ABSTRACT
Growth responses of trees and shrubs to climate often exhibit unexplained variation in alpine regions, making it difficult to predict how they will respond to future changes in climate. We sought to characterize and explain this variability in southwest Yukon, a topographically complex region of subarctic Canada. We collected cores and sections from 360 spruce trees and 480 willow shrubs across treelines on north and south aspects in six valleys spanning two mountain ranges. We compared growth rates, growth patterns, and climate-growth responses between species and topographic factors. South aspects had wider tree rings and higher tree and shrub interseries correlations than north aspects, likely because of shallow active layers on the latter. Growth patterns and responses to climate did not vary between aspects or elevations but differed slightly between mountain ranges, likely because of differences in spring soil moisture content between ranges. Growth responses of both species to summer temperature were positive, but tree growth was negatively correlated to spring temperature and shrub growth was negatively correlated to summer precipitation, both of which are projected to increase along with summer temperature. Future changes in climate could therefore reduce the growth of one or both species in southwest Yukon.
Introduction
Satellite imagery has revealed that many regions in the circumpolar north have experienced an increase in plant productivity in recent decades, much of which has been attributed to changes in the abundance and growth of woody vegetation (Frost, Epstein, and Walker Citation2014; Goetz et al. Citation2005; Jia, Epstein, and Walker Citation2009). Future change is expected to be particularly pronounced near the treeline, where forest transitions into tundra (Grace Citation2002). Using a combination of dendroecology, repeat photography, and repeat surveys, field studies have confirmed that tall deciduous shrubs are rapidly advancing into what was previously dwarf shrub or tussock tundra, likely in response to rising air temperatures associated with climate change (Forbes, Fauria, and Zetterberg Citation2010; Sturm, Racine, and Tape Citation2001; Tape, Sturm, and Racine Citation2006; Tremblay, Lévesque, and Boudreau Citation2012). Simultaneously, dendrochronological studies on both trees and shrubs have revealed predominantly positive relationships between annual ring widths and rising air temperatures, especially near the elevational or latitudinal range limits of the study species (Salzer et al. Citation2009; Forbes, Fauria, and Zetterberg Citation2010; Blok et al. Citation2011; Buras, Hallinger, and Wilmking Citation2012; Macias-Fauria and Johnson Citation2013; Buchwal et al. Citation2013; Jørgensen et al. Citation2015).
These changes have the potential to alter the composition of tundra plant communities and initiate a positive feedback loop between rising air temperatures and increasing woody plant abundance by reducing tundra surface albedo (Chapin, Sturm, and Serreze Citation2005; Greenwood and Jump Citation2014). However, rates of change in the distribution and productivity of trees and shrubs often exhibit substantial spatial variability, particularly in topographically complex regions (Danby and Hik Citation2007; Leonelli et al. Citation2009; Ropars and Boudreau Citation2012; Young et al. Citation2016). A global meta-analysis revealed that only half of all treelines worldwide have advanced within the past century, despite nearly ubiquitous warming (Harsch et al. Citation2009), and several studies have found evidence that the ring widths of trees growing at treeline are becoming decoupled from growing-season air temperatures, particularly among white spruce populations in northwestern North America (D’Arrigo et al. Citation2008). These results suggest that future increases in woody plant productivity will be contingent on variables other than air temperature, even at the northern or elevational limits of tree and shrub distributions.
Shrubs tend to respond rapidly to ambient and experimental warming and, accordingly, shrub advance has generally occurred over much shorter time periods than treeline advance (Chapin et al. Citation1995; Hobbie and Chapin Citation1998; Myers-Smith et al. Citation2011; Walker et al. Citation2006). Most studies that have investigated drivers of shrub growth have measured primary growth over time periods of less than twenty years (e.g., Bret-Harte, Shaver, and Chapin Citation2002; Kaarlejärvi et al. Citation2012). Measuring secondary growth (annual ring widths) of shrubs over longer time periods can provide a more comprehensive record of growth responses to changing climatic conditions, but the study of shrub rings is an underdeveloped field, having only gained momentum in the past decade or so. This is in large part the restult of the difficulties associated with measuring shrub secondary growth. Rings are often extremely narrow or difficult to identify, and stems frequently contain missing rings and highly irregular growth patterns (Schmidt et al. Citation2010; Zalatan and Gajewski Citation2006). For these reasons, conventional dendrochronological techniques are currently being adapted to the study of shrub rings (Myers-Smith et al. Citation2015). Although there is a growing body of knowledge on shrub growth responses to climate across the circumpolar north (recently summarized by Myers-Smith et al. Citation2015), the viability of many shrub species for ring-width analyses remains uncertain.
Comparisons between the ring-width patterns and climate-growth responses of trees and shrubs from the same location are uncommon (although, see García-Cervigón Morales et al. Citation2012; Meinardus, Weinert, and Löffler Citation2011). Several studies have compared the growth patterns and responses of two or more species of deciduous shrub growing within the same site (Blok et al. Citation2011; Buras, Hallinger, and Wilmking Citation2012; Jørgensen et al. Citation2015), and experimental studies have identified important competitive interactions between tree cover and shrub growth (Boudreau and Villeneuve-Simard Citation2012) or between tree seedling growth and shrub cover near treeline (Grau et al. Citation2012). Longer-term direct comparisons between mature tree and shrub ring-width patterns will improve our understanding of past differences in their respective responses to climate, and will help identify the extent to which their responses to future climate are likely to differ.
Given the high deciduous shrub cover across the forest-tundra ecotones in southwest Yukon, Canada, particularly on south-facing slopes, we broadly aimed to compare tree and shrub growth at treeline. We also sought to describe and explain variation in the growth of both species across different topographic features so that predictions of northern ecosystem productivity change can be improved for alpine regions. Our questions were: (1) How do growth patterns and growth responses to climate compare between the dominant tree and shrub species occupying the forest-tundra ecotone in southwest Yukon?; and (2) How do the growth rates, patterns, and responses to climate for the two species vary among elevations, aspects, and mountain ranges?
Methods
Site description
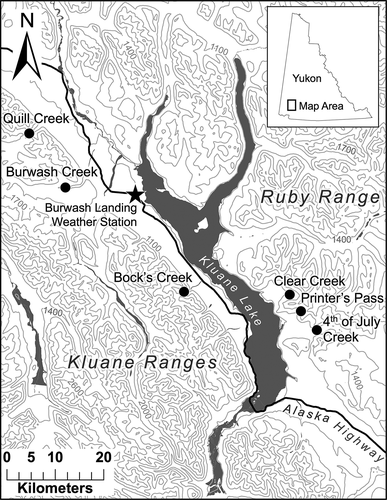
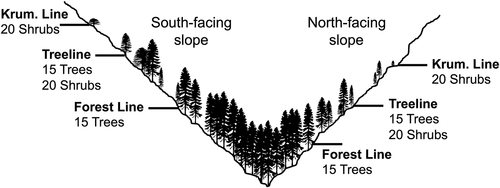
The Kluane Region of southwest Yukon contains several different mountain ranges in a small geographic area. The Ruby and Kluane Ranges to the east and west of Kluane Lake, respectively, have different geologic origins and, as such, their topography differs markedly (Smith, Meikle, and Roots Citation2004). The Ruby Range is characterized by rolling topography with slope angles often below 20° at treeline, while slopes in the Kluane Ranges are rugged and steep in comparison, with angles often greater than 30° (). The dominant tree species comprising the forest-tundra ecotone in this region is Picea glauca ([Moench] Voss), although Populus balsamifera (L.) and Populus tremuloides (Michx.) can occur locally in patches alongside stands of P. glauca on south-facing slopes. The dominant tall shrub species occupying the forest-tundra ecotone is Salix glauca (L.), which occurs in particularly high abundance on south-facing slopes, often forming dense thickets and frequently growing well over a meter in height. Other common tall shrub species occupying the ecotone include Alnus crispa (Drylander ex Ait.), Salix pulchra (Cham.), Salix richardsonii (Hook.), and Betula glandulosa (Michx.). Treeline, the upper limit of 2 m trees, generally occurs at 1,300 m a.s.l.; forest line, the limit of 30 percent canopy cover, occurs approximately 100 m below treeline; and krummholz line, the upper limit of tree-forming species regardless of growth form, occurs up to 200 m above treeline (Dearborn and Danby Citation2017).
Sampling
We obtained cores from fifteen P. glauca trees at treeline and another fifteen at forest line on a north- and a south-facing slope in six alpine valleys: three in the Ruby Range (Printer’s Pass [PP], Clear Creek [CC], and Fourth of July Creek [FJ]) and three in the Kluane Ranges (Bock’s Creek [BC], Burwash Creek [BW], and Quill Creek [QC]; ). In the same six valleys, we sampled twenty S. glauca shrubs at krummholz line and another twenty at treeline on each aspect (). We staggered our sampling elevations such that we could compare the growth of each species at or near their range limits (treeline for trees, krummholz line for shrubs) with the growth of individuals at lower elevations within the ecotone (forest line for trees, treeline for shrubs). We selected ostensibly old, representative trees and shrubs to extend each chronology as far back in time as possible and to ensure that the growth patterns of sampled individuals were not being influenced by local disturbances or abnormal site conditions (e.g., we avoided trees growing next to large stumps or shrubs growing in creek channels). Two cores were obtained from each tree at breast height (1.3 m), although cores at treeline were often obtained closer to the ground to maximize series length (as low as 60 cm; Oberhuber Citation2004). We avoided sampling at ground level because of the frequent presence of growth abnormalities and excessive branching. Cores were taken perpendicular to the slope to minimize compression wood (Leonelli et al. Citation2009; Peterson and Peterson Citation1994). A single 10 cm long stem section was obtained from the dominant upright stem of each shrub within 10–30 cm of the base of the stem. Care was taken to ensure that each shrub we sampled was genetically distinct (i.e., not a clonal stem from a previously sampled shrub) by ensuring a distance of 50 m between shrubs and visually assessing the likelihood that any two sampled shrubs were connected below ground.
Laboratory work
We mounted, sanded, and visually cross-dated P. glauca tree cores using standard dendrochronological techniques (Speer Citation2010). We cut thin sections of S. glauca shrub stems using a Leica sliding microtome (Model SM2010R) after soaking them in water for 24 h, and then wet-mounted them onto glass microscope slides to facilitate the identification of ring boundaries using transmitted light (Myers-Smith et al. Citation2014). Ring widths of both species were measured to the nearest 0.001 mm using a Velmex sliding measurement stage under an Olympus stereo microscope (Model SZ61, up to 45× magnification). On each shrub section we measured two (occasionally three) paths at 90° angles from one another to ensure sufficient representation of ring-width patterns.
We statistically verified cross-dating of both species using the program COFECHA (Grissino Citation2001) with default settings (thirty-two-year spline and fifty-year segments with twenty-five-year overlap). In the interest of reducing noise in the chronologies because of anomalous series, we removed all tree and shrub series with low correlations to their respective site chronology (<0.4; similar to Chavardès et al. Citation2013; Leonelli et al. Citation2009). Because shrub series tended to have lower correlations to site chronologies than tree series, we permitted shrub series with correlations as low as 0.3 to be retained in the chronologies so long as we were convinced that they were dated correctly. Overall, this resulted in fifteen out of a total of 678 measured tree series removed, and 328 out of a total of 914 measured shrub series removed. We also removed the first two years of growth from each tree and shrub series because of their frequently anomalous values.
Chronology development
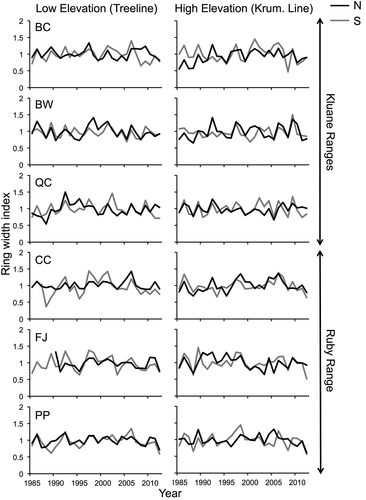
We used the interactive detrending option available in ARSTAN (Cook and Holmes Citation1986) to visually determine the best method of detrending and standardization for each individual ring-width series. We used negative exponential curves, negative linear curves, or straight lines through the mean, while being careful not to use a curve that exaggerated growth near the end of a series (Cook and Peters Citation1997). We combined detrended series into chronologies using the robust (biweight) mean option in ARSTAN, then constructed a chronology at each elevation (high and low) on each aspect (north and south) at each of our six sites, and truncated the chronologies such that there were at least three individual trees or two individual shrubs making up each chronology (as in Jørgensen et al. Citation2015; Leonelli et al. Citation2009). For each species, this resulted in a total of three replicate chronologies for every combination of topographic factors (elevation, aspect, and mountain range). Because shrub series were often too short to adequately assess low-frequency growth patterns (patterns that occur during decadal time scales), we used only “residual” chronologies in our analyses, which are made up of series that have had all temporal autocorrelation removed and reflect only high frequency (year-to-year) ring-width patterns (Speer Citation2010).
Data analysis
We extracted several descriptive statistics for each tree and shrub chronology in ARSTAN to describe the growth characteristics of trees and shrubs in different topographic positions. These included the mean raw ring-width of series in each chronology, the mean interseries correlation of each chronology (mean correlation of residual series to their site chronology) as a measure of synchrony between series in a chronology, and the subsample signal strength (SSS) of each chronology, which is a measure of a chronology’s suitability for climate-growth analysis and is based on the chronology’s sample depth and mean interseries correlation (Buras 2017; Wigley, Briffa, and Jones Citation1984). We also extracted the mean standard deviation of residual series in each chronology as a measure of overall responsiveness to climate (Bunn et al. Citation2013).
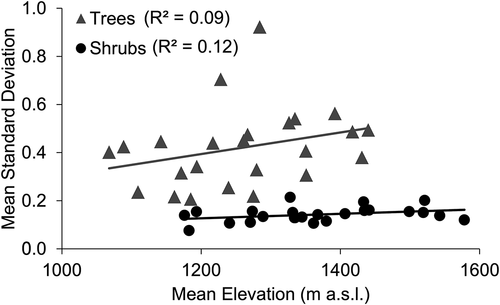
Statistics were calculated using the full length of each residual tree or shrub series because we wished to characterize growth traits over as much of each tree’s or shrub’s lifetime as possible. We tested for significant differences in raw ring widths and correlations to master chronologies between individual trees and shrubs on different aspects and at different elevations (fixed factors) using two-way analysis of variance (ANOVA) in SPSS v.21 with site as a random factor to account for any within-site similarities in growth characteristics. Because series from the same individual tree or shrub are not independent of one another, we averaged series from the same individual together prior to running these tests. Finally, we used linear regressions to describe the relationship between mean standard deviation and mean elevation of each chronology, because the growth of individuals at higher elevations should theoretically be more sensitive to climate (particularly temperature) than the growth of individuals at lower elevations (Leonelli et al. Citation2009; Oberhuber Citation2004; Villalba, Boninsegna, and Veblen Citation1997).
To compare ring-width patterns between topographic positions, we calculated Pearson’s correlation coefficients between all possible pairs of residual chronologies (separately for each species) for a common time period shared by all chronologies (1985–2012). To compare ring-width patterns between species, we calculated correlation coefficients between tree and shrub chronologies obtained from the same sampling location (at treeline on the same aspect at the same site, see ). To examine the relationship between ring widths and climate during this time period, we calculated correlation coefficients between each chronology and seasonal temperature and precipitation values obtained from the Burwash Landing Environment Canada weather station (61°22ʹ N, 139°03ʹ W, 806.2 m a.s.l.), which is within 45 km of all study sites (). We used 95 percent confidence intervals generated with 1,000 bootstrapped simulations to test for significant climate-growth relationships. Seasonal winter temperature was defined as the average of December, January, and February; spring as the average of March, April, and May; summer as the average of June, July, and August; and fall as the average of September, October, and November. We used the same seasonal definitions for precipitation, but with summed monthly values rather than averaged values.
Results
Viability of S. glauca for climate-growth analysis
We briefly investigated the viability of S. glauca for dendroclimatological analysis in southwest Yukon, because only two previous studies have examined climate-growth relationships in S. glauca populations, both of which were conducted in western Greenland (Jørgensen et al. Citation2015; Young et al. Citation2016). We encountered several difficulties in analyzing S. glauca shrub ring widths. There were occasionally sections of stems containing multiple extremely narrow or missing rings that made cross-dating impossible, forcing us to discard an average of five out of the twenty stems we sampled at a given location (). We also encountered several series from each sampling location that appeared to be dated correctly but had very low correlations to their respective site chronologies (r < 0.3). These series were removed from the final chronologies to maximize a common signal (328 out of a total of 914 series were removed). Finally, shrub series were sometimes short in length (less than twenty years), particularly on north-facing slopes at treeline, making cross-dating difficult and limiting the time period over which we could assess growth characteristics and climate-growth relationships.
Figure 3. Photos of shrub stem microsections showing a sample that did not cross-date and had to be discarded, with an arrow pointing to a particularly difficult series of rings (right), and a sample that did cross-date and was viable for dendroclimatological analysis (left)
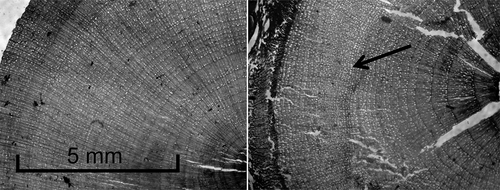
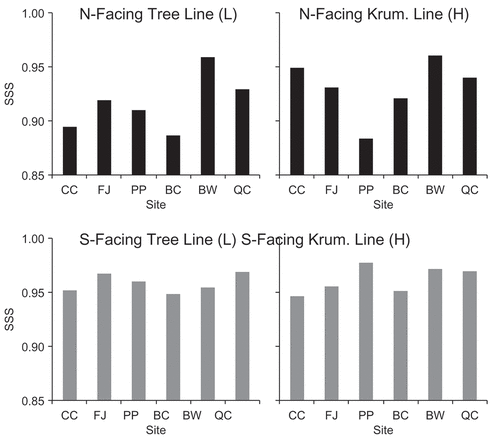
Despite these challenges, we found that S. glauca shrubs on south-facing slopes were viable for dendroclimatological analysis, because SSS values were consistently 0.95 or higher (Appendix 1). South-facing krummholz-line chronologies had particularly high SSS values that were comparable to those of tree chronologies (tree SSS values not shown). In contrast, north-facing S. glauca chronologies frequently had SSS values below 0.95 (sometimes below 0.90), particularly at treeline, and were generally less suitable for climate-growth analysis. However, we still investigated 1985–2012 climate-growth relationships on both aspects with the caveat that our results would not be quite as reliable for shrub chronologies on north aspects as on south aspects.
Effects of elevation on radial growth of P. glauca and S. glauca
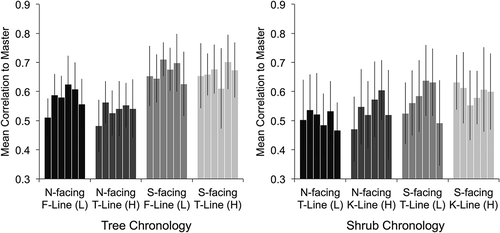
Tree rings were significantly wider at lower elevations (forest line compared to treeline, F = 12.155, p = 0.018), while shrub rings were actually wider at higher elevations, although not significantly (krummholz line compared to treeline, F = 5.050, p = 0.074; ). Overall, tree and shrub interseries correlations did not vary with elevation (F = 2.671, p = 0.163 for trees; F = 2.464, p = 0.176 for shrubs; ). However, tree interseries correlations did decrease with elevation on north-facing slopes (there was a significant interaction between aspect and elevation, F = 17.374, p = 0.009). Linear regression revealed no significant relationship between mean standard deviations and elevations of tree or shrub chronologies (p = 0.164 and 0.100, respectively; ).
Figure 4. Mean raw ring widths of tree (left) and shrub (right) series at each of the six sites with shades of grey representing different topographic positions. The order of sites from left to right within each topographic group is Bock’s Creek (BC), Burwash Creek (BW), Quill Creek (QC), Clear Creek (CC), Fourth of July Creek (FJ), and Printer’s Pass (PP). Note the different y axis scales for trees and shrubs. Error bars represent standard deviations
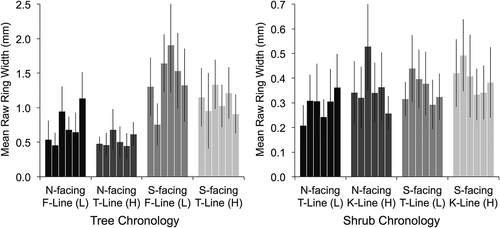
Figure 5. Mean correlation of standardized tree (left) and shrub (right) series to their respective site chronologies at each of the six sites with shades of grey representing different topographic positions. The order of sites from left to right within each topographic group is BC, BW, QC, CC, FJ, PP (see for site abbreviations). Error bars represent standard deviations
Effects of aspect on radial growth of P. glauca and S. glauca
Tree rings were significantly wider on south-facing compared to north-facing slopes (F = 31.818, p = 0.002), while shrub ring widths were not significantly different between aspects (F = 2.957, p = 0.146). Interseries correlations of both trees and shrubs were significantly higher on south-facing compared to north-facing slopes (F = 61.494, p = 0.001 for trees; F = 64.610, p < 0.001 for shrubs; ). However, residual ring-width patterns were very similar on north compared to south aspects. Tree chronologies on opposing aspects had mean correlations of 0.66 ± 0.11 to one another in the Kluane Ranges and 0.67 ± 0.09 in the Ruby Range, while shrub chronologies had mean correlations between aspects of 0.42 ± 0.16 in the Kluane Ranges and 0.46 ± 0.19 in the Ruby Range (note that while these are lower than the correlations between tree chronologies, they are relatively high for shrub chronologies in our study area).
Effects of mountain range on the radial growth of P. glauca and S. glauca
There were no notable differences in raw ring widths or interseries correlations between the Ruby and Kluane Ranges, but we did find substantial differences in the growth patterns of trees and shrubs between ranges. On average, correlations between tree chronologies were strongly positive between pairs of chronologies within a mountain range (mean r = 0.70 ± 0.12 in the Kluane Ranges and 0.71 ± 0.10 in the Ruby Range), but weak between chronologies in different mountain ranges (mean r = 0.53 ± 0.15). Similarly, correlations were weak between shrub chronologies in different mountain ranges (mean r = 0.34 ± 0.2) compared to those in the same range (mean r = 0.45 ± 0.17 in the Kluane Ranges and 0.46 ± 0.17 in the Ruby Range).
Comparison of P. glauca and S. glauca growth patterns
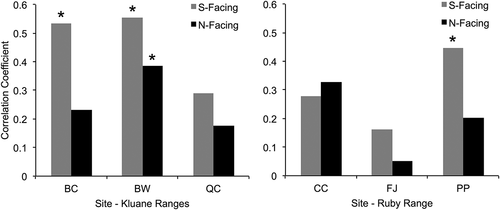
Shrub chronologies were positively correlated to tree chronologies obtained from the same sampling location (i.e., at treeline, see ), although the strengths of these correlations varied between aspects and mountain ranges (). In general, tree and shrub chronologies had stronger correlations to one another on south-facing than north-facing slopes. Correlations between shrub and tree chronologies were also weaker overall at sites in the Ruby Range than in the Kluane Ranges. Additionally, shrub chronologies had considerably lower mean standard deviations than tree chronologies (). See Appendix 2 (Figures 1 and 2) and Appendix 3 (Tables 1 and 2) for tree and shrub residual chronologies and associated chronology descriptive statistics, respectively.
Figure 7. Correlations between tree and shrub chronologies at treeline on each aspect at each site for the common period 1985–2012. The three Kluane Range sites are shown in the left panel, while the three Ruby Range sites are in the right panel (see for site abbreviations). Correlations between south-facing chronologies are in grey, while correlations between north-facing chronologies are in black and asterisks indicate significant correlations at the p < 0.05 level (according to bootstrapped confidence intervals)
Climatic drivers of P. glauca and S. glauca growth
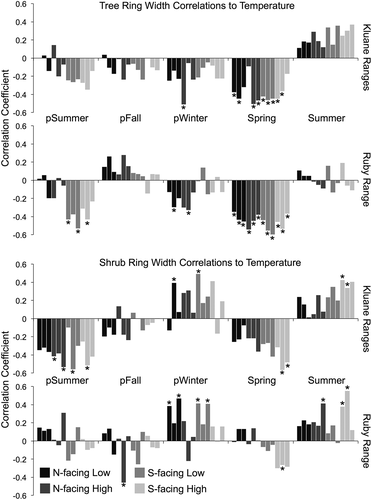
Growth responses of trees and shrubs to climate between 1985 and 2012 were similar overall, although there were some notable differences between species and mountain ranges. Trees generally responded negatively to previous summer and spring temperatures and positively to current summer temperatures. Shrubs responded similarly to temperature in these seasons, although many shrub chronologies in the Ruby Range had weaker negative correlations to previous summer and spring temperatures than those in the Kluane Ranges (). Temperature-growth responses differed most between species in winter, when trees responded weakly and negatively to temperature, while shrubs generally responded positively.
Figure 8. Correlations between residual tree and shrub chronologies and seasonal temperature from 1985 to 2012, shown separately for each mountain range (Kluane Ranges on top, Ruby Range on bottom). Asterisks indicate significant correlations at the p < 0.05 level (according to bootstrapped confidence intervals), and shades of grey represent different topographic positions
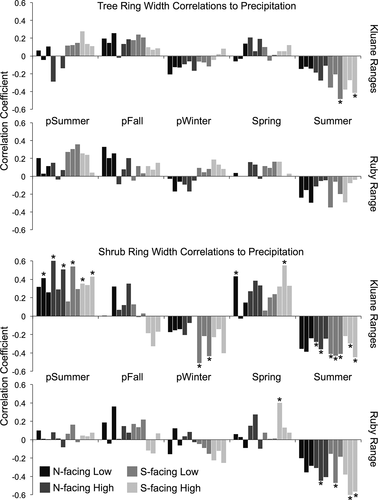
In general, tree ring widths were positively correlated to previous summer precipitation and negatively correlated to current summer precipitation, although these correlations were weak and usually insignificant. Shrubs exhibited similar precipitation-growth responses, although Ruby Range shrubs did not respond to previous summer precipitation at all, while Kluane Range shrubs responded strongly and positively. Negative shrub growth responses to current summer precipitation in both ranges were also notably stronger than tree growth responses to current summer precipitation ().
Figure 9. Tree and shrub ring-width correlations to seasonal precipitation from 1985 to 2012 shown separately for each mountain range (Kluane Ranges on top, Ruby Ranges on bottom). Asterisks indicate significant correlations at the p < 0.05 level (based on bootstrapped confidence intervals), and shades of grey represent different topographic positions
Discussion
In general, the growth rates and patterns of woody species occupying the forest-tundra ecotone were influenced by topographic factors, but their degree of influence varied depending on the growth characteristic and species under consideration. Elevation influenced the raw ring widths of trees but not the raw ring widths of shrubs nor the interseries correlations of either species. Slope aspect influenced raw tree ring widths as well as tree and shrub interseries correlations. In contrast, residual ring-width patterns of trees and shrubs were similar between elevations and aspects but differed notably between mountain ranges. In combining these findings with previous measurements of late growing-season active-layer depth, soil temperature, and soil moisture at each of the six sites between 2013 and 2015 (see Dearborn and Danby Citation2017), we have several hypotheses as to the drivers of topographic and interspecies variability in growth.
Effects of elevation on radial growth of P. glauca and S. glauca
Raw ring widths of white spruce trees were 24 percent wider at forest line than treeline. This is undoubtedly the result of the reduction in average growing-season temperature of more than half a degree between forest line and treeline elevations at our sites (air-temperature data obtained from HOBO loggers installed at Printer’s Pass between 2013 and 2015). In contrast, S. glauca shrub rings were 14 percent wider at high elevations (krummholz line compared to treeline), although the difference was not significant. This was not an artifact of decreasing shrub age or density with elevation, because shrub series lengths were similar at krummholz line and treeline and shrub cover did not decline with elevation on north-facing slopes where the increase in mean raw ring width with elevation was most pronounced. Instead, these results suggest that shrub growth rates are not limited by air temperature to the extent that tree growth rates are, perhaps because shrubs are closer to the ground and their growth is less coupled to prevailing atmospheric conditions (Körner Citation2012) and is influenced more by soil temperature and microtopography. This is supported by the fact that soil temperatures do not decline (in fact, they tend to increase) between treeline and krummholz line in this region (Dearborn and Danby Citation2017).
The lack of any relationship between standard deviation and elevation was unexpected given the strong increase in tree-ring sensitivity (another widely used metric of responsiveness to climate) with elevation over similar elevational ranges across treelines in Europe and South America (Leonelli et al. Citation2009; Oberhuber Citation2004; Villalba, Boninsegna, and Veblen Citation1997). In the interest of a direct comparison with these studies we calculated the mean sensitivities of all of our chronologies, but we found even weaker relationships between sensitivity and elevation than we did between standard deviation and elevation (sensitivity results not shown). While we do not have a definitive explanation for these results, we suspect that the range of conditions over which we sampled, combined with the range of tree and shrub ages at each sampling location, confounded any overall relationship between elevation and responsiveness to climate as measured by these metrics.
Effects of aspect on radial growth of P. glauca and S. glauca
Tree rings were 49 percent wider on south-facing than north-facing slopes, while shrub ring widths did not vary significantly with aspect. Aspect-related differences in raw tree ring widths are likely related to the significantly shallower active layers (often less than 50 cm) and colder soil temperatures on north-facing slopes (measured at each of the six sites between 2013 and 2015 by Dearborn and Danby Citation2017). It is unclear why cold soil temperatures do not restrict shrub growth to the same extent, although perhaps shrub rooting depths are shallower and therefore less influenced by active-layer depth. Alternatively, it is possible that lower photosynthetically active radiation on north aspects restricts tree growth; although, again, it is unclear why this does not affect shrub growth.
Significantly higher interseries correlations of both species on south- compared to north-facing slopes suggest that growth patterns on south aspects are governed by broad-scale climatic patterns that influence the growth of all individuals on the slope. In contrast, low interseries correlations of trees and shrubs on north-facing slopes suggest that growth patterns are influenced more by microsite conditions. Previously collected field data from these sites suggest that there is considerably higher variability in soil moisture content from one location to the next on north aspects, likely the result of the hummocky microtopography associated with shallow active layers (Dearborn and Danby Citation2017). Wolken et al. (Citation2016) found similarly low synchrony in black spruce growth patterns near valley bottoms in Alaska, where frost heaving of shallow active layers created high fine-scale variability in soil moisture content compared to valley sides.
Effects of mountain range on radial growth of P. glauca and S. glauca
Despite differences in raw ring widths and interseries correlations between north- and south-facing slopes, the growth patterns of residual tree and shrub chronologies were remarkably similar between aspects. However, growth patterns of both species differed notably between mountain ranges. These results were unexpected given the substantial aspect-related differences in tree and shrub growth patterns that have been observed in other topographically complex regions (Leonelli et al. Citation2009; Young et al. Citation2016) and the close proximity of the two mountain ranges (20–70 km between sites in different ranges). Range-related differences in growth patterns of spruce at these sites have become particularly pronounced in the past several decades (see Dearborn Citation2017) and are likely related to variation in early growing-season soil moisture content between the two ranges. It is likely that shrub growth patterns are similarly influenced by variation in spring soil moisture content. The stark differences in Kluane and Ruby Range shrub growth responses to previous summer precipitation may lend some support to this hypothesis. Shrubs in the steeper Kluane Ranges exhibited positive responses to previous summer precipitation, while shrubs in the Ruby Range showed no response, possibly because shallower slopes retain soil moisture from the previous growing season more effectively, and thus shrub growth in the Ruby Range does not suffer from a lack of spring soil moisture when the preceding summer is dry.
Comparison of P. glauca and S. glauca growth patterns
Interseries correlations of shrub chronologies were often lower than those of tree chronologies. There is a possibility that herbivory or disease are more prevalent among shrub populations than tree populations and disrupt any common signal between individual shrub ring-width patterns, but we found no evidence of this at any of our sites. Alternatively, our sampling strategy might have contributed to low shrub interseries correlations. Ropars et al. (Citation2017) found that series obtained from the stems of B. glandulosa exhibited far weaker responses to climatic variables than those obtained from root collars in northwestern Quebec. They postulated that the rings in shrub stems primarily reflect the influences of microtopography and competition with other shrubs, or other stems from the same shrub, whereas rings in root collars incorporate all of the factors influencing growth, including climatic factors. We found that the ring widths in stems of S. glauca were highly correlated to certain climate variables, but perhaps these correlations, as well as the interseries correlations of shrub chronologies, would have improved had we sampled shrubs at root collar.
We found predominantly positive correlations between spruce and willow growth patterns at our sites, indicating that trees and shrubs are responding similarly to climate near treeline in southwest Yukon. Although there are few comparable studies, similar positive correlations between tree and shrub chronologies were observed at treelines in southern Norway (Meinardus, Weinert, and Löffler Citation2011). Competition between mature trees and shrubs growing immediately adjacent to each other would theoretically result in negative correlations between their ring-width patterns, but because we only sampled open-growing individuals we were unable to assess the degree to which this occurs. Previous work has shown that shrubs readily outcompete spruce seedlings in this region when shrub cover is high (Kambo Citation2017), and we suspect that a shift toward more favorable climatic conditions will eventually lead to stronger competitive interactions between adult trees and shrubs, particularly if the densities of either increase. These competitive interactions would likely be most prominent on south-facing slopes, where S. glauca cover is already high (Dearborn and Danby Citation2017) and growth rates (raw ring widths) of both species are high. Additionally, tall deciduous shrubs tend to respond rapidly to experimental warming (Chapin et al. Citation1995; Wahren et al. 2005; Walker et al. Citation2006), can readily reproduce clonally (Bret-Harte, Shaver, and Chapin Citation2002), and tend to possess the competitive advantage over gymnosperms at the seedling stage in favorable environments (Becker Citation2000; Bond Citation1989). It is therefore possible that S. glauca populations will outcompete adult P. glauca individuals in addition to P. glauca seedlings on south-facing slopes if growing conditions improve.
Climatic drivers of P. glauca and S. glauca growth
We found positive correlations between summer temperature and ring widths of both species. Similar results have been found at many northern locations worldwide (Blok et al. Citation2011; Buras, Hallinger, and Wilmking Citation2012; Forbes, Fauria, and Zetterberg Citation2010; Macias-Fauria and Johnson Citation2013), but in the Kluane Region positive relationships to summer temperature were weak compared to negative relationships with spring temperature. Jørgensen et al. (Citation2015) and Young et al. (Citation2016) found similarly strong negative responses to spring temperatures in populations of A. crispa and S. glauca in west Greenland, and proposed that the negative influence of warming springs on snow depth was resulting in a spring meltwater shortage. These findings support our earlier supposition that spring soil moisture is an important limiting factor for growth, and is likely responsible for the range-related differences we observed in the growth patterns of both species.
Negative responses to summer precipitation among both P. glauca and S. glauca chronologies suggest that neither species is suffering from temperature-induced drought stress in the summer. This result was unexpected, as evidence for temperature-induced drought stress is widespread among P. glauca populations in northwestern North America (D’Arrigo et al. Citation2008). Evidence for summer drought stress is less commonly observed in northern shrub populations. Young et al. (Citation2016) found that positive correlations between S. glauca ring widths and summer temperatures were actually stronger at their dry sites than their more mesic sites, and a meta-analysis of shrub climate-growth responses across the circumpolar north revealed mainly negative relationships between shrub growth and summer precipitation (Myers-Smith et al. Citation2015). The fact that many northern shrub populations, including those sampled in this study, are not suffering from temperature-induced drought stress may be because the majority of growth occurs early in the season and is therefore more dependent on spring than summer soil moisture content (Young et al. Citation2016).
Future responses of P. glauca and S. glauca to climate change
Although tree and shrub growth responses to climate were broadly similar, there were notable differences that could have implications for the future growth of these species. Negative growth responses to spring temperature were considerably stronger among trees than shrubs, and tree growth responses to winter temperature were generally negative while shrub responses were positive. Winter and spring maximum temperatures in southwest Yukon are projected to increase by 2.4–4.1°C and 1.7–2.7°C, respectively, by 2100 (Price, McKenney, and Joyce Citation2011). These changes are more likely to slow the growth of trees than shrubs, because negative shrub-growth responses to spring temperature were weak, and responses to winter temperature were weakly positive. However, summer precipitation is also projected to increase by 12–26 mm (4–9%) during the same time period (Price, McKenney, and Joyce Citation2011). Because negative correlations between shrub rings and current summer precipitation were considerably stronger than they were for trees, it remains unclear whether the growth of one species will be more heavily impacted by future changes in climate than the other.
In summary, future directions and rates of change in woody plant growth will vary depending on topographic context and on the species under consideration. The growth of trees and shrubs will likely continue to be more rapid and synchronous on south- compared to north-facing slopes because of the influence of cold soil temperatures and shallow active layers on the latter. However, future feedbacks between shrub abundance and permafrost thaw could complicate these trends (Blok et al. Citation2010; Sturm et al. Citation2005). Our results further suggest that trees and shrubs growing on shallow slopes may have an advantage over those on steep slopes as climate warms because of the higher potential for early season drought stress to negatively affect individuals on the latter. Finally, despite weakly positive relationships between summer temperature and ring widths of both species, climate warming may not result in a net increase in growth. Warm springs and wet summers throughout the past several decades have generally slowed tree and shrub growth, and both variables are projected to increase along with summer temperatures throughout the next century in southwest Yukon (Price, McKenney, and Joyce Citation2011). Based on our results, there is a possibility that future changes in climate could actually reduce the growth of one or both species in this region.
Acknowledgments
The authors thank Allison Slater, Laura Kitchen, Courtenay Jacklin, Lucas Brehaut, and Daz Kambo for help with fieldwork; Sian Williams and Lance Goodwin for logistical support; and the Kluane First Nation for kind permission to conduct research on their traditional territory. Funding for this research was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), the W. Garfield Weston Foundation, Queen’s University, and the Northern Scientific Training Program (NSTP).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Becker, P. 2000. Competition in the regeneration niche between conifers and angiosperms: Bond’s slow seedling hypothesis. Functional Ecology 14:1–18.
- Blok, D., M. M. P. D. Heijmans, G. Schaepman-Strub, A. V. Kononov, T. C. Maximov, and F. Berendse. 2010. Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Global Change Biology 16:1296–305.
- Blok, D., U. Sass-Klaassen, G. Schaepman-Strub, D. Heijmans, M. M. P. P. Sauren, and F. Berendse. 2011. What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences 8:1169–79.
- Bond, W. J. 1989. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Biological Journal of the Linnean Society 36:227–49.
- Boudreau, S., and M.-P. Villeneuve-Simard. 2012. Dendrochronological evidence of shrub growth suppression by trees in a subarctic lichen woodland. Botany 90:151–56.
- Bret-Harte, M. S., G. R. Shaver, and F. S. Chapin. 2002. Primary and secondary stem growth in arctic shrubs: Implications for community response to environmental change. Journal of Ecology 90:251–67.
- Buchwal, A., G. Rachlewicz, P. Fonti, P. Cherubini, and H. Gärtner. 2013. Temperature modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard). Polar Biology 36:1305–18.
- Bunn, A. G., E. Jansma, M. Korpela, R. D. Westfall, and J. Baldwin. 2013. Using simulations and data to evaluate mean sensitivity (ζ) as a useful statistic in dendrochronology. Dendrochronologia 31:250–54.
- Buras, A. 2012. A comment on the expressed population signal. Dendrochronologia 44:130–130. doi:https://doi.org/10.1088/1748-9326/7/4/044031.
- Buras, A., M. Hallinger, and M. Wilmking. 2012. Can shrubs help to reconstruct historical glacier retreats? Environmental Research Letters 7:1–8. doi:https://doi.org/10.1088/1748-9326/7/4/044031.
- Chapin, F. S., G. Shaver, A. Giblin, K. J. Nadelhoffer, and J. A. Laundre. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711.
- Chapin, F. S., M. Sturm, and M. Serreze. 2005. Role of land-surface changes in Arctic summer warming. Science 310:657–60.
- Chavardès, R. D., L. D. Daniels, P. O. Waeber, J. L. Innes, and C. R. Nitschke. 2013. Unstable climate−growth relations for white spruce in southwest Yukon, Canada. Climatic Change 116:593–611.
- Cook, E. R., and R. L. Holmes. 1986. Users manual for program ARSTAN. In Tree-ring chronologies of Western North America, California, Eastern Oregon and Northern Great Basin with procedures used in the chronology development work including user manuals for computer programs COFECHA and ARSTAN, R. L. Holmes, R. K. Adams, and H. C. Fritts, eds., 50–65. Tucson: Laboratory of Tree-Ring Research, University of Arizona.
- Cook, E. R., and K. Peters. 1997. Calculating unbiased tree-ring indices for the study of climatic and environmental change. The Holocene 7:361–70.
- D’Arrigo, R. D., R. Wilson, B. Liepert, and P. Cherubini. 2008. On the ‘divergence problem’ in northern forests: A review of the tree-ring evidence and possible causes. Global and Planetary Change 60:289–305.
- Danby, R. K., and D. S. Hik. 2007. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology 95:352–63.
- Dearborn, K. D., 2017: Landscape-scale variability in the composition, growth and pattern of alpine treeline vegetation. Ph.D. thesis, Department of Geography and Planning, Queen’s University, Kingston, 138 pp.
- Dearborn, K. D., and R. K. Danby. 2017. Aspect and slope influence plant community composition more than elevation across forest-tundra ecotones in subarctic Canada. Journal of Vegetation Science 28:595–604.
- Forbes, B. C., M. M. Fauria, and P. Zetterberg. 2010. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Global Change Biology 16:1542–54.
- Frost, G. V., H. E. Epstein, and D. A. Walker. 2014. Regional and landscape-scale variability of Landsat-observed vegetation dynamics in northwest Siberian tundra. Environmental Research Letters 9:1–11. doi:https://doi.org/10.1088/1748-9326/9/2/025004.
- García-Cervigón Morales, A. I., J. M. Olano Mendoza, M. Eugenio Gozalbo, and J. J. Camarero Martínez. 2012. Arboreal and prostrate conifers coexisting in Mediterranean high mountains differ in their climatic responses. Dendrochronologia 30:279–86.
- Goetz, S. J., A. G. Bunn, G. J. Fiske, and R. A. Houghton. 2005. Satellite-observed photosynthetic trends across boreal North America associated with climate and fire disturbance. Proceedings of the National Academy of Sciences of the United States of America, 102: 13521–25.
- Grace, J. 2002. Impacts of climate change on the tree line. Annals of Botany 90:537–44.
- Grau, O., J. M. Ninot, J. M. Blanco-Moreno, R. S. P. van Logtestijn, J. H. C. Cornelissen, and T. V. Callaghan. 2012. Shrub-tree interactions and environmental changes drive treeline dynamics in the Subarctic. Oikos 121:1680–90.
- Greenwood, S., and A. S. Jump. 2014. Consequences of treeline shifts for the diversity and function of high altitude ecosystems. Arctic, Antarctic, and Alpine Research 46:829–40.
- Grissino, H. D. 2001. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Research 57:205–21.
- Harsch, M. A., P. E. Hulme, M. S. McGlone, and R. P. Duncan. 2009. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters 12:1040–49.
- Hobbie, S. E., and F. S. Chapin. 1998. The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology 79:1526–44.
- Jia, G. J., H. E. Epstein, and D. A. Walker. 2009. Vegetation greening in the Canadian Arctic related to decadal warming. Journal of Environmental Monitoring: JEM 11:2231–38.
- Jørgensen, R. H., M. Hallinger, S. Ahlgrimm, J. Friemel, J. Kollmann, and H. Meilby. 2015. Growth response to climatic change over 120 years for Alnus viridis and Salix glauca in West Greenland. Journal of Vegetation Science 26:155–65.
- Kaarlejärvi, E., R. Baxter, A. Hofgaard, H. Hytteborn, O. Khitun, U. Molau, S. Sjögersten, P. Wookey, and J. Olofsson. 2012. Effects of warming on shrub abundance and chemistry drive ecosystem-level changes in a forest–tundra ecotone. Ecosystems 15:1219–33.
- Kambo, D. S. 2017. Fine-scale mechanisms influencing germination success, seedling growth and survival in an alpine forest-tundra ecotone. Ph.D. thesis, Department of Geography and Planning, Queen’s University, Kingston, 193 pp.
- Körner, C. 2012. Treelines will be understood once the functional difference between a tree and a shrub is. Ambio 41:197–206.
- Leonelli, G., M. Pelfini, G. Battipaglia, and P. Cherubini. 2009. Site-aspect influence on climate sensitivity over time of a high-altitude Pinus cembra tree-ring network. Climatic Change 96:185–201.
- Macias-Fauria, M., and E. A. Johnson. 2013. Warming-induced upslope advance of subalpine forest is severely limited by geomorphic processes. Proceedings of the National Academy of Sciences, 110: 8117–22.
- Meinardus, C., B. Weinert, and J. Löffler. 2011. The potential of the dwarf shrub Betula nana L. as a climate indicator above the tree line in the southern Norwegian Scandes. Tree Rings in Archaeology, Climatology, and Ecology 9:181–86.
- Myers-Smith, I. H., S. C. Elmendorf, P. S. A. Beck, M. Wilmking, M. Hallinger, D. Blok, K. D. Tape, S. A. Rayback, M. Macias-Fauria, B. C. Forbes, et al. 2015. Climate sensitivity of shrub growth across the tundra biome. Nature Climate Change. doi:https://doi.org/10.1038/nclimate2697.
- Myers-Smith, I. H., B. C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, M. Macias-Fauria, U. Sass-Klaassen, E. Lévesque, et al. 2011. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters 6:1–15. doi:https://doi.org/10.1088/1748-9326/6/4/045509.
- Myers-Smith, I. H., M. Hallinger, D. Blok, U. Sass-Klaassen, S. A. Rayback, S. Weijers, A. J. Trant, K. D. Tape, A. T. Naito, S. Wipf, et al. 2014. Methods for measuring arctic and alpine shrub growth: A review. Earth-Science Reviews 140:1–13.
- Oberhuber, W. 2004. Influence of climate on radial growth of Pinus cembra within the alpine timberline ecotone. Tree Physiology 24:291–301.
- Peterson, D. W., and D. L. Peterson. 1994. Effects of climate on radial growth of subalpine conifers in the North Cascade Mountains. Canadian Journal of Forest Research 24:1921–32.
- Price, D., D. McKenney, and L. Joyce. 2011. High-resolution interpolation of climate scenarios for Canada derived from general circulation model simulations. Edmonton: Canadian Forestry Service. 104.
- Ropars, P., S. Angers-Blondin, M. Gagnon, I. H. Myers-Smith, E. Lévesque, and S. Boudreau. 2017. Different parts, different stories: Climate sensitivity of growth is stronger in root collars versus stems in tundra shrubs. Global Change Biology 23:3281–91.
- Ropars, P., and S. Boudreau. 2012. Shrub expansion at the forest–tundra ecotone: Spatial heterogeneity linked to local topography. Environmental Research Letters 7:1–9. doi:https://doi.org/10.1088/1748-9326/7/1/015501.
- Salzer, M. W., M. K. Hughes, A. G. Bunn, and K. F. Kipfmueller. 2009. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proceedings of the National Academy of Sciences of the United States of America, 106: 20348–53.
- Schmidt, N. M., C. Baittinger, J. Kollmann, and M. C. Forchhammer. 2010. Consistent dendrochronological response of the dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arctic, Antarctic, and Alpine Research 42:471–75.
- Smith, C. A. S., J. C. Meikle, and C. F. Roots eds. 2004. Ecoregions of the Yukon Territory: Biophysical properties of Yukon landscapes, 313. Summerland, British Columbia: Agriculture and Agri-Food Canada, PARC Technical Bulletin No. 04-01.
- Speer, J. 2010. Fundamentals of tree-ring research. Tucson: University of Arizona Press. 333.
- Strackee, J., and E. Jansma. 1992. The statistical properties of mean sensitivity - a reappraisal. Dendrochronologia 10:121–35.
- Sturm, M., C. Racine, and K. Tape. 2001. Increasing shrub abundance in the Arctic. Nature 411:546–47.
- Sturm, M., J. Schimel, G. Michaelson, J. M. Welker, S. F. Oberbauer, G. E. Liston, J. Fahnestock, and V. E. Romanovsky. 2005. Winter biological processes could help convert Arctic tundra to shrubland. BioScience 55:17–26.
- Tape, K., M. Sturm, and C. Racine. 2006. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology 12:686–702.
- Tremblay, B., E. Lévesque, and S. Boudreau. 2012. Recent expansion of erect shrubs in the Low Arctic: Evidence from Eastern Nunavik. Environmental Research Letters 7:1–11. doi:https://doi.org/10.1088/1748-9326/7/3/035501.
- Villalba, R., J. Boninsegna, and T. Veblen. 1997. Recent trends in tree-ring records from high elevation sites in the Andes of northern Patagonia. Climatic Change 36:425–54.
- Wahren, C.-H. A., M. D. Walker, and M. S. Bret-Harte. 2005. Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Global Change Biology 11: 537–552. doi:https://doi.org/10.1111/j.1365-2486.2005.00927.x.
- Walker, M., C. Wahren, R. Hollister, G. Henry, L. Ahlquist, J. Alatalo, M. S. Bret-Harte, M. P. Calef, T. V. Callaghan, A. B. Carroll, et al. 2006. Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America, 103: 1342–46.
- Wigley, T. M. L., K. R. Briffa, and P. D. Jones. 1984. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. Journal of Climate and Applied Meteorology 23:201–13.
- Wolken, J. M., D. H. Mann, T. A. Grant, A. H. Lloyd, T. S. Rupp, and T. N. Hollingsworth. 2016. Climate-growth relationships along a black spruce toposequence in interior Alaska. Arctic, Antarctic, and Alpine Research 48:637–52.
- Young, A. B., D. A. Watts, A. H. Taylor, and E. Post. 2016. Species and site differences influence climate-shrub growth responses in West Greenland. Dendrochronologia 37:69–78.
- Zalatan, R., and K. Gajewski. 2006. Dendrochronological potential of Salix alaxensis from the Kuujjua River area, western Canadian Arctic. Tree-Ring Research 62:75–82.
Appendix 1
Figure 1. Subsample signal strength (SSS) of Salix glauca shrub chronologies at each of the six sites (along the x axis), with each panel representing a different topographic position. Ruby Range sites are shown in the three left-most bars and Kluane Range sites in the three right-most bars (CC = Clear Creek, FJ = Fourth of July Creek, PP = Printer’s Pass, BC = Bock’s Creek, BW = Burwash Creek, and QC = Quill Creek). Note that these values are based on only the series that we included in the final chronologies (total of 586 series out of 914 were retained). Please see the methods of the main article for series exclusion criteria
Appendix 2
Appendix 2 (Continued)
Appendix 3
Table 1. Tree residual chronology statistics
Table 2. Shrub residual chronology statistics