 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
High mountain areas above the alpine zone are, despite the low-temperature conditions, inhabited by evolutionary and functionally differing organism groups. We compared the abundance and species richness of vascular plants, oribatid mites, springtails, spiders, and beetles, as well as bacterial and methanogenic archaeal prokaryotes (only abundance), at 100 m vertical intervals from 2,700–3,400 m in the Central Alps. We hypothesized that the less mobile microarthropods and microorganisms are more determined by and respond in similar ways to soil properties as do vascular plants. In contrast, we expected the more mobile surface-dwelling groups to forage also in places devoid of vegetation and thus to show patterns that deviate from that of vascular plants.
Surprisingly, the observed patterns were diametrically opposed to our expectations: soil-living oribatid mites and springtails showed high individual numbers at high elevations, even where vascular plants barely occurred. Springtails also showed a rather constant species richness throughout the entire gradient. In contrast, patterns of surface-dwelling organisms and of archaeal prokaryotes did not differ significantly from vascular plants, because of either comparable climate sensitivity or their dependency on vegetated habitats.
This study may serve as a baseline to estimate the risks of biodiversity losses in response to climate change across different biotic ecosystem components and to explore the potential and limitations of vascular plants as proxy for other organism groups that are far more challenging to monitor.
Introduction
The alpine zone of humid temperate mountains is defined by the occurrence of mostly closed treeless vegetation, which disintegrates in its upper part into open, scattered plant assemblages, interspersed by unvegetated screes, patches of bare soil, and rock (Nagy and Grabherr Citation2009). This alpine-nival ecotone is one of the most distinct ecological transition zones in high mountain environments (Gottfried, Pauli, and Grabherr Citation1998), which was found to coincide with the elevation of the summer snow line at about 2,900 m (Gottfried et al. Citation2011).
Besides the conspicuous organism group of vascular plants, alpine to nival ecosystems are composed of several taxonomically and functionally differing biota, such as arthropods, microarthopods, and microorganisms. Their degree of co-occurrence with and dependence on plants as primary producers may vary among organism groups, even though a general decrease in abundance and species diversity with increasing elevation, low-temperature conditions, and the thinning of plant biomass can be expected and was reported in invertebrate and microbiological studies. A gradual decrease in species numbers and activity-density values with elevation and a stepwise decline at the major ecotones (treeline, alpine-nival ecotone) was found for spiders in the Alps (Thaler Citation2003), whereas this was not the case for carabid beetles in a study from the southern Alps, where the studied gradient did not, however, extend into the nival zone (Pizzolotto et al. Citation2016). Although insects often co-occur with vascular plants in alpine environments, their physiology is differently affected by high-elevation and low-temperature conditions. For instance, the management of metabolic resources of insects through diapause nutrient regulation depends on low-temperature regimes (Hahn and Denlinger Citation2011). Furthermore, thermoregulation of insects can be facilitated by lower air density at high elevation through a substantial reduction of convective heat loss (Dillon, Frazier, and Dudley Citation2006).
Soil and surface-dwelling microarthropods such as oribatid mites and springtails were found at extreme high-elevation habitats (Körner Citation2011; Mani Citation1968). Nonetheless, taxonomic richness and community structure of soil microarthropod groups along forest to alpine gradients were found to be affected by elevation, vegetation, soil properties (Jiang, Yin, and Wang Citation2015), habitat quality, and food availability (Mitchell et al. Citation2016). For oribatid mites, species diversity was shown to be higher in montane forests than in alpine tundra vegetation (Leonov, Rakhleeva, and Sidorchuk Citation2015); nevertheless, they are considered important decomposers in alpine soils (Heggen Citation2010). Their presence may enhance fungal diversity and abundance and can stimulate microbial growth by reducing nutrient loss from alpine ecosystems (Maraun, Visser, and Scheu Citation1998).
Patterns of soil prokaryote distributions along alpine elevation gradients showed that bacterial taxon richness decreases monotonically with elevation in the Rocky Mountains, while plants showed a unimodal pattern with a peak at mid elevations (Bryant et al. Citation2008). In the Alps, abundance and diversity of soil microorganisms were found to be strongly governed by site characteristics, especially organic-matter content and pH (Siles and Margesin Citation2016). These studies and similar microbiological investigations along elevation gradients, however, hardly reach the lowest elevation at which the present study starts. At our study site, strong decreases of microbial abundance and activities from alpine to subnival and further to nival soils were observed, with bacteria decreasing at higher elevations than archaea (Hofmann et al. Citation2016a). A distinct impact of the specific type of vegetation on the abundance of methanogenic archaea and on their potential to form methane within upland soils was found across the Tyrolean Alps (Hofmann et al. Citation2016c).
Comparisons of distribution patterns of vascular plants, arthropod groups, and microbes along a gradient from closed alpine grassland to the climatic limits of plant life have, to our knowledge, not yet been conducted. Knowing the current abundance and diversity patterns across organism groups with different ecological requirements, however, would be fundamental for designing experimental studies to elucidate possible interdependencies and potential responses to ongoing climatic changes.
Recent climate warming led to shifts in vascular plant distributions in high mountain environments across Europe (Pauli et al. Citation2012; Steinbauer et al. Citation2018), including declines in the abundance of high-elevation species in our study region (Pauli et al. Citation2007). Other organism groups may remain resistant or may be similarly affected, which would be of particular concern owing to the above-average level of endemic invertebrates compared to elevations below the timberline in the Alps (Rabitsch et al. Citation2016). For alpine soil arthropod abundance, experimental warming was most effective when combined with nutrient addition (Hågvar and Klanderud Citation2009), whereas a long-term warming experiment had no effect on richness and abundance of springtails in alpine subarctic ecosystems (Alatalo, Jägerbrand, and Čuchta Citation2015). Changes in occurrence and activities of microorganisms would be especially relevant to climate change considering their attributes as key players for methane formation and consumption, belonging to methanogenic archaea and methanotrophic bacteria, respectively (Crutzen and Lelieveld Citation2001; Hofmann et al. Citation2016b; Praeg, Wagner, and Illmer Citation2017).
Aiming to determine common and deviating occurrence patterns among organism groups and to contribute to the assessment of their “indicator value” regarding the ecological implications of climate change, we used a uniform slope system on the southwest side of Mt. Schrankogel in the Tyrolean Central Alps, which extends uninterruptedly from the alpine grassland into the nival zone. Abundance and diversity patterns of vascular plant species, soil-dwelling oribatid mites (Oribatida), soil- and surface-dwelling springtails (Collembola), spiders, and beetles as well as of bacterial and archaeal prokaryotes (only abundance) are compared along the elevational gradient from 2,700 m to 3,400 m and along ecological gradients (thermic vegetation indicator and soil moisture indicator, maximum water-holding capacity, soil organic matter, soil C and N content, pH).
Patterns of vascular plant cover and species richness above the treeline are negatively correlated with elevation and soil moisture (McCain and Grytnes Citation2010; Theurillat et al. Citation2003) and positively correlated with biotic soil properties (Hofmann et al. Citation2016a) and the thermic vegetation indicator (Gottfried et al. Citation2012). We hypothesize that diversity and/or abundance patterns of
more mobile surface-dwelling organism groups (i.e., spiders and beetles) are not significantly related to vascular plant patterns, because they also may feed in places devoid of vegetation.
predominantly ground-dwelling soil microarthropods (oribatid mites and springtails) are more determined by soil properties and, thus, show similar patterns as vascular plants.
soil microorganisms are similarly related to vascular plant patterns, but to a different extent for methanotrophic bacteria compared to methanogenic archaea.
Materials and methods
Samples were taken along an elevation gradient across the alpine-nival ecotone on the southwest slope of Mt. Schrankogel (Tyrol, Austria, 3,497 m a.s.l.) at eight elevation-level intervals of 100 m from 2,700 m to 3,400 m, ranging from alpine grassland across scarcely vegetated areas to bare nival habitats. At each elevation level, standard soil properties, microbial activity and abundance, soil and surface arthropod diversity and abundance, and vascular plant species diversity and cover were recorded in 2014 (if not stated otherwise). The following organism groups were recorded: vascular plants, springtails (Collembola; in the soil and on the soil surface separately), soil oribatid mites (Oribatida), beetles (Coleoptera), spiders (Araneae), bacteria, and archaea, where Methanocella were considered separately.
Soil characteristics
Soil properties were determined to assess possible links between soil biota and vegetation. Soil samples for analyzing soil properties and microbial activity and abundance were taken at three different locations approximately 10 m apart at each elevation level by sampling and merging from five to ten single probes at each location. The three replicate sites were analyzed separately (with three technical replicates) and the means of each elevation level were used for further calculations.
Methods were performed according to the standard procedures given in Schinner et al. (Citation1996). Maximum water-holding capacity (MWHC) was determined gravimetrically using glass cylinders with pores at one side. Soil pH was determined in 10 mM CaCl2 at a mixing ratio of 1:2.5 (w/v) after two hours of incubation at room temperature. Determination of soil organic-matter content (OM) was carried out using the loss-on-ignition method at 430°C. Total C and N content of the soils were measured on a CHN analyzer (Truspec CHN, Leco, MI, USA).
Microbial activity and abundance: DNA extraction, spiking, and quantitative real-time PCR (qpcr)
Genomic DNA was isolated from 0.25 g of sieved and untreated soil using a commercially available kit (NucleoSpin® Soil, Macherey-Nagel, Germany) as recommended by the manufacturer. Quantification of bacterial and archaeal 16S rRNA gene copies was conducted on a Corbett Life Science (Quiagen, Netherlands) Rotor-Gene Q system using the primer pairs 338 F/518 R and 787 F/1059 R, respectively, according to Hofmann, Reitschuler, and Illmer (Citation2013). Archaea PCR conditions for bacterial primers can be found in Reitschuler, Lins, and Illmer (Citation2014). To determine the exact abundance of the five dominant groups of methanogenic archaea (Methanosarcinales, Methanobacteriales, Methanococcales, Methanomicrobiales, and Methanocellales) and to avoid biased results because of interactions, we used a spiking method described in Hofmann et al. (Citation2016c). Only the abundance of Methanocella is shown separately in the results because it may stand for the copy number of all methanogenic archaea, and was the only group that was found in all soil samples of a study investigating more than thirty sites across Tyrol (Hofmann et al. Citation2016c). For more details concerning the microbial parameters measured along the elevation gradient of our site, see Hofmann et al. (Citation2016b).
Arthropod diversity and abundance
Seven large pitfall traps (6 cm diameter) with a plastic roof were placed about 10 m apart along a horizontal transect at each elevation level. Traps were filled with ethylene glycol (50%), ethanol (50%), and a drop of odorless detergent. After an exposure of four weeks in August 2014 the traps were removed, and all arthropods transferred to 70 percent ethanol and stored until identification. Springtails, spiders, and beetles were found in sufficient numbers and species in the pitfalls; they were counted and identified to species level for further analysis.
In early August 2014, seven soil samples were taken along the same horizontal transect as the pitfalls at each elevation level with a distance of about 10 m between samples. Steel tubes (57 × 57 mm2) were inserted to a depth of 100 mm, and the soil was stored in plastic bags and cooled until extraction. Sampling points were selected with and without vegetation. All soil samples were extracted in a modified Berlese-Tullgren device for seven days into a saturated salt solution. From the soil samples springtails and oribatid mites were sorted, transferred to 70 percent ethanol, and identified with taxonomic keys to species level.
Species identifications of springtails were done by P. Querner, oribatid mites by B.M. Fischer, beetles by J. Schied, and spiders by K.-H. Steinberger; reference material is stored in the collection of each expert.
Vascular plant diversity and abundance and ecological indicator values
Between 2011 and 2015, all vascular plant species occurring in each of five 1 m × 1 m plots at each elevation level were recorded and their percent cover estimated visually following Pauli et al. (Citation2015).
Ecological properties of elevation levels were derived indirectly from known ecological preferences and altitudinal distributions of vascular plant species. For each plot, mean Landolt indicator values (Landolt et al. Citation2010), describing species’ ecological requirements in terms of soil moisture (F), weighted with the respective species cover values were calculated. Similarly, the thermic vegetation indicator (TVI), that is, weighted species altitudinal ranks, was calculated for each plot following Gottfried et al. (Citation2012). TVI was highly correlated with temperature sums derived from soil temperature measurements carried out at the monitoring plots on the southwest slope of Mt. Schrankogel (Supplemental Figure S1) and was therefore considered as a suitable surrogate for temperature, because no temperature measurements were available for the elevation levels 2,700 m and 2,800 m. Landolt indicator value and thermic vegetation indicator plot means were averaged over each sampling elevation.
Data analysis
The influence of elevation and ecological site properties (TVI, F, MWHC, OM, N, C, pH) on overall abundance and alpha diversity of the organism groups were investigated using generalized linear mixed-effects models (GLMMs) with a penalized quasi-likelihood estimation and a Poisson distribution (function glmm.PQL as implemented in R; R Core Team Citation2016) package MASS (Venables and Ripley Citation2002). Because most ecological site properties and elevation were highly correlated (Supplemental Figure S2), a model with a single ecological factor as fixed effect and elevation as random intercept term was built for each organism group and ecological factor combination separately. P values were corrected using the algorithm proposed by Benjamini and Hochberg (Citation1995), implemented in the R-function p.adjust of the library stats to avoid type I error inflation caused by multiple testing. The effect of elevation was tested using generalized linear models with a quasi-Poisson distribution and log-link (GLMs; function glm, package stats).
As an additional measure of diversity that does not rely solely on presence or absence, but takes into account abundances of species, the Shannon index (Hill Citation1973) was calculated using the R package vegan (Oksanen et al. Citation2015):
where pi is the cover of the ith species. Given equal species richness, H′ increases with increasing evenness and reaches its maximum when all species are equally abundant.
To assess whether overall abundance and species richness patterns along ecological gradients coincided among organism groups, ecological site properties, groups, and their interactions were incorporated as fixed-effect predictors and elevation as random intercept term in GLMMs, with the baseline level of the fixed-effect organism group set to vascular plants. To assess patterns of the Shannon index along the TVI gradient, linear mixed-effect models (LMMs; R-package lme4; Bates et al. Citation2015) were used. For patterns along the elevation gradient, GLMs (abundance and species richness) or linear models (Shannon index) with the same structure were built. A significant ecological factor:group interaction means that abundance or diversity patterns of the respective group along the respective gradient are significantly deviating from that of vascular plants. As abundance values differed among organism groups by a factor of 109, they were rescaled to values between 0 and 100. Raw data of abundance and diversity of organism groups along the elevation gradient and species lists are available in Supplemental Table S.
Table 1. Effect of elevation and ecological factors (TVI = thermic vegetation indicator, F = soil moisture indicator, MWHC = maximum water-holding capcity, OM = soil organic-matter content, C = soil C content, N = soil N content, and pH = soil pH) on (a) abundance of vascular plants, animals, and microbial groups and (b) diversity of vascular plants and animal groups along an elevational gradient on Mt. Schrankogel, Tyrol. Shown are p values from generalized linear models for elevation and for generalized linear mixed-effects models with a quasi-Poisson distribution and log-link for ecological factors with a single predictor each. P values are corrected for multiple testing (Benjamini and Hochberg Citation1995) and those significant at the 0.05 level are printed in bold
Results
Along the elevation gradient at Mt. Schrankogel, TVI, soil moisture (F), MWHC, OM, C content, and N content showed distinct and significant negative correlations with increasing elevation. Soil pH ranged from 4.1 to 5.2 and did not follow any consistent trend (Supplemental Figure S2).
Abundance of organism groups along elevational and ecological gradients
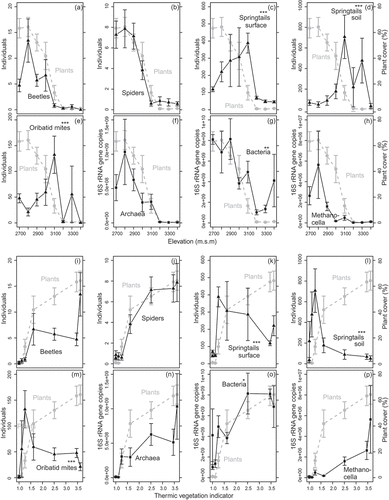
Vegetation cover (vascular plants) decreased with increasing elevation and F, and increased with increasing TVI, MWHC, OM, C content, and N content (Supplemental Figure S3a–g), whereas there was no trend along the pH gradient (Supplemental Figure S3h). However, only elevation (GLM), F, and TVI (GLMMs with the respective ecological factor as the only fixed effect) had a statistically significant effect on vascular plant abundance ().
Elevation had a significant effect on the abundance of archaea, bacteria, beetles, and spiders (GLMs; ). In springtails and oribatid mites no ecological factor had a significant effect on abundance. The abundance of bacteria was statistically significantly influenced by F and TVI. All ecological factors, except pH, had a significant effect on archaea abundance and, except pH and F, on Methanocella abundance, respectively. F, MWHC, and TVI had a significant effect on spider abundance, whereas none of the measured factors had a significant effect on the abundance of beetles (single fixed-effect GLMMs, ).
The abundance of springtails (both surface and soil) and oribatid mites deviated significantly from the vascular plant pattern (i.e., showed a significant ecological indicator:groups interaction in penalized quasi-likelihood models) along all ecological gradients. The abundance of bacteria deviated from the vascular plant pattern along the elevational and TVI gradients only. All other organism groups (i.e., beetles, spiders, archaea, and Methanocella) showed an abundance pattern along the ecological gradients similar to that of vascular plants (, ; Supplemental Figure S4; Supplemental Table S2).
Table 2. Predictors of (a) generalized linear models and (b) fixed effects of generalized linear mixed-effects models with a quasi-Poisson distribution and log-link comparing patterns of abundance of plant, animal, and microbial groups along (a) elevational and (b) thermic vegetation indicator (TVI) on Mt. Schrankogel, Tyrol, Austria. Abundance data were rescaled to values between 0 and 100. The baseline level of the fixed effect organism group is vascular plants. A significant ecological factor:group interaction means that abundance patterns of the respective group along the respective gradient are significantly deviating from that of vascular plants. The predictor organism group is not shown for clarity (see Supplemental Table S2 for the full table). P values significant at the 0.05 level are printed in bold
Figure 1. Abundance of animal and microbial group compared to vascular plant abundance along the (a–h) elevational and (i–p) thermic vegetation indicator gradient. (a,i) Beetles, (b,j) spiders, (c,k) springtails from pitfall traps, (d,l) springtails from soil samples, (e,m) oribatid mites, (f,n) archaea, (g,o) bacteria, and (h,p) Methanocella. Values are means ± standard error of raw data. Vascular plant abundance (percent cover) is illustrated in each subplot in grey. Significant deviations of the abundance patterns of animal and microbial groups from that of vascular plants are indicated with asterisks (significance levels: *p < 0.05, **p < 0.01, ***p < 0.001; generalized linear models with a quasi-Poisson distribution and penalized quasi-likelihood mixed models with a Poisson distribution; )
Diversity of organism groups along elevational and ecological gradients
Vascular plant species richness decreased with increasing elevation and increased with increasing TVI, MWHC, OM, C content, and N content (Supplemental Figure S5a,b,d–g), whereas there was no trend for pH (Supplemental Figure S5h). Vascular plant species richness increased with F between indicator values of 2.2–2.7 and then decreased at higher values (Supplemental Figure S5c). In GLMMs/GLMs with single fixed-effect ecological factors, only elevation, F, and TVI had a statistically significant effect on vascular plant species richness ().
Elevation had a significant effect on the species richness of all animal groups except soil springtails (GLMs, ). F and TVI had a significant effect on spider species richness. All other ecological factors except pH had a significant effect on beetle species richness, whereas none had a significant effect on springtails and oribatid mites species richness (single fixed-effect GLMMs, ).
The species richness patterns of spiders coincided with that of vascular plants along all gradients. Springtails (both surface and soil) deviated significantly from the vascular plant pattern along all ecological gradients except pH, and that of beetles and oribatid mites along all gradients except elevation, soil moisture, and pH, respectively (, ; Supplemental Figure S6; Supplemental Table S).
Table 3. Predictors of (a) generalized linear models and (b) fixed effects of generalized linear mixed-effects models with a quasi-Poisson distribution and log-link comparing diversity patterns of plant and animal groups along (a) elevational and (b) thermic vegetation indicator (TVI) on Mt. Schrankogel, Tyrol, Austria. The baseline level of the fixed effect organism group is vascular plants. A significant ecological factor:group interaction means that diversity patterns of the respective group along the respective gradient are significantly deviating from that of vascular plants. The predictor organism group is not shown for clarity (see Supplemental Table S3a–b for the full table). P values significant at the 0.05 level are printed in bold
Figure 2. Diversity of animal groups compared to vascular plant species richness along the (a–h) elevational and (i–p) thermic indicator gradient. (a,e,i,m) Beetles, (b,f,j,n) spiders, (c,g,k,o) springtails from pitfall traps and soil samples, (d,h,l,p) oribatid mites. Values are means ± standard error of raw data of species richness (a–d, i–l) and Shannon index (e–h, m–p). Vascular plant diversity is illustrated in each subplot in grey. Significant deviations of the diversity patterns of animal groups from that of vascular plants are indicated with asterisks (significance levels: *p < 0.05, **p < 0.01, ***p < 0.001; species richness: generalized linear models with a quasi-Poisson distribution and penalized quasi-likelihood mixed models with a Poisson distribution, Shannon index: linear models and linear mixed-effects models; )
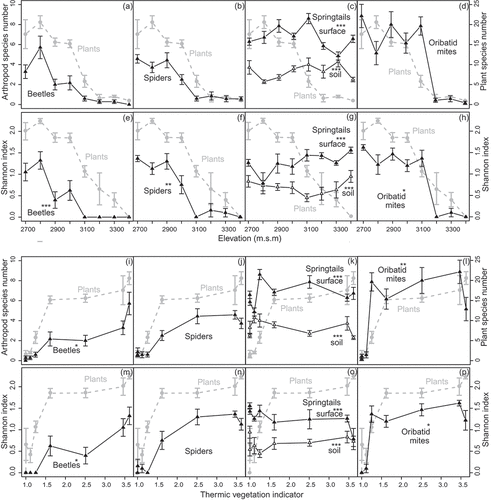
Vascular plant species richness and Shannon index patterns were similar up to an elevation of approximately 3,000 m. Above this elevation, species richness declined sharply and remained more or less at the same level at greater than 3,100 m, whereas the Shannon index showed a continuous linear decline. The Shannon index patterns of all animal groups deviated from that of vascular plants along the elevation gradient, in contrast to species richness where only springtails deviated from the pattern of vascular plants. Alternatively, plant species richness and Shannon index patterns were similar along the entire TVI gradient. Consequently, as with species richness, only the Shannon index patterns of spiders followed the pattern of vascular plants (, , Supplemental Table S).
Table 4. Predictors of (a) linear models and (b) fixed effects of linear mixed-effects models comparing Shannon index patterns of plant and animal groups along (a) elevational and (b) thermic vegetation indicator (TVI) on Mt. Schrankogel, Tyrol, Austria. The baseline level of the fixed effect organism group is vascular plants. A significant ecological factor:group interaction means that diversity patterns of the respective group along the respective gradient are significantly deviating from that of vascular plants. The predictor organism group is not shown for clarity (see Supplemental Table S4 for the full table). P values significant at the 0.05 level are printed in bold
Discussion
Vascular plants proved to be a suitable indicator group to characterize ecological conditions along environmental gradients: Their cover and species numbers decreased with increasing elevation and along a gradient of decreasing soil organic compounds (Supplemental Figure S3a, d, f–g, Figure S5a, d, f–g). The community mean soil moisture indicator F was positively related to elevation (Supplemental Figure S2), reflecting increasing precipitation and decreasing evapotranspiration with elevation at mid-latitude mountains (Barry Citation2008). The thermic vegetation indicator was significantly related to measured temperature at our site (Supplemental Figure S1) and on summits across European mountain ranges (Gottfried et al. Citation2012). Vascular plants responded to recent climate warming through directional changes of their species composition and/or abundance at our study site (Pauli et al. Citation2007), elsewhere in the Alps (Wipf et al. Citation2013), and in other mountain systems (Pauli et al. Citation2012; Steinbauer et al. Citation2018). They may therefore also provide a suitable reference for the response of less visible organism groups in the context of temperature gradients and climate change. Our results, however, show partly consistent and partly significantly divergent patterns of these organism groups from that of vascular plants.
Surface-dwelling beetles and spiders
Contrary to our hypothesis, the more mobile surface-dwelling spiders and beetles did not significantly deviate from the vascular plant cover and diversity patterns along the elevation gradient ( and ). Both species and individual numbers of beetles and spiders significantly decreased with increasing elevation, along with vascular plant species richness and cover. Similar results were found by Chatzaki et al. (Citation2005) for spiders and by Hågvar (Citation1976) for beetles. Meyer and Thaler (Citation1995) described spiders and beetles as more abundant and species-rich in the alpine zone (compared to the subnival and nival zones), where high vegetation cover is an important habitat feature. Although Meyer and Thaler (Citation1995) do not provide a causal explanation of the rapid decline of spiders and beetles at higher elevations where closed vegetation disintegrates, they describe the mesofauna of the nival zone as being mainly composed of springtails and mites, besides microfauna groups such as Protozoa, rotifers, nematodes, tardigrades, and Enchytreidae.
The Shannon diversity index of vascular plants versus beetles and spiders differed along the elevation gradient (), which appeared to be mostly because of a more rapid decline of the two arthropod groups than of vascular plants. Although vascular plant assemblages also consisted of only a few species at higher elevations, they were obviously more evenly distributed. In contrast, species diversity and Shannon index showed consistent patterns along the TVI gradient. Although low-temperature conditions and short seasons are expected to be limiting determinants for the occurrence of beetles and spiders, it is difficult to separate climatic effects from biotic factors, such as the presence of vegetation (Thaler Citation2003). For carabid beetles of the alpine zone, however, Pizzolotto et al. (Citation2016) found that habitat type was more important than elevation, suggesting that the occurrence of plants is an important habitat requirement. Both the investigated spiders and beetles are mostly predators and may not immediately depend on but benefit from vegetation for finding prey on which to feed. Spiders and beetles, however, were previously hardly investigated in the Alps along elevation gradients extending into the nival zone, so our study provides confirmation of their actual absence from the zone above 3,100 m in unvegetated habitats. The only exceptions among the spiders were two Linyphiidae species and the Lycosidae Pardosa nigra, the latter known from scree fields in the Central Eastern Alps up to 3,500 m (Thaler and Buchar Citation1996), and among the beetles the carabid Nebria germari, a pioneer species of open habitats feeding on springtails (Brandmayr et al. Citation2003; Gereben-Krenn, Krenn, and Strodl Citation2011; cf. Supplemental Table S).
Microarthropods
The remarkably high abundance and species numbers of microarthropods, especially of springtails, throughout the elevation gradient suggests that they are governed by other determinants than are vascular plants, surface-dwelling arthropods, and microorganisms. Although systematic gradient studies of springtails and oribatid mites from alpine to nival environments are scarce for the Alps, springtails at high-elevation habitats were found to have a specially adapted lifecycle and to develop cold hardiness (Janetschek Citation1956; Schatz Citation2008). They can endure strong frost without freezing and can be active below the snow layer (Eisenbeis and Meyer Citation1999; Kopeszki Citation2000; Zhang et al. Citation2014). Higher individual numbers of oribatid mites and springtails, compared to other arthropod groups, were also found in high-alpine habitats in southeastern Tibet, China, and thus were characterized as being the least limited groups in terms of low-temperature conditions (Shen et al. Citation2005). The oribatid mite abundance peak at 3,300 m was caused by the high abundance of a single species, Ceratozetes spitzbergenzis, which was only found at this elevation and was the first record of the species outside of the Arctic (Fischer et al. Citation2016). In the Alps, Körner (Citation2011) found springtails, mites, and fungi close to one of the highest mountain peaks at 4,507 m in cushions of Saxifraga oppositifolia, and suggested that these soil animals and fungal decomposer communities depend on the occurrence of higher plant life. This would to some extent be in contradiction to our results, where springtails and oribatid mites obviously did not depend on adjacent vascular plants. One possible explanation for the persistence of springtails in bare-soil habitats is their euryoecious feeding behavior, consuming unicellular algae, fungal mycelium, and lichens as well as plant litter and living plants (Bokhorst et al. Citation2007; Hodkinson et al. Citation1996; Minor et al. Citation2016). This applies also to the carnivorous or omnivorous oribatid mites, with their species-specific and habitat-dependent feeding preferences (Diaz-Aguilar and Quideau Citation2013; Fischer, Meyer, and Maraun Citation2014; Schneider et al. Citation2004), which, however, showed lower diversity at higher elevations. Yet, springtails were reported to be wind-dispersed more often than oribatid mites (Freeman Citation1952; Glick Citation1939; Gressitt et al. Citation1961; Johnson Citation1957), which could explain their high species numbers at higher elevations and their more even distribution across elevation and ecological gradients. Although wind dispersal can also be relevant for oribatid mites (Lehmitz et al. Citation2011), we trapped them only sporadically at the wind-exposed surface. In contrast, seventeen species of springtails were captured in the pitfall traps, nine of which also occurred in the soil (Supplemental Table S).
Microorganisms
Both domains of prokaryotic life, that is, bacteria and archaea, were distinctly influenced by elevation and additionally by the content of organic matter in soils, which in turn is positively correlated with vascular plant cover (; Supplemental Figure S4c). Archaeal distribution is more pronouncedly affected by elevation than bacteria, leading to an increasing bacteria/archaea ratio with increasing elevation (Hofmann et al. Citation2016b). The ratio between methane-producing archaea and methane-oxidizing bacteria (mainly belonging to the phylum of proteobacteria) was surprisingly constant throughout the entire elevation gradient at Mt. Schrankogel, whereas no connection of procaryotes to soil animals, neither to total individual numbers nor to species richness, could be established. This suggests an unaffected coexistence of these groups without close and direct interrelations. The abundance of soil fungi was not determined within the present investigation, but from similar investigations it is known that the ratio of fungal to prokaryotic cells usually increases with C/N ratios and elevation (Siles et al. Citation2016; Thébault et al. Citation2014). Effects of elevation on fungal community structure, abundance, and activity, however, distinctly vary with season, abiotic factors such as pH, and the dominant vegetation (Siles and Margesin Citation2016).
Conclusions
Comparisons of abundance and diversity patterns of functionally and taxonomically strongly differing organism groups from alpine to nival habitats beyond the limits of closed vegetation are unprecedented so far in the European Alps. We could show that the surface-dwelling invertebrate fauna such as spiders and beetles mostly stay side-by-side with vascular plant vegetation. In contrast, soil microarthropods, springtails in particular, occur well beyond the vegetation limits and show high individual numbers at high elevations and similar species numbers throughout the gradient. A number of soil microarthropod species, thus, appear to be able to survive in the absence of living vascular plants as food source or shelter. Soil microorganism abundance patterns were more strongly related to vascular plant vegetation than the patterns of soil microarthropods, with no significant deviations from vascular plants for archaea, but measurable differences for bacteria, which can be quite abundant even in nival habitats.
Elevation and the thermic vegetation gradient could serve as a space-for-time substitute and, thus, we may draw conclusions on the potential effects of climate change on diversity and abundance across organism groups. The observed decline of high-elevation specialist vascular plant species and a concurrent increase of species centred at lower elevations (Pauli et al. Citation2007) should also influence the distribution of other organisms, especially of surface-dwelling invertebrates. Caution is advised, however, when inferring processes and causal relationships from patterns. The adaptive metabolic mechanisms to low-temperature regimes may strongly vary among invertebrates and vascular plants, but could have similar detrimental consequences under warmer conditions. For example, the limited ability of some invertebrates to invoke diapause for nutritional regulation in the absence of sufficiently low temperatures (Hodkinson Citation2005) and the inability of cryophilic vascular plants for respiratory adjustments to higher temperatures (Larigauderie and Körner Citation1995) both would limit population growth rates.
An upward advancement of vegetation would favor methane-producing microorganisms, which can cause feedback effects influencing atmospheric greenhouse gas concentrations, although less relevant in the Alps than in the large low-temperature environments of the Arctic.
Our results may provide a first indication that other organism groups concurrently come under pressure with vascular plants. Further, multiorganism group studies along climatic gradients would help to estimate the potential risk of biodiversity losses of the whole ecosystem. Our study may also elucidate the potential and the limitations of using vascular plant patterns as a proxy for abundance and diversity changes in other organism groups, which are far more difficult to sample and monitor. Understanding the interdependencies and causal relationships among organism groups and individual species under changing thermal conditions, such as shifts in facilitation versus competition and effects on physiological adaptation capabilities, however, would require both experimental studies and continued monitoring across different organism groups.
Supplemental Material
Download Zip (1.8 MB)Acknowledgments
Notably, we acknowledge Karl-Heinz Steinberger, Innsbruck, for the identification of the spiders in the pitfall samples, and we thank Philipp R. Semenchuk for statistical advice. We thank the Government of Tyrol for providing permission to set up a field camp during the field seasons 2014 and 2015. The study site is part of the LTSER Platform Tyrolean Alps.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website here.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Harald Pauli
MW, PI, PQ, and HP conceived and designed this study. AL, KS, and HP conducted vegetation, PI and NP soil for microbiological, PQ and BMF soil for arthropod and pitfall trap sampling. KH, NP, and PI ran the microbiological analyses. PQ identified Collembola species, BMF Oribatida, and JS beetles. MW analyzed the data, and HP and MW wrote the manuscript with contributions from all other authors.
References
- Alatalo, J. M., A. K. Jägerbrand, and P. Čuchta. 2015. Collembola at three alpine subarctic sites resistant to twenty years of experimental warming. Scientific Reports 5(18161):1–13.
- Barry, R. G. 2008. Mountain weather and climate. Cambridge, UK: Cambridge University Press.
- Bates, D., M. Maechler, B. M. Bolker, and S. Walker. 2015. lme4: Linear mixed-effects models using Eigen and S4. Journal of Statistical Software 67:1–48.
- Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Methodological 57:289–300.
- Bokhorst, S., C. Ronfort, A. Huiskes, P. Convey, and R. Aerts. 2007. Food choice of Antarctic soil arthropods clarified by stable isotope signatures. Polar Biology 30:983–90.
- Brandmayr, P., R. Pizzolotto, S. Scalercio, M. C. Algieri, and T. Zetto. 2003. Diversity patterns of carabids in the Alps and the Apennines. In Alpine biodiversity in Europe - A Europe-wide assessment of biological richness and change, eds., 307–17. Berlin: Springer.
- Bryant, J. A., C. Lamanna, H. Morlon, A. J. Kerkhoff, B. J. Enquist, and J. L. Green. 2008. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proceedings of the National Academy of Sciences of the United States of America 105:11505–11.
- Chatzaki, M., P. Lymberakis, G. Markakis, and M. Mylonas. 2005. The distribution of ground spiders (Araneae, Gnaphosidae) along the altitudinal gradient of Crete, Greece: Species richness, activity and altitudinal range. Journal of Biogeography 32:813–31.
- Crutzen, P. J., and J. Lelieveld. 2001. Human impacts on atmospheric chemisty. Annual Review of Earth and Planetary Sciences 29:17–45.
- Diaz-Aguilar, I., and S. A. Quideau. 2013. Trophic ecology of mesostigmatan and oribatid mites in harvested and control coniferous and deciduous stands of the boreal mixedwood forest determined using N-15 stable isotopes. Soil Biology & Biochemistry 67:147–54.
- Dillon, M. E., M. R. Frazier, and R. Dudley. 2006. Into thin air: Physiology and evolution of alpine insects. Integrative and Comparative Biology 46:49–61.
- Eisenbeis, G., and E. Meyer. 1999. Ecophysiological and morphological features of glacier-dwelling Collembola. In Cold-adapted organisms - ecology, physiology, enzymology and molecular biology, eds., 197–218. Berlin: Springer.
- Fischer, B. M., E. Meyer, and M. Maraun. 2014. Positive correlation of trophic level and proportion of sexual taxa of oribatid mites (Acari: Oribatida) in alpine soil systems. Experimental and Applied Acarology 63:465–79.
- Fischer, B. M., H. Schatz, P. Querner, and H. Pauli. 2016. Ceratozetes spitsbergensis Thor, 1934: An Arctic mite new to continental Europe (Acari: Oribatida). International Journal of Acarology 42:135–39.
- Freeman, J. A. 1952. Occurence of Collembola in the air. Proceedings of the Royal Entomological Society of London 27A:1–28.
- Gereben-Krenn, B.-A., H. W. Krenn, and M. A. Strodl. 2011. Initial colonization of new terrain in an alpine glacier foreland by carabid beetles (Carabidae, Coleoptera). Arctic, Antarctic, and Alpine Research 43:397–403.
- Glick, P. A. 1939. The distribution of insects, spiders and mites in the air. Technical Bulletin No. 673, U.S. Department of Agriculture, Washington.
- Gottfried, M., M. Hantel, C. Maurer, R. Toechterle, H. Pauli, and G. Grabherr. 2011. Coincidence of the alpine-nival ecotone with the summer snowline. Environmental Research Letters 6(014013):1–12.
- Gottfried, M., H. Pauli, A. Futschik, M. Akhalkatsi, P. Barancok, J. L. Benito Alonso, G. Coldea, J. Dick, B. Erschbamer, M. R. Fernandez Calzado, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2:111–15.
- Gottfried, M., H. Pauli, and G. Grabherr. 1998. Predicting of vegetation patterns at the limits of plant life: A new view of the alpine-nival ecotone. Arctic and Alpine Research 30:207–21.
- Gressitt, J. L., R. E. Leech, T. S. Leech, J. Sedlacek, and K. A. J. Wise. 1961. Trapping of air-borne insects in the antarctic area (part 2). Pacific Insects 3:559–62.
- Hågvar, S. 1976. Altitudinal zonation of the invertebrate fauna on branches of birch (Betula pubescens Ehrh.). Norwegian Journal of Entomology 23:61–74.
- Hågvar, S., and K. Klanderud. 2009. Effect of simulated environmental change on alpine soil arthropods. Global Change Biology 15:2972–80.
- Hahn, D. A., and D. L. Denlinger. 2011. Energetics of insect diapause. Annual Review of Entomology, M. R.Berenbaum, R. T. Carde, and G. E. Robinson eds., Vol.56, 103–21.
- Heggen, M. P. 2010. Oribatid mites of alpine fennoscandia. Norwegian Journal of Entomology 57:38–70.
- Hill, M. O. 1973. Diversity and evenness: A unifying notation and its consequences. Ecology 54:427–32.
- Hodkinson, I. D. 2005. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biological Reviews 80:489–513.
- Hodkinson, I. D., S. J. Coulson, N. R. Webb, and W. Block. 1996. Can high arctic soil microarthropods survive elevated summer temperatures? Functional Ecology 10:314–21.
- Hofmann, K., A. Lamprecht, H. Pauli, and P. Illmer. 2016a. Distribution of prokaryotic abundance and microbial nutrient cycling across a high-alpine altitudinal gradient in the Austrian Central Alps is affected by vegetation, temperature, and soil nutrients. Microbial Ecology 72:704–16.
- Hofmann, K., H. Pauli, N. Praeg, A. O. Wagner, and P. Illmer. 2016b. Methane-cycling microorganisms in soils of a high-alpine altitudinal gradient. FEMS Microbiology Ecology 92:1–10 (fiw009).
- Hofmann, K., N. Praeg, M. Mutschlechner, A. O. Wagner, and P. Illmer. 2016c. Abundance and potential metabolic activity of methanogens in well-aerated forest and grassland soils of an alpine region. FEMS Microbiology Ecology 92:1–11 (fiv171).
- Hofmann, K., C. Reitschuler, and P. Illmer. 2013. Aerobic and anaerobic microbial activities in the foreland of a receding glacier. Soil Biology & Biochemistry 57:418–26.
- Janetschek, H. 1956. Das Problem der inneralpinen Eiszeitüberdauerung durch Tiere (Ein Beitrag zur Geschichte der Nivalfauna). Österreichische Zoologische Zeitschrift 6:421–506.
- Jiang, Y. F., X. Q. Yin, and F. B. Wang. 2015. Composition and spatial distribution of soil mesofauna along an elevation gradient on the north slope of the Changbai Mountains, China. Pedosphere 25:811–24.
- Johnson, C. G. 1957. The distribution of insects in the air and the empirical relation of density to height. Journal of Animal Ecology 26:479–94.
- Kopeszki, H. 2000. Auf der Suche nach roten Gletscherflöhen. Funde hochalpiner Springschwänze (Collembola). Vorarlberger Naturschau 8:133–44.
- Körner, C. 2011. Coldest places on earth with angiosperm plant life. Alpine Botany 121:11–22.
- Landolt, E., B. Bäumler, A. Erhardt, O. Hegg, F. Klötzli, W. Lämmler, M. Nobis, K. Rudmann-Maurer, F. H. Schweingruber, J.-P. Theurillat, E. Urmi, M. Vust, and T. Wohlgemuth. 2010. Flora indicativa: Ökologische Zeigerwerte und biologische Kennzeichen zur Flora der Schweiz und der Alpen/Ecological indicator values and biological attributes of the Flora of Switzerland and the Alps. 2nd ed. Bern: Haupt Verlag.
- Larigauderie, A., and C. Körner. 1995. Acclimation of leaf dark respiration to temperature in alpine and lowland plant-species. Annals of Botany 76:245–52.
- Lehmitz, R., D. Russell, K. Hohberg, A. Christian, and W. E. R. Xylander. 2011. Wind dispersal of oribatid mites as mode of migration. Pedobiologia 54:201–07.
- Leonov, V. D., A. A. Rakhleeva, and E. A. Sidorchuk. 2015. Distribution of oribatid mites (Acari: Oribatida) along an altitudinal profile of Mount Vud’yavrchorr (the Khibiny Mountains). Eurasian Soil Science 48:1257–67.
- Mani, M. S. 1968. Ecology and biogeography of high altitude insects. The Hague: Dr. W. Junk N. V. Publ.
- Maraun, M., S. Visser, and S. Scheu. 1998. Oribatid mites enhance the recovery of the microbial community after a strong disturbance. Applied Soil Ecology 9:175–81.
- McCain, C. M., and J.-A. Grytnes. 2010. Elevational gradients in species richness, encyclopedia of life sciences. 1–10. Chichester: John Wiley & Sons.
- Meyer, E., and K. Thaler. 1995. Animal diversity at high altitudes in the Austrian Central Alps. In Arctic and alpine biodiversity: Patterns, causes and ecosystem consequences, eds., 97–108. Berlin: Springer.
- Minor, M. A., A. B. Babenko, S. G. Ermilov, A. A. Khaustov, and O. L. Makarova. 2016. Effects of cushion plants on high-altitude soil microarthropod communities: Cushions increase abundance and diversity of mites (Acari), but not springtails (Collembola). Arctic, Antarctic, and Alpine Research 48:485–500.
- Mitchell, R. J., H. M. Urpeth, A. J. Britton, H. Black, and A. R. Taylor. 2016. Relative importance of local- and large-scale drivers of alpine soil microarthropod communities. Oecologia 182:913–24.
- Nagy, L., and G. Grabherr. 2009. The biology of alpine habitats. 336. Oxford, New York: Oxford University Press.
- Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, and H. Wagner. 2015. vegan: community ecology package. R package version 2.3-0.
- Pauli, H., M. Gottfried, S. Dullinger, O. Abdaladze, M. Akhalkatsi, J. L. B. Alonso, G. Coldea, J. Dick, B. Erschbamer, R. F. Calzado, et al. 2012. Recent plant diversity changes on Europe’s mountain summits. Science 336:353–55.
- Pauli, H., M. Gottfried, A. Lamprecht, S. Niessner, S. Rumpf, M. Winkler, K. Steinbauer, and G. Grabherr. 2015. The GLORIA field manual – Standard multi-summit approach, supplementary methods and extra approaches. 5th ed. Vienna: GLORIA-Coordination, Austrian Academy of Sciences & University of Natural Resources and Life Sciences Vienna.
- Pauli, H., M. Gottfried, K. Reiter, C. Klettner, and G. Grabherr. 2007. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global Change Biology 13:147–56.
- Pizzolotto, R., A. Albertini, M. Gobbi, and P. Brandmayr. 2016. Habitat diversity analysis along an altitudinal sequence of alpine habitats: The Carabid beetle assemblages as a study model. Periodicum Biologorum 118:241–54.
- Praeg, N., A. O. Wagner, and P. Illmer. 2017. Plant species, temperature, and bedrock affect net methane flux out of grassland and forest soils. Plant and Soil 410:193–206.
- R Core Team. 2016. R: A language and environment for statistical computing. http://www.R-project.org/.
- Rabitsch, W., W. Graf, P. Huemer, M. Kahlen, C. Komposch, W. Paill, A. Reischütz, P. L. Reischütz, D. Moser, and F. Essl. 2016. Biogeography and ecology of endemic invertebrate species inAustria: A cross-taxon analysis. Basic and Applied Ecology 17:95–105.
- Reitschuler, C., P. Lins, and P. Illmer. 2014. Primer evaluation and adaption for cost-efficient SYBR green-based qPCR and its applicability for specific quantification of methanogens. World Journal of Microbiology and Biotechnology 30:293–304.
- Schatz, H. 2008. The Schlern/Sciliar massif (Southern Alps, Italy) – A biodiversity hotspot for oribatid mites (Acari, Oribatida). In Integrative acarology. Proceedings of the 6th European Congress, European Association of Acarologists 2008 July 21–25, eds. M. Bertrand, S. Kreiter, K. D. McCoy, A. Migeon, M. Navajas, M. S. Tixier, and L. Vial, 24–31. Montpellier.
- Schinner, F., R. Öhlinger, E. Kandeler, and R. Margesin. 1996. Methods in soil biology. 426. Heidelberg: Springer.
- Schneider, K., S. Migge, R. A. Norton, S. Scheu, R. Langel, A. Reineking, and M. Maraun. 2004. Trophic niche differentiation in soil microarthropods (Oribatida, Acari): Evidence from stable isotope ratios (N-15/N-14). Soil Biology & Biochemistry 36:1769–74.
- Shen, J., T. Solhøy, H. F. Wang, T. I. Vollan, and R. M. Xu. 2005. Differences in soil arthropod communities along a high altitude gradient at Shergyla Mountain, Tibet, China. Arctic, Antarctic, and Alpine Research 37:261–66.
- Siles, J. A., T. Cajthaml, S. Minerbi, and R. Margesi. 2016. Effect of altitude and season on microbial activity, abundance and community structure in alpine forest soils. FEMS Microbiology Ecology 92:1–12 (fiw008).
- Siles, J. A., and R. Margesin. 2016. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient in alpine forest soils: What are the driving factors? Microbial Ecology 72:207–20.
- Steinbauer, M. J., J.-A. Grytnes, G. Jurasinski, A. Kulonen, H. Pauli, C. Rixen, M. Winkler, M. Bardy-Durchhalter, E. Barni, A. D. Bjorkman, et al. 2018. Climate warming accelerates the increase in plant species richness on European mountain summits. Nature 556:231–234.
- Thaler, K. 2003. The diversity of high altitude Arachnids (Araneae, Opiliones, Pseudoscorpiones) in the Alps. In Alpine biodiversity in Europe - A Europe-wide assessment of biological richness and change, eds., 281–96. Berlin: Springer.
- Thaler, K., and J. Buchar. 1996. Die Wolfspinnen von Österreich 3: Gattungen Aulonia, Pardosa (p. p.), Virata, Xerolycosa (Arachnida, Araneae: Lycosidae) - Faunistischtiergeographische Übersicht. Carinthia II 186/106:393–410.
- Thébault, A., J.-C. Clément, S. Ibanez, J. Roy, R. A. Geremia, C. A. Pérez, A. Buttler, Y. Estienne, and S. Lavorel. 2014. Nitrogen limitation and microbial diversity at the treeline. Oikos 123:729–40.
- Theurillat, J.-P., A. Schlüssel, P. Geissler, A. Guisan, C. Velluti, and L. Wiget. 2003. Vascular plant and bryophyte diversity along elevation gradients in the Alps. In Alpine biodiversity in Europe - A Europe-wide assessment of biological richness and change, eds., 185–93. Berlin: Springer.
- Venables, W. N., and B. D. Ripley. 2002. Modern applied statistics with S. Fourth edition. New York: Springer.
- Wipf, S., V. Stöckli, K. Herz, and C. Rixen. 2013. The oldest monitoring site of the Alps revisited: Accelerated increase in plant species richness on Piz Linard summit since 1835. Plant Ecology and Diversity 6:447–55.
- Zhang, B., L. Chang, N. Zhen, H. Wu, X. Sun, and D. Wu. 2014. A review of the snow-living collembola. Acta Ecologica Sinica 34:1922–36.
