ABSTRACT
Climate change is shifting species distributions and altering plant community composition worldwide. For instance, with rising temperatures shrubs are encroaching into alpine ecosystems, resulting in important implications for ecosystem functioning. In particular, woody-plant encroachment could slow decomposition in systems traditionally dominated by herbaceous species. To evaluate how litter decomposition responded jointly to warming and shrub presence, we conducted a passive warming chamber experiment in subalpine and alpine plant communities in the White Mountains of California. Passive warming chambers were placed over plots with and without the range-expanding sagebrush Artemisia rothrockii at two elevations. Litter from A. rothrockii and the common perennial herb Trifolium andersonii decomposed for two years under the experimental treatments. Nitrate availability was measured with ion-exchange resins during the same time period. Warming decreased decomposition rates overall, associated with decreased soil moisture, but did not influence soil nitrate availability. Sagebrush presence decreased both decomposition rates and nitrate availability. Hence, future warming in this system will likely reduce decomposition rates, both directly and indirectly, via shrub encroachment. However, impacts on nutrient mineralization are less clear. These findings highlight how shifting species composition, through processes such as range expansions, can influence ecosystem responses to climate change.
Introduction
High-altitude and high-latitude biomes are predicted to experience disproportionate amounts of warming in the twenty-first century (IPCC Citation2007; Nogués-Bravo et al. Citation2007). Warming may alter the rate of nutrient cycling and the associated release of carbon dioxide (CO2) to the atmosphere and thus have important consequences for the balance of carbon (C) sequestered versus released in these cold biomes (Gorham Citation1991; Mack et al. Citation2004; Schuur et al. Citation2009; Väisänen et al. Citation2014; Webb et al. Citation2016; Welker et al. Citation2004). Plant litter is a C pool that has the potential to contribute to this balance (Grogan et al. Citation2001), and litter decomposition rates may be directly or indirectly changed by global warming.
Rising temperatures associated with climatic warming are predicted to have strong, direct consequences for the physical processes governing decomposition. Low temperatures limit decomposition rates in alpine systems (Seastedt and Adams Citation2001; Seastedt, Walker, and Bryant Citation2001; Withington and Sanford Citation2007); therefore, warmer temperatures are predicted to increase enzymatic activities and nutrient cycling rates (Coûteaux, Bottner, and Berg Citation1995; Davidson et al. Citation2006). Alternatively, warming could cause earlier snowmelt and greater water stress in the latter part of the summer (Parida and Beurmann Citation2014). Reduced soil moisture can strongly limit litter decomposition (Bunnell et al. Citation1977; Swift, Heal, and Anderson Citation1979), especially in the alpine (Aerts Citation2006; Bryant et al. Citation1998; Fisk, Schmidt, and Seastedt Citation1998; O’Lear and Seastedt Citation1994; Seastedt, Walker, and Bryant Citation2001; Webber et al. Citation1976; Withington and Sanford Citation2007). Hence, warming has the potential to either increase or decrease decomposition rates in alpine systems, mediated through an effect on soil moisture.
In addition to the direct effects of warming, shifts in species composition can indirectly influence ecosystem process rates. As montane systems warm, some species are able to shift their elevational ranges upward to track climatic niches (Doak and Morris Citation2010; Gavazov Citation2010; Gottfried et al. Citation1999; Klanderud and Birks Citation2003; Kopp and Cleland Citation2014). For instance, woody shrubs are encroaching from lower elevation areas into the alpine, with important implications for ecosystem processes (Myers-Smith et al. Citation2011; Wookey et al. Citation2009), such as decomposition. Shrub litter generally decomposes more slowly than forb and graminoid litter, and hence warming may indirectly slow decomposition in alpine systems by facilitating shrub encroachment (Cornelissen et al. Citation2007).
Shrub encroachment may also alter decomposition rates via effects on the microenvironment. For instance, shrubs tend to have higher stature than the prostrate species that typically dominate alpine zones; thus, shrubs could reduce the photodegradation of litter through shading (Sturm et al. Citation2000) and slow decomposition (Austin and Vivanco Citation2006; Henry, Brizgys, and Field Citation2008). Shrubs could also enhance snow accumulation (Essery and Pomeroy Citation2004; Liston et al. Citation2002; Pomeroy et al. Citation2006), which may promote increased microbial activity and faster nutrient cycling via enhanced thermal insulation from snow trapped beneath the shrub canopy (Sturm et al. Citation2000, Citation2005; Seastedt and Adams Citation2001; Schimel, Bilbrough, and Welker Citation2004; but also see DeMarco, Mack, and Bret-Harte Citation2014). Shrub encroachment into alpine ecosystems is likely to alter decomposition rates, yet the direction depends both on how shrubs influence the microenvironment and the relative decomposition rates of shrubs versus resident species.
The White Mountain range of California provides an appropriate study site for investigating the impact of warming and shrub encroachment on nutrient cycling. Located in the rain shadow of the Sierra Nevada Mountains, the alpine and subalpine vegetation zones receive less precipitation than many other montane systems and the plant communities have a long history of study (Hall Citation1991; Rundel, Gibson, and Sharifi Citation2005). In a forty-nine-year resurvey of sites initially characterized by Mooney et al. (1962), Kopp and Cleland (Citation2014) documented an increase in the elevational range margin and abundance of Artemisia rothrockii, an endemic species of sagebrush common in the subalpine area of the White Mountains, during a period of rising temperature (0.98°C increase in mean growing season temperature) and decreasing precipitation (53 mm decrease in mean annual precipitation). In contrast to A. rothrockii, the previously abundant nitrogen (N)-fixing perennial herb Trifolium andersonii experienced a range contraction with decreased abundance at the lower range margin and no corresponding expansion of its upper limit (Kopp and Cleland Citation2014). The expansion of Betula, a genus common in mesic alpine and Arctic tundra systems, has been the subject of several decomposition studies (e.g., DeMarco, Mack, and Bret-Harte Citation2014; McLaren et al. Citation2017; Myers-Smith and Hik Citation2013), but the impact of shrub expansion in more xeric alpine systems, and across a wider range of taxa, such as Artemisia, needs to be better characterized.
To understand the sensitivity of alpine litter dynamics to the direct and indirect (shrub encroachment) effects of climate warming, we investigated the impact of experimental warming and shrub presence on the early stages of litter decomposition, when mass loss and mineralization rates are most rapid. While several studies have examined how warming or shrub encroachment affect litter decomposition independently, we also examined the interaction of these factors. During two years (September 2013–September 2015) and at two elevations (subalpine and alpine zones), we examined decomposition rates of two species with ranges that are responding differently to climate change: A. rothrockii and T. andersonii. We predicted that warming, induced by passive open-top chambers (OTCs), could produce one of two responses: (1) warming could lead to greater decomposition via increased microbial activity or (2) warming could decrease decomposition due to the soil moisture limitation of microbial processes. We expected that the presence of A. rothrockii would indirectly affect decomposition rates via microclimate changes: shrubs could promote increased microbial activity through enhanced snow accumulation and thermal insulation or shrubs could reduce photodegradation of litter through shading (Sturm et al. Citation2000). A separate shading treatment allowed us to test this last hypothesis.
Materials and methods
Experimental design
This experiment was conducted at two elevations in the White Mountains (Inyo County, CA, USA) utilizing the University of California White Mountain Research Station. The subalpine site was located at 3,100 m (37° 29.9ʹ N, 118° 10.3ʹ W) and the alpine site at 3,750 m (37° 34.1ʹ N, 118° 14.3ʹ W). Historical averages (1956–1985) from weather stations near both study sites show that the low elevation Crooked Creek Station (3,094 m) has a mean annual precipitation of 327 mm yr−1 and a mean annual temperature of 0.9°C. The high elevation Barcroft Station (3,800 m) has a mean annual precipitation of 456 mm yr−1 and a mean annual temperature of −1.7°C (Hall Citation1991).
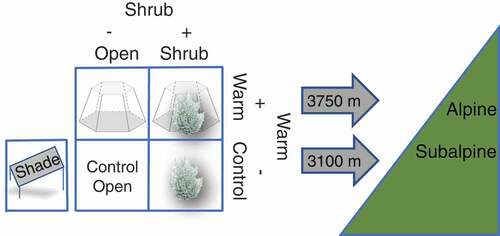
In 2011, experimental plots were established in the subalpine (3,100 m) and alpine (3,750 m) to evaluate the influence of warming and sagebrush encroachment on plant communities at both elevations. Plots were established such that they had similar species composition, except for the presence or absence of A. rothrockii. With the exception of the presence of A. rothrockii, treatments were assigned randomly: (1) warmed plots with A. rothrockii (Warm Shrub), (2) warmed plots without A. rothrockii (Warm Open), (3) unwarmed plots with A. rothrockii (Control Shrub), (4) unwarmed plots without A. rothrockii (Control Open), and (5) shaded plots without A. rothrockii (Shade; ). Each treatment had four replicates at each elevation for a total of forty circular plots, each with an area of 0.785 m2. Plots were established at least 2 m apart and shared a common granitic-derived soil substrate. Following methods developed by the International Tundra Experiment (Molau and Mølgaard Citation1996), OTCs were 1 m in diameter and were constructed with 5 oz clear Crystalite fiberglass (thickness = 1.1 mm, light transmission = 90%; Ridout Plastics Company Inc., San Diego, CA, USA). Open-top chambers were in place year-round. The shading treatment was built by suspending a fiberglass window screen 20 cm above the soil surface. Shading structures intercepted 60 percent of the light, and the shade structures were removed for the nongrowing season (from October 13, 2013 to May 24, 2014, and from September 15, 2014 to May 5, 2015) to prevent impacts on snow cover.
Surface temperature and soil moisture
To monitor surface temperature, iButton temperature loggers were secured in 5 cm PVC pipe (to block solar radiation) and were placed in three plots per treatment at each elevation. Temperatures were recorded hourly. Volumetric soil moisture content (0–10 cm depth) was measured in all plots in each treatment at the low elevation, taking three samples per plot using a Spectrum Field Scout TDR 100 portable volumetric soil moisture meter (Spectrum Technologies, Aurora, IL, USA). This sensor could only be used at the low elevation site because the rocky substrate at the high elevation site prevented insertion of the soil moisture probes.
Litter decomposition
To compare litter decomposition across treatments, on October 13, 2013, litterbags containing 1.5 g of dry litter were deployed within the experimental framework. Litterbags contained material from either T. andersonii or A. rothrockii, using litter collected during August 2013. Litterbags were constructed using 1 mm mesh nylon netting material to contain the small leaves. Eight replicates were collected for both litter types from each treatment at both elevations (two litterbags per litter type per plot) on September 15, 2014 and September 17, 2015, resulting in 320 litterbags. On collection, litter was separated from the mesh, dried, and weighed. A subsample was ground and ashed in a muffle furnace at 600°C for six hours to calculate ash-free mass loss. Ash-free mass at deployment was subtracted from ash-free mass at a collection date and divided by ash-free mass at deployment to get a value for mass loss. The decay constant (k, y−1) was fitted to the data using a single exponential model, Xt/X0 = e−kt with Xt and X0 being the initial ash-free mass and the mass at time t, respectively (Adair, Hobbie, and Hobbie Citation2010). We used the nls function to fit nonlinear least squares to estimate k on untransformed data.
Nitrate availability
Treatment effects on soil nutrient availability were assessed using ion-exchange resin bags (Giblin et al. 1994) deployed during four time periods. These four time periods consisted of growing (from May 24, 2014 to September 15, 2014, and from May 5, 2015 to September 17, 2015) and nongrowing (from October 13, 2013 to May 24, 2014, and from September 15, 2014 to May 5, 2015) season time points. Resin bags were constructed using 2 g of Dowex Marathon MR-3 mixed ion-exchange resins (Giblin et al. 1994). At deployment, resin bags were buried 5 cm below the soil surface in each treatment. On collection, bags were washed with deionized water to remove soil particles, then extracted using a 2.0 M NaCl/0.1 M HCl solution, which was shaken along with the resins for one hour, filtered through pre-leached Whatman #1 filters, and analyzed with subsequent colorimetric analyses (Doane and Horwáth Citation2003).
Statistical analysis
Statistical analyses were performed in R v. 3.3.2 (R Development Core Team Citation2017). Data were tested for normality and homogeneity of variance before analysis. The k constants were analyzed with mixed-effects models where species, warming, shrub presence, and elevation were included as fixed effects (excluding the shading treatment) and plot was a random effect. The effect of shading on decomposition was analyzed using two mixed-effects models on subsets of data that excluded the warming treatment and used two levels of Shrub treatment (Control Open and Shade; Control Shrub and Shade). The comparison between the Control Open and Shade treatments was drawn to determine the effect of shading on litter decomposition, while the comparison between Control Shrub and Shade treatments was made to evaluate whether their effects on decomposition were similar, which would confirm that shading was the mechanism behind the effect of shrubs.
Nitrate availability was log-transformed to meet assumptions of normality. We ran a mixed-effects model to analyze nitrate availability with warming, shrub presence, and elevation included as fixed effects (excluding the shading treatment) and plot as a random effect. The effect of litter decomposition rate on nitrate availability was tested with a linear model where log-transformed nitrate availability was predicted by k values, both averaged at the plot level.
Significance of all factors was evaluated with Type II tests using the ANOVA function in the car package (Fox and Weisberg Citation2011). When appropriate, multiple comparisons of specific treatments were made using Tukey’s post hoc analyses via the lsmeans package (Lenth Citation2016).
Results
Climate data
The average mean temperature and precipitation throughout the study duration (2013–2015) was warmer and drier than the historical averages (see the “Materials and Methods” section for historical climate means). During the study, the average mean temperature was 2.97 ± 0.50°C at the low elevation site (2.07°C higher than the historical average) and 0.60 ± 0.50°C at the high elevation site (2.30°C higher than the historical average; PRISM Climate Group Citation2018). Annual precipitation was 279 ± 104 mm at the low elevation site (14.7 percent lower than the historical average) and was 300 ± 119 mm at the high elevation site (34.2 percent lower than the historical average; PRISM Climate Group Citation2018). These deviations from the long-term average climate were likely the result of documented warming at these sites (Kopp and Cleland Citation2014) and the historic drought that California experienced between 2012 and 2015 (Margulis et al. Citation2016).
Surface temperature and soil moisture
Surface temperatures were on average 1.63°C higher in the warmed treatment compared to the unwarmed treatment (; F1,16 = 39.9, P < 0.001) and on average 1.11°C higher at the low elevation compared to the high elevation (; F1,16 = 15.9, P = 0.001). The presence of A. rothrockii (hereafter referred to as shrub presence) did not statistically influence temperature in the plots. While soil moisture was only examined at the low elevation, there was an interaction between the warmed treatment and shrub presence (F1,14 = 5.48, P = 0.035) such that there was 24.5 percent lower soil moisture in the warmed treatment containing shrubs (Warm Shrub) compared to the unwarmed treatment with shrubs (Control Shrub; Tukey’s HSD, P = 0.021).
Table 1. The surface temperature (°C) and soil moisture (percent volumetric water content) across treatments. Soil-moisture data were only collected at 3,100 m
Litter decomposition
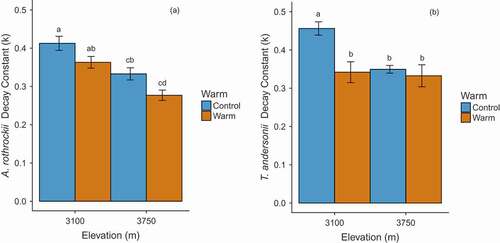
Averaged across all elevations, species, and treatments, litter mass loss was approximately 41.1 ± 8.69 percent during the two-year study period. Shrub presence slowed decomposition, leading to 13.0 percent slower decomposition compared to open plots (). While warming tended to slow litter decomposition (), the magnitude of the effect depended on both elevation and litter species identity (, ). Artemisia rothrockii decay was not affected by warming at either elevation (), while T. andersonii decomposition was lower in the warmed treatment at the low elevation (). See for average k values across treatments.
Table 2. Mixed-effects model analysis of decay constants (k y−1). The factorial design consisted of two species of litter (A. rothrockii and T. andersonii), two temperature treatments (Warm; warmed and control), and two shrub treatments (Shrub; with and without) across two elevations (Elevation; subalpine at 3,100 m and alpine at 3,750 m). Significant effects (P < 0.05) are in bold
Shading treatment
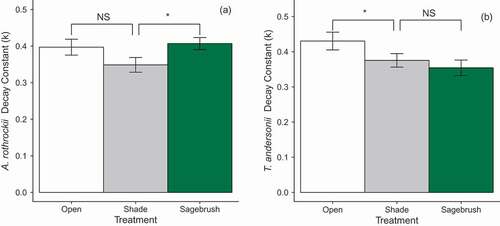
To determine whether shading was a mechanism behind the significantly lower decay constants observed with shrub presence, two planned comparisons were made. One compared the Shade treatment to the unwarmed treatment without shrubs (Control Open) for both litter types at both elevations, while the second made the same comparison between the Shade treatment and the unwarmed treatment with shrubs (Control Shrub). There was no main effect of the Shade treatment in either model, but there was an interaction between the Shade treatment and litter type in both models (; Shade × Species: Shade vs. Shrub, X2 = 13.85, P < 0.001; Shade vs. Open, X2 = 17.81, P < 0.001). In the model comparing Shade and Control Shrub treatments, there was higher A. rothrockii decomposition in the Shade treatment (), while T. andersonii was unaffected (). However, in the comparison between the Shade and the Control Open treatment, there was lower T. andersonii decomposition in the Shade treatment (), while A. rothrockii was unaffected ().
Figure 3. The decay constants (k y−1) for A. rothrockii (a) and T. andersonii (b) in a comparison between Shade (gray bars) and Control Open (white bars) and also Shade (gray bars) and Control Shrub (green bars) as per the two models run. *P < 0.05 (Tukey's HSD). Error bars indicate ± 1 SE of the mean
Nitrate availability
There was a positive relationship between decomposition and nitrate (NO3−) availability (adjusted R2 = 0.21, P = 0.005). Shrub presence decreased NO3− ppm/g (), resulting in 41.2 percent lower NO3− availability in shrub plots compared to open. Additionally, there was 53.8 percent lower NO3− at the low elevation (). There was no effect of warming on NO3− availability; see for average NO3− ppm/g across treatments.
Table 3. Decay constants (k y−1) across treatments grouped by species
Table 4. Mixed-effects model analysis of log-transformed NO3− ppm/g. The factorial design consisted of two temperature treatments (Warm; warmed and control) and two shrub treatments (Shrub; with and without) across two elevations (Elevation; subalpine at 3,100 m and alpine at 3,750 m). Significant effects (P < 0.05) are in bold
Table 5. Untransformed NO3− ppm/g across treatments grouped by season
Discussion
Overall, shrub encroachment decreased decomposition and NO3− availability. Alternatively, warming did not influence NO3− availability, and its effect on decomposition was dependent on elevation and litter type. We found that T. andersonii, a common alpine plant in this region, experienced more variable decomposition responses to warming than A. rothrockii, which did not show warming-driven differences in decomposition.
Litter decomposition
Although warming decreased decomposition rates as a main effect, we also found a higher-order interaction among warming, litter type, and elevation, suggesting that the impact of warming on decomposition rates can vary across species and environmental contexts. Litter from N-fixing species, such as T. andersonii, often decomposes more quickly in comparison to non-fixing species (Vitousek and Walker Citation1989). Hence, as expected, T. andersonii litter decomposed more quickly than A. rothrockii, overall. However, the two litter types responded differently to the Warm treatment; A. rothrockii was not impacted by warming and T. andersonii decomposition was only lowered by warming at the low elevation site. This result highlights the variability of litter decomposition responses to predicted warming, and suggests that future experimentation should employ a wider array of taxa litter to better evaluate the generality of these patterns.
Furthermore, that T. andersonii litter decomposed more slowly only at the low elevation site suggests that a characteristic of the low elevation interacted with warming to slow decomposition. One potential mechanism is the lower precipitation experienced at this elevation compared to the high elevation, especially during the drought throughout this study. This effect may have been compounded by the presence of OTCs, which can decrease soil moisture (Allison and Treseder Citation2008; Zhang et al. Citation2014), as we found at our low elevation site. Low soil moisture strongly limits decomposition (Aerts Citation2006; Bryant et al. Citation1998; Shaw and Harte Citation2001); hence, soil-water evaporation, as a result of increased temperatures, can limit potential temperature-induced increases in decomposition (Allison and Treseder Citation2008; Arft et al. Citation1999; Mooney et al. Citation1999). Although the low precipitation during this study was unusual across both elevations, particularly at the low elevation, droughts may become increasingly common with climate change (Fischer, Beyerle, and Knutti Citation2013; Wetherald Citation2010). Accordingly, we predict a greater sensitivity of decomposition at the low elevation compared to the high elevation associated both with warming and drought-associated climatic change.
A potential artifact of warming experiments using OTCs is snow accumulation (Dorrepaal et al. Citation2003; Aerts, Cornelissen, and Dorrepaal Citation2006; but also see Marion et al. Citation1997), but this effect can vary interannually depending on snowfall (DeMarco, Mack, and Bret-Harte Citation2011). Although we did not explicitly measure snowpack in our experiment, we expect that the OTCs played a negligible role in snow accumulation because of the strong drought and minimal snowpack during the study period. Qualitative observations suggested that warming chambers did not accumulate more snow than the surrounding areas ().
Figure 4. An open-top chamber in the center of the photo (square) and a shrub in the bottom right (circle). Photo taken March 5, 2013, when snow cover is typically high during non-drought years. Notice the lack of snow accumulation with both of these treatments

As with warming, shrub presence reduced decomposition rates as a main effect. We found no difference in soil moisture due to shrub presence, and a past study at the same location demonstrated variable differences in soil moisture under A. rothrockii versus in open areas (Collins et al. Citation2016). Consequently, the influence of shrub presence on soil moisture is not likely to mechanistically explain the decrease in litter decomposition rates under shrubs. Results from our separate shading treatment demonstrated litter-specific responses, suggesting that shading is not the mechanism linking consistently lower decomposition with shrub presence. On the other hand, many species of Artemisia in North America produce secondary chemical compounds such as terpenes and polyphenols (Turi, Shipley, and Murch Citation2014). These compounds can decrease the turnover of organic matter and the rate of mineralization (Kuiters Citation1990). Therefore, it is possible that shrub presence led to lower rates of decomposition and nitrate availability (discussed further on) because of the leaching of phenolics into the soil. Shrubs decreased decomposition regardless of litter type and elevation, suggesting that the impact is less variable than is warming. Because warming is accompanied by shrub encroachment, the interaction between the two is a net decrease in decomposition.
Nitrate availability
While NO3− availability and litter decomposition were positively related, the proportion of explained variance was low. The low explanatory power of decomposition for NO3− availability may stem from the apparent decoupling of NO3− availability and litter decomposition in the warming treatment. Warming did not influence NO3− availability, even with lower decomposition seen in the warming treatment. While C and N mineralization are typically linked (McGill and Cole Citation1981), our results are similar to those found by Allison and Treseder (Citation2008), who saw a decoupling of soil respiration and N availability under experimental warming in the arctic tundra.
There was, however, a negative effect of shrub presence on NO3− availability. One possible explanation is the increased rates of nitrate uptake by plots with shrubs compared to open plots. Our finding is in contrast to studies that have found greater NO3− and net N mineralization under shrubs resulting from snow trapping by shrubs and consequent soil insulation by snow (Sturm et al. Citation2005). Because this study took place during a drought and resultant low snow levels, there was likely little shrub–snow accumulation. Instead, lower NO3− availability may result from greater immobilization; higher microbial biomass N under the same shrub species was observed at this site (Collins et al. Citation2016), likely the result of the presence of phenolics (Bowman et al. 2004; Turi, Shipley, and Murch Citation2014). Our results highlight the variability in strength of the coupling of C and N cycles at this site, making predictions about the consequences of climate on nutrient cycling more challenging. Furthermore, they suggest a greater need for the study of climate-change effects on xeric montane systems.
Conclusion
In the subalpine and alpine of the White Mountain range in California, shrub presence and warming decreased decomposition, although the effect of the latter was variable by litter type and elevation. Continued warming may allow further A. rothrockii expansion into the alpine, which may in turn lower decomposition. While a direct impact of lower decomposition rates is less C released into the atmosphere, there may be indirect impacts on other aspects of C cycling. In plots containing shrubs, there was both lower decomposition and NO3− availability. These alterations to nutrient cycling may impact primary production and species relative abundances at the community level. In sum, this study demonstrates the complex potential for indirect effects of climate change via shifting species composition and important ramifications for ecosystem-level functioning.
Acknowledgments
This work was performed at the University of California Natural Reserve System’s White Mountain Research Station, and we are grateful for the research infrastructure provided by the station managers. We thank Elizabeth Premo and the Fall 2015 undergraduate Ecology Lab (BIEB 121) students at the University of California, San Diego, who assisted in processing samples from this experiment. The comments of several anonymous reviewers greatly improved the manuscript. This material is based on work supported by the National Science Foundation Graduate Research Fellowship under Grant No. (DGE-1144086), a White Mountain Research Center Minigrant, and a Mary DeDecker Botanical grant (CNPS Bristlecone Chapter). Publication of this chapter was funded by the University of Colorado Boulder Libraries Open Access Fund. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adair, E. C., S. E. Hobbie, and R. K. Hobbie. 2010. Single-pool exponential decomposition models: Potential pitfalls in their use in ecological studies. Ecology 91 (4):1–10.
- Aerts, R. 2006. The freezer defrosting: Global warming and litter decomposition rates in cold biomes. Journal of Ecology 94:713–24.
- Aerts, R., J. H. C. Cornelissen, and E. Dorrepaal. 2006. Plant performance in a warmer world: General responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecology 182:65–77.
- Allison, S. D., and K. K. Treseder. 2008. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Global Change Biology 14:2898–909.
- Arft, A. M., M. D. Walker, J. Gurevitch, J. M. Alatalo, M. S. Bret-Harte, M. Dale, M. Diemer, F. Gugerli, G. H. R. Henry, M. H. Jones, et al. 1999. Responses of tundra plants to experimental warming: Meta-analysis of the international tundra experiment. Ecological Monographs 69 (4):491–511.
- Austin, A. T., and L. Vivanco. 2006. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442 (3):555–58.
- Bowman, W. D., H. Steltzer, T. N. Rosenstiel, C. C. Cleveland, and C. L. Meier. 2004. Litter effects of two co-occurring alpine species on plant growth, microbial activity and immobilization of nitrogen. Oikos 104 (2):336–344.
- Bryant, D. M., E. A. Holland, T. R. Seastedt, and M. D. Walker. 1998. Analysis of litter decomposition in an alpine tundra. Canadian Journal of Botany 76:1295–304.
- Bunnell, F. L., D. E. N. Tait, P. W. Flanagan, and K. van Clever. 1977. Microbial respiration and substrate weight loss-I. A general model of the influences of abiotic variables. Soil Biology and Biochemistry 9 (1):33–40.
- Collins, C. G., C. J. Carey, E. L. Aronson, C. W. Kopp, J. M. Diez, and A. Austin. 2016. Direct and indirect effects of native range expansion on soil microbial community structure and function. Journal of Ecology 104:1271–83.
- Cornelissen, J. H. C., P. M. van Bodegom, R. Aerts, T. V. Callaghan, R. S. P. van Logtestijn, J. Alatalo, F. Stuart Chapin, R. Gerdol, J. Gudmundsson, D. Gwynn-Jones, et al. 2007. Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecology Letters 10:619–27.
- Coûteaux, M. M., P. Bottner, and B. Berg. 1995. Litter decomposition, climate and litter quality. Trends in Ecology & Evolution 10 (2):63–66.
- Davidson, E. A. J. E. A., I. A. Janssens, D. Marks, M. M. D. Murdock, R. S. Ahl, S. W. Woods, H. R. Zuuring, A. C. Balisky, P. J. Burton, R. D. Bardgett, et al. 2006. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440 (7081):165–73.
- DeMarco, J., M. C. Mack, and M. S. Bret-Harte. 2011. The effects of snow, soil microenvironment, and soil organic matter quality on N availability in three Alaskan arctic plant communities. Ecosystems 14 (5):804–17.
- DeMarco, J., M. C. Mack, and M. S. Bret-Harte. 2014. Effects of arctic shrub expansion on biophysical vs. biogeochemical drivers of litter decomposition. Ecology 95 (7):1861–75.
- Doak, D. F., and W. F. Morris. 2010. Demographic compensation and tipping points in climate-induced range shifts. Nature 467 (7318):959–62.
- Doane, T. A., and W. R. Horwáth. 2003. Spectrophotometric determination of nitrate with a single reagent. Analytical Letters 36 (12):2713–22.
- Dorrepaal, E., R. Aerts, J. H. C. Cornelissen, T. V. Callaghan, and R. S. P. van Logtestijn. 2003. Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Global Change Biology 10:93–104.
- Essery, R., and J. Pomeroy. 2004. Vegetation and topographic control of wind-blown snow distributions in distributed and aggregated simulations for an arctic tundra basin. Journal of Hydrometeorology 5 (5):735–44.
- Fischer, E. M., U. Beyerle, and R. Knutti. 2013. Robust spatially aggregated projections of climate extremes. Nature Climate Change 3 (12):1033–38.
- Fisk, M. C., S. K. Schmidt, and T. R. Seastedt. 1998. Topographic patterns of above- and belowground production and nitrogen cycling in alpine tundra. Ecology 79 (7):2253–66.
- Fox, J., and S. Weisberg. 2011. An R companion to applied regression. 2nd ed. Thousand Oaks: Sage.
- Gavazov, K. S. 2010. Dynamics of alpine plant litter decomposition in a changing climate. Plant and Soil 337 (1):19–32.
- Giblin, A. E., J. A. Laundre, K. J. Nadelhoffer, and G. R. Shaver. 1994. Measuring nutrient availability in arctic soils using ion exchange resins: a field test. Soil Science Society of America Journal 58 (4):1154–1162.
- Gorham, E. 1991. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecological Applications 1 (2):182–95.
- Gottfried, M., H. Pauli, K. Reiter, and G. Grabherr. 1999. A fine-scaled predictive model for changes in species distribution patterns of high mountain plants induced by climate. Diversity and Distributions 5 (6):241–51.
- Grogan, P., L. Illeris, A. Michelsen, and S. Jonasson. 2001. Respiration of recently-fixed plant carbon dominates mid-winter ecosystem CO2 production in sub-arctic heath tundra. Climatic Change 50:129–42.
- Hall, C. 1991. Natural history of the White-Inyo Range, eastern California. Berkeley, CA: University of California Press.
- Henry, H. A. L., K. Brizgys, and C. B. Field. 2008. Litter decomposition in a California annual grassland: Interactions between photodegradation and litter layer thickness. Ecosystems 11 (4):545–54.
- IPCC. 2007. Working group I contribution to the IPCC fourth assessment report. Climate change 2007: The physical science basis. New York: Cambridge University Press.
- Klanderud, K., and H. J. B. Birks. 2003. Recent increases in species richness and shifts in altitudinal distributions of Norwegian mountain plants. The Holocene 13 (1):1–6.
- Kopp, C. W., and E. E. Cleland. 2014. Shifts in plant species elevational range limits and abundances observed over nearly five decades in a western North America mountain range. Journal of Vegetation Science 25 (1):135–46.
- Kuiters, A. T. 1990. Role of phenolic substances from decomposing forest litter in plant-soil interactions. Acta Botanica Neerlandica 39 (4):329–48.
- Lenth, R. V. 2016. Least-squares means : The R package lsmeans. Journal of Statistical Software 69 (1):1–33.
- Liston, G. E., J. P. Mcfadden, M. Sturm, and R. A. Pielke. 2002. Modelled changes in arctic tundra snow, energy and moisture fluxes due to increased shrubs. Global Change Biology 8 (1):17–32.
- Mack, M. C., E. A. G. Schuur, M. S. Bret-Harte, G. R. Shaver, and F. S. Chapin. 2004. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431 (7007):440–43.
- Margulis, S. A., G. Cortés, M. Girotto, L. S. Huning, D. Li, and M. Durand. 2016. Characterizing the extreme 2015 snowpack deficit in the Sierra Nevada (USA) and the implications for drought recovery. Geophysical Research Letters 43 (12):6341–49.
- Marion, G. M., G. H. R. Henry, D. W. Freckman, J. Johnstone, G. Jones, M. H. Jones, E. Lévesque, U. Molau, P. Mølgaard, A. N. Parsons, et al. 1997. Open-top designs for manipulating field temperature in high-latitude ecosystems. Global Change Biology 3 (S1):20–32.
- McGill, W. B., and C. V. Cole. 1981. Comparative aspects of cycling of organic C, N, S and P through soil organic matter. Geoderma 26:267–86.
- McLaren, J. R., K. M. Buckeridge, M. J. van de Weg, G. R. Shaver, J. P. Schimel, and L. Gough. 2017. Shrub encroachment in arctic tundra: Betula nana effects on above- and belowground litter decomposition. Ecology 98 (5):1361–76.
- Molau, U., and P. Mølgaard. 1996. International tundra experiment (ITEX) manual. Copenhagen, Denmark: Danish Polar Center.
- Mooney, H., G. Andre, and R. Wright. 1962. Alpine and subalpine vegetation patterns in the White Mountains of California. American Midland Naturalist 68 (2):257–273.
- Mooney, H. A., J. Canadell, F. S. Chapin III, J. R. Ehleringer, C. Korner, R. E. Mcmurtrie, W. J. Parton, L. F. Pitelka, and E.-D. Schulze. 1999. Ecosystem physiology responses to global change. In The terrestrial biosphere and global change: Implications for natural and managed ecosystems, eds. B. H. Walker, W. Steffen, J. Canadell, and J. Ingram, 141–89. Cambridge: Cambridge University Press.
- Myers-Smith, I. H., B. C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K. D. Tape, M. MacIas-Fauria, U. Sass-Klaassen, E. Lévesque, et al. 2011. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters 6:045509.
- Myers-Smith, I. H., and D. S. Hik. 2013. Shrub canopies influence soil temperatures but not nutrient dynamics: An experimental test of tundra snow-shrub interactions. Ecology and Evolution 3 (11):3683–700.
- Nogués-Bravo, D., M. B. Araújo, M. P. Errea, and J. P. Martínez-Rica. 2007. Exposure of global mountain systems to climate warming during the 21st century. Global Environmental Change 17 (3–4):420–28.
- O’Lear, H. A., and T. R. Seastedt. 1994. Landscape patterns of litter decomposition in alpine tundra. Oecologia 99 (1):95–101.
- Parida, B. R., and W. Beurmann. 2014. Increasing summer drying in North American ecosystems in response to longer nonfrozen periods. Geophysical Research Letters 41 (8):2851–57.
- Pomeroy, J. W., D. S. Bewley, R. L. H. Essery, N. R. Hedstrom, T. Link, R. J. Granger, J. E. Sicart, C. R. Ellis, and J. R. Janowicz. 2006. Shrub tundra snowmelt. Hydrological Processes 20 (4):923–41.
- PRISM Climate Group. 2018. PRISM climate group. Accesses. http://www.prism.oregonstate.edu/.
- R Development Core Team. 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria.
- Rundel, P. W., A. C. Gibson, and M. R. Sharifi. 2005. Plant functional groups in alpine fellfield habitats of the White Mountains, California. Arctic, Antarctic, and Alpine Research 37 (3):358–65.
- Schimel, J. P., C. Bilbrough, and J. M. Welker. 2004. Increased snow depth affects microbial activity and nitrogen mineralization in two arctic tundra communities. Soil Biology and Biochemistry 36 (2):217–27.
- Schuur, E. A. G., J. G. Vogel, K. G. Crummer, H. Lee, J. O. Sickman, and T. E. Osterkamp. 2009. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature 459 (7246):556–59.
- Seastedt, T. R., and G. A. Adams. 2001. Effects of mobile tree islands on alpine tundra soils. Ecology 82 (1):8–17.
- Seastedt, T. R., M. D. Walker, and D. M. Bryant. 2001. Controls on decomposition processes in the alpine tundra. In Structure and function of an alpine ecosystem: Niwot Ridge, Colorado, eds. W. D. Bowman, and T. R. Seastedt, 222–36. New York: Oxford University Press.
- Shaw, R., and J. Harte. 2001. Control of litter decomposition in a subalpine meadow-sagebrush steppe ecotone under climate change. Ecological Applications 11 (4):1206–23.
- Sturm, M., J. P. Mcfadden, G. E. Liston, F. S. Chapin III, C. Racine, and J. Holmgreen. 2000. Snow-shrub interactions in arctic tundra: A hypothesis with climatic implications. Journal of Climate 14:336–44.
- Sturm, M., J. Schimel, G. Michaelson, J. M. Welker, S. F. Oberbauer, G. E. Liston, and J. Fahnestock. 2005. Winter biological processes could help convert arctic tundra to shrubland. BioScience 55 (1):17–26.
- Swift, M. J., O. W. Heal, and J. M. Anderson. 1979. Decomposition in terrestrial ecosystems. 5th ed. Oxford: Blackwell Scientific.
- Turi, C. E., P. R. Shipley, and S. J. Murch. 2014. North American Artemisia species from the subgenus Tridentatae (Sagebrush): A phytochemical, botanical and pharmacological review. Phytochemistry 98:9–26.
- Väisänen, M., H. Ylänne, E. Kaarlejärvi, S. Sjögersten, J. Olofsson, N. Crout, and S. Stark. 2014. Consequences of warming on tundra carbon balance determined by reindeer grazing history. Nature Climate Change 4 (5):384–88.
- Vitousek, P. M., and L. R. Walker. 1989. Biological invasion by Myrica faya in Hawai’i: Plant demography, nitrogen fixation, ecosystem effects. Ecological Monographs 59 (3):247–65.
- Webb, E. E., E. A. G. Schuur, S. M. Natali, K. L. Oken, R. Bracho, J. P. Krapek, D. Risk, and N. R. Nickerson. 2016. Increased wintertime CO2 loss as a result of sustained tundra warming. Journal of Geophysical Research: Biogeosciences 121:249–65.
- Webber, P. J., J. C. Emerick, D. C. Ebert May, and V. Komarkova. 1976. The impact of increased snowfall on alpine vegetation. In Ecological impacts of snowpack augmentation in the San Juan Mountains, Colorado. Final Report of the San Juan Ecology Project, eds.Steinhoff, H. W. and Ives, J. D. Prepared for the Division of Atmospheric Water Resources Management, Bureau of Reclamation, U.S. Department of Interior, Denver, Colorado, by Colorado State University, Fort Collins, Colorado. Report No. CSU-FNR-7052-1, Contract No. 14-06-D-7052, 201–64.
- Welker, J. M., J. T. Fahnestock, G. H. R. Henry, K. W. O’Dea, and R. A. Chimner. 2004. CO2 exchange in three Canadian High Arctic ecosystems: Response to long-term experimental warming. Global Change Biology 10 (12):1981–95.
- Wetherald, R. T. 2010. Changes of time mean state and variability of hydrology in response to a doubling and quadrupling of CO2. Climatic Change 102 (3):651–70.
- Withington, C. L., and R. L. Sanford. 2007. Decomposition rates of buried substrates increase with altitude in the forest-alpine tundra ecotone. Soil Biology and Biochemistry 39 (1):68–75.
- Wookey, P. A., R. Aerts, R. D. Bardgett, F. Baptist, K. Bråthen, J. H. C. Cornelissen, L. Gough, I. P. Hartley, D. W. Hopkins, S. Lavorel, et al. 2009. Ecosystem feedbacks and cascade processes: Understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Global Change Biology 15 (5):1153–72.
- Zhang, B., S. Chen, X. He, W. Liu, Q. Zhao, L. Zhao, and C. Tian. 2014. Responses of soil microbial communities to experimental warming in alpine grasslands on the Qinghai-Tibet Plateau. PLoS One 9 (8):e103859.