al. Citation2013; Formica et al. Citation2014; Myers-Smith et al. Citation2011). In Japanese mountains, dwarf bamboo is the most common and dominant shrub (Oshima Citation1961a, Citation1961b, Citation1962). This extends beyond the Taisetsu Mountains where our study takes place. For example, the expansion of dwarf bamboo into the alpine zone has also been reported in the Tateyama Mountains of central Japan (Yoshida, Takahashi, and Ohmiya Citation2016). That said, because our study is limited to a single mountain without site replication, future studies across multiple mountain regions will enable a stronger generalization of the patterns observed. Given the large impact of dwarf bamboo on the edaphic conditions where they grow, the high levels of shade they create, and the relatively massive amounts of biomass they produce, the dynamics of dwarf bamboo should be carefully monitored to better predict the ecological impacts of dwarf bamboo invasions in alpine ecosystems.
Acknowledgments
Special thanks to Y. Amagai and Y. Kawai for field support, and A. Shibata for assistance of analysis.
Additional information
Funding
References
- Ballantyne, M., and C. M. Pickering. 2015. Shrub facilitation is an important driver of alpine plant community diversity and functional composition. Biodiversity Conservation 24:1–11.
- Berdanier, A. B., and J. A. Klein. 2011. Growing season length and soil moisture interactively constrain high elevation aboveground net primary production. Ecosystems 14:963–74.
- Bliss, L. C. 1971. Arctic and alpine plant life cycles. Annual Reviews of Ecology and Systematics 2:405–38.
- Brandt, J. S., M. A. Haynes, T. Kuemmerle, D. M. Waller, and V. C. Radeloff. 2013. Regime shift on the roof of the world: Alpine meadows converting to shrublands in the southern Himalayas. Biological Conservation 158:116–27.
- Bret-Harte, M.S., G.R. Shaver, J.P. Johnstone, J.L. Wagner, A.S. Chavez, R.F. Gunkelman IV, S.C. Lipert, and J.A. Laundre. 2001. Developmental plasticity allows Betula nana to dominate tundra subjected to an altered environment. Ecology 82:18–32.
- Bülmann, T., E. Hiltbrunner, and C. Körner. 2014. Alnus viridis expansion contributes to excess reactive nitrogen release, reduces biodiversity and constrains forest succession in the Alps. Alpine Botany 124:187–91.
- Cannone, N., S. Sgorbati, and M. Guglielmin. 2007. Unexpected impacts of climate change on alpine vegetation. Frontiers in Ecology and the Environment 5:360–64.
- Colautii, R. I., and S. C. H. Barrett. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342:364–66.
- De Kroon, H., and F. Schieving. 1991. Resource allocation patterns as a function of clonal morphology: A general model applied to a foraging clonal plant. Journal of Ecology 79:519–30.
- Dullinger, S., T. Dirnbock, and G. Grabherr. 2003. Patterns of shrub invasion into high mountain grasslands of the Northern Calcareous Alps, Austria. Arctic, Antarctic, and Alpine Research 35:434–41.
- Falster, D. S., and M. Westby. 2003. Leaf size and angle vary widely across species: What consequences for light interception? New Phytologist 158:509–25.
- Formica, A., E. C. Farrer, I. W. Ashton, and K. N. Suding. 2014. Shrub expansion over the past 62 years in Rocky Mountain alpine tundra: Possible causes and consequences. Arctic, Antarctic, and Alpine Research 46:616–31.
- Frost, G. V., and H. E. Epstein. 2014. Tall shrub and tree expansion in Siberian tundra ecotones since the 1960s. Global Change Biology 20:1264–77.
- Hallinger, M., M. Manthey, and M. Wilmking. 2010. Establishing a missing link: Warm summer and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist 186:890–99.
- Isard, S. A. 1986. Factors influencing soil moisture and plant community distribution on Niwot Ridge, Front Range, Colorado, U.S.A. Arctic and Alpine Research 18:83–96.
- Ishii, H. T., T. Kobayashi, S. Uemura, K. Takahashi, Y. T. Hanba, A. Sumida, and T. Hara. 2008. Removal of understory dwarf bamboo (Sasa kurilensis) induces changes in water-relations characteristics of overstory Betula ermanii trees. Journal of Forest Research 13:101–09.
- Kawai, Y., and G. Kudo. 2014. Current changes in alpine vegetation and their impacts on biodiversity in the Daisetsuzan National Park. Chikyu-Kankyo 19:23–32. in Japanese.
- Konno, Y. 2002. Effects of competitive exclusion by the dominant Sasa tsuboiana on associate species. Vegetation Science 19:1–10.
- Körner, C. 2003. Alpine Plant Life, 2nd ed. Berlin, Heidelberg: Springer-Verlag.
- Körner, C., M. Neumayer, S. P. Menendez-Riedl, and A. Smeets-Scheel. 1989. Functional morphology of mountain plants. Flora 182:353–83.
- Körner, C., and U. Renhardt. 1987. Dry matter partitioning and root length/leaf area ratios in herbaceous perennial plants with diverse altitudinal distribution. Oecologia 74:411–18.
- Kudo, G. 2014. Monitoring of mountain ecosystems under climate change: Its significance and perspective. Chikyu-Kankyo 19:3–11. in Japanese.
- Kudo, G., Y. Amagai, B. Hoshino, and M. Kaneko. 2011. Invasion of dwarf bamboo into alpine snow-meadows in northern Japan: Pattern of expansion and impact on species diversity. Ecology and Evolution 1:85–96.
- Kudo, G., Y. Kawai, Y. Amagai, and D. E. Winkler. 2017. Degradation and recovery of alpine plant community: Experimental removal of an encroaching dwarf bamboo. Alpine Botany 127:75–83.
- Kullman, L. 2002. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology 90:68–77.
- Ma, W.-L., P.-L. Shi, W.-H. Li, Y.-T. He, X.-Z. Zhang, Z. Shen, and S.-Y. Chai. 2010. Changes in individual plant traits and biomass allocation in alpine meadow with elevation variation on the Qinghai-Tibetan Plateau. Science China Life Sciences 53:1142–51.
- Marshall, B., and P. V. Biscoe. 1980. A model for C3 leaves describing the dependence of net photosynthesis on irradiance. Journal of Experimental Botany 31:29–39.
- Myers-Smith, I.H., B.C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K. D. Tape, M. Macias-Fauria, U. Sass-Klaassen, E. Lévesque, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters 6:045509.
- Nakashizuka, T., and M. Numata. 1982. Regeneration process of climate beech forests. I. Structure of a beech forest with the undergrowth of Sasa. Japanese Journal of Ecology 32(5):2–67.
- Narukawa, Y., and S. Yamamoto. 2002. Effects of dwarf bamboo (Sasa sp.) and forest floor microsites on conifer seedling recruitment in a subalpine forest, Japan. Forest, Ecology and Management 163:61–70.
- Oshima, Y. 1961a. Ecological studies of Sasa communities I. Productive structure of some of Sasa communities in Japan. Botanical Magazine, Tokyo 74:199–210.
- Oshima, Y. 1961b. Ecological studies of Sasa communities II. Seasonal variations of productive structure and annual net production in Sasa communities. Botanical Magazine, Tokyo 74:280–90.
- Oshima, Y. 1962. Ecological studies of Sasa communities V. Influence of light intensity, snow depth and temperature upon the development of Sasa kurilensis community. Botanical Magazine, Tokyo 75:43–48.
- Pistón, N., C. Schöb, C. Armas, I. Prieto, and F. I. Pugnaire. 2016. Contribution of co-occurring shrub species to community richness and phylogenetic diversity along an environmental gradient. Perspectives in Plant Ecology, Evolution, and Systematics 19:30–39.
- Poorter, L., F. Bongers, F. Sterck, and H. Wöll. 2003. Architecture of 53 rain forest tree species differing in adult stature and shade tolerance. Ecology 84:602–08.
- Qing, L., L. Yunxiang, and Z. Zhangcheng. 2004. Effect of moisture availability on clonal growth in bamboo Pleioblastus maculata. Plant Ecology 17:107–13.
- R Development Core Team. 2016. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Richards, C. L., L., O. Bossdorf, N. Z. Muth, J. Gurevitch, and M. Pigliucci. 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters 9:981–93.
- Sakai, T., T. Akiyama, N. Saigusa, S. Yamamoto, and Y. Yasuoka. 2006. The contribution of gross primary production of understory dwarf bamboo, Sasa senanensis, in a cool-temperate deciduous broadleaved forest in central Japan. Forest Ecology and Management 236:259–67.
- Sanz-Elorza, M., E. D. Dana, A. Gonzalez, and E. Sobrino. 2003. Changes in the high-mountain vegetation of the central Iberian Peninsula as a probable sign of global warming. Annals of Botany 92:273–80.
- Strum, M., C. Racine, and K. Tape. 2001. Increasing shrub abundance in the Arctic. Nature 411:546–47.
- Takahashi, K. 1997. Regeneration and coexistence of two subalpine conifer species in relation to dwarf bamboo in the understory. Journal of Vegetation Science 8:529–36.
- Takahashi, K., S. Uemura, J. Suzuki, and T. Hara. 2003. Effects of understory dwarf bamboo on soil water and the growth of overstory trees in a dense secondary Betula ermanii forest, northern Japan. Ecological Research 18:767–74.
- Toyooka, H., A. Satoh, and M. Ishizuka, 1983: Distribution map of Sasa group in Hokkaido. Explanatory Note. Forestry and Forest Products Research Institute, Hokkaido Branch, Sapporo, Japan, 36 pp. [in Japanese]
- van der Bijl, L., K. Sand-Jensen, and A. L. Hjermind. 1989. Photosynthetic and canopy structure of a submerged plant, Potamogeton pectinatus, in a Danish lowland stream. Journal of Ecology 77:947–62.
- Winkler, D. E., Y. Amagai, T. E. Huxman, M. Kaneko, and G. Kudo. 2016a. Seasonal dry-down rates and high stress tolerance promote bamboo invasion above and below treeline. Plant Ecology 217:1219–34.
- Winkler, D. E., K. J. Chapin, and L. M. Kueppers. 2016b. Soil moisture mediates alpine life form and community productivity responses to warming. Ecology 97:1553–63.
- Yashiro, Y., Y. Shizu, M. Hirota, A. Shimono, and T. Ohtsuka. 2010. The role of shrub (Potentilla fruticosa) on ecosystem CO2 fluxes in an alpine shrub meadow. Journal of Plant Ecology 3:89–97.
- Yoshida, M., K. Takahashi, and T. Ohmiya. 2016. Expansion of dwarf bamboo communities in Murodo-daira, Tateyama Mountains, as revealed by aerial photography. Bulletin of the Botanical Garden of Toyama 22:9–17. [in Japanese with English summary].