ABSTRACT
Nieves penitentes are pinnacle-shaped ice structures found at high elevations in the dry Andes. Here we report, using molecular and microscopic approaches, the first description of snow algae communities inhabiting penitente ice at 5,277 m a.s.l., demonstrating a new habitat for snow algae in one of the most extreme environments on Earth. Red ice patches on penitentes contained a microbial community dominated by algae in the genera Chlamydomonas and Chloromonas, both of which were closely related to known snow algae from alpine and polar environments. In contrast, we obtained few snow algae sequences from clear ice, but we did find cyanobacteria sequences and evidence of aeolian-deposited organic matter. Tephra (“soil”) within and just downhill from the penitente field hosted more complex bacterial and eukaryotic communities that were significantly different from ice communities in terms of both alpha and beta diversity. In this environment penitentes provide both water and shelter from harsh winds, high UV radiation, and thermal fluctuations, creating an oasis in an otherwise extreme landscape. Intriguingly, recent planetary investigations have suggested the existence of penitente-like structures on other planetary bodies of our solar system. Therefore, penitentes and the harsh environment that surrounds them provide a new terrestrial analog for astrobiological studies of life beyond Earth.
Introduction
Nieves penitentes are surface ablation ice forms that are commonly found in the dry Andes at high elevations (above 4,000 m a.s.l.; Matthes Citation1934; Lliboitry Citation1954), and were first mentioned in the scientific literature by Darwin (Citation1839). The name “penitente” is a Spanish word (meaning “penitent one”) that comes from the resemblance of a field of penitentes to a procession of monks in white robes doing penance (Betterton Citation2001). They form when snowfields are subjected to the unique combination of high radiation, low humidity, and dry winds, causing differential ablation that leads to the formation of spire-like ice pinnacles that can range in size from a few centimeters to more than 5 m in height. The key climatic condition that leads to their formation is that the dew point temperature is always below 0°C (Corripio and Purves Citation2005). Penitente initiation and morphogenesis require cold temperature and once the process starts the surface geometry of the evolving penitente produces a positive feedback mechanism and radiation is trapped by multiple reflections among the walls (Corripio and Purves Citation2005). Thus, once they start to form, further growth in height can occur by the melting downward of the wells between the spires (Bergeron, Berger, and Betterton Citation2006).
The high-elevation desert areas where penitentes form are characterized by some of the most life-limiting conditions found on Earth, such as extreme solar radiation and dryness, dramatic temperature fluctuations, and a thin atmosphere (Lynch et al. Citation2012). The study of this harsh environment and penitentes has relevance to the field of astrobiology because this environment is perhaps the best analog for Mars-like soils on Earth (Schmidt et al. Citation2018) and penitentes have been detected on Pluto and other planetary bodies, such as Europa (Hobley, Moore, and Howard Citation2013; Moores et al. Citation2017). Given the harshness of the environments where they are found, nieves penitentes may represent oases for life, because, along with fumaroles, they represent intermittent water sources in these very arid environments (Costello et al. Citation2009; Solon et al. Citation2018).
There is currently no information on microbial life inhabiting these ice structures, although a preliminary study of volcanic soils (tephra) within two penitente fields indicated that they increased microbial diversity in tephra associated with a penitente field at 5,277 m a.s.l. on Volcán Llullaillaco, but not in a penitente field at 5,825 m a.s.l. on nearby Volcán Socompa (Solon et al. Citation2018). However, no previous studies have explored the microbial diversity in and on the ice of penitentes. The present study is the first sampling of life in penitente ice and was undertaken because we noticed snow algae–like patches of red ice on the sides of some penitente spires and in the so-called wells between penitentes on Volcán Llullaillaco in March 2016. Here we show that these red ice patches were dominated by algae whose 18S rRNA gene sequences most closely match sequences of snow algae from other sites around the world. Additionally, we describe the total eukaryotic and bacterial microbial community found within these unique ice structures and in the tephra soils that receive meltwater from penitentes.
Materials and methods
Site location and sample collection
The penitente field described here () is situated at 5,277 m a.s.l. on the Chilean side of Volcán Llullaillaco (24°43′40″ S, 68°34′20″W, 6,739 m a.s.l.), the second highest volcano in the world. All penitente fields on Llullaillaco occur above 5,000 m a.s.l., which is approximately the elevational limit for vascular plants on this mountain as well (Arroyo et al. Citation1988). The penitentes were roughly 1–1.5 m in height (). A total of seven samples were collected from the ice structures on March 12, 2016: two samples from clear ice and five samples from visibly red patches (). Ice samples were collected by scraping the ice wall with a sterile stainless-steel spoon and they were placed in sterile Whirl Pak bags. These ice samples were melted and filtered onto 0.22 µm Whatman Nucleopore membranes (GE Healthcare, Pittsburgh, PA, USA) the same day that they were collected.
Figure 1. (a, b) Photographs of Llullaillaco penitente field at 5,277 m a.s.l., Jack Darcy and Lara Vimercati are pictured for scale in (b). (c, d) Closeup pictures of red patches in penitente ice close to the tephra ground. and have been modified with Adobe Photoshop CS5 to enhance the color of the red patch. Input levels of the image were adjusted using the quick selection tool to select the area of interest (red ice) and selective color percentages were adjusted by increasing magenta and yellow to make the red ice stand out. Photos by G. Zimmerman and S. K. Schmidt.

A total of nine tephra samples were collected within and downhill from the penitente field; five samples were from wet soils between the penitentes structures and four samples were from a horizontal transect 10 m downslope from the penitente field. All soil samples consisted of the top 4 cm of soil. All samples were collected with a sterile stainless-steel spoon and were placed in sterile Whirl Pak bags. They were stored on ice until the descent from the mountain and were transported to Antofagasta where they were frozen at −20°C until they were shipped to the University of Colorado at Boulder, where they were stored at −80°C until being processed as described further on.
DNA extraction, polymerase chain reaction (PCR), and sequencing
The total environmental genomic DNA from the samples collected within the penitente field was extracted using a PowerWater® DNA Isolation Kit for clear ice and filtered red ice samples and a PowerSoil® DNA Isolation Kit for soil samples adjacent to penitentes (MoBio Inc., Carlsbad, CA, USA), following the manufacturer’s instructions. Extracted genomic DNA was amplified using the oligonucleotide primer set F515 and R806 for Bacteria (Caporaso et al. Citation2012), and the 1391F-EukBr primer set for Eukarya. Both primer sets can be accessed from the Earth Microbiome Project website (http://press.igsb.anl.gov/earthmicrobiome/emp-standard-protocols). Amplified DNA was pooled, normalized to equimolar concentrations using SequalPrep Normalization Plate Kits (Invitrogen Corp., Carlsbad, CA, USA), barcoded, and then sequenced using the Illumina MiSeq V2 pair-end technology (2x150 bp chemistry) at the BioFrontiers Sequencing Core Facility at the University of Colorado at Boulder.
Bioinformatics and statistical analysis
Raw 16S/18S rRNA gene sequences were demultiplexed, quality filtered, and processed using the QIIME v1.9.1 bioinformatics package (Caporaso et al. Citation2010b). 16S rRNA gene paired-end reads were joined, but this process did not work for 18S rRNA gene reads because of insufficient overlap, so only the read corresponding to the 1391F primer was used (150 bp). This read was selected because it overlaps more with most sequences within the NCBI and SILVA databases (Darcy and Schmidt Citation2016). Singletons were excluded from further analysis and sequences with more than 97 percent similarity were clustered into an operational taxonomic unit (OTU) via UCLUST (Edgar Citation2010). OTU clustering was performed rather than using exact sequence variants (ESV) to previous work (Solon et al. Citation2018), and because the short reads used in this study are not long enough to assign most OTUs to the species level. Representative sequences for each OTU were chosen for classification and the GreenGenes 13.5 (DeSantis et al. Citation2006) and SILVA Ref NR 97 database (Quast et al. Citation2012) for 16S and 18S rRNA gene sequences, respectively, were employed to assign taxonomy identification to each single OTU. All mitochondrial and chloroplast OTUs based on this classification were removed from the bacterial data set and all bacterial OTUs were removed from the eukaryotic data set. All sequences belonging to the Enterobacteriales order, a lineage commonly seen in the gut, were also removed from the data set as likely reflecting lab or sampling contaminants. The taxonomic assignments of the top OTUs from each habitat were verified by using BLAST to search NCBI, and were refined as needed. Sequences were aligned with PyNAST (Caporaso et al. Citation2010a) and a phylogeny was built with the FastTree algorithm (Price, Dehal, and Arkin Citation2009). OTU tables were rarefied to the number of sequences in the lowest populated sample to make comparisons more robust, and were used to assess alpha diversity and relative abundance of all taxa. Rarefaction curves, which plot the number of OTUs as a function of the number of samples, were also calculated using the QIIME v1.9.1 bioinformatics package. Alpha diversity significance was determined by a one-way analysis of variance (ANOVA) with function AOV. A community-level distance matrix with pairwise weighted Unifrac beta-diversity values (Lozupone and Knight Citation2005; Lozupone et al. Citation2007) was generated and analyzed using an ADONIS model (Anderson Citation2001; Oksanen et al. Citation2013) to partition the variance in community composition. Test of homogeneity of multivariate dispersion (PERMDISP) was determined with function BetaDisper to evaluate whether the difference supported by ADONIS was because of differences between clusters or differences between the variations within clusters. Principal coordinate analysis (PCoA) ordination was constructed based on weighted Unifrac distance matrices to visualize differences among community compositions of soil and ice samples. All statistical tests were performed using the Vegan package of R (Oksanen et al. Citation2013).
Nucleotide sequence accession numbers
16S rRNA gene sequences from Illumina MiSeq libraries determined by this study were deposited in the GenBank database under accession numbers MK242070–MK243365, while 18S rRNA gene sequences were deposited in the SRA (Short Read Archive) database under Bioproject PRJNA530025.
Results
Alpha and beta diversity patterns
Alpha and beta diversity patterns were significantly different between ice and soil samples for both eukaryotic (18S) and bacterial (16S) communities. Rarefaction curves () show that soils within and close (10 m downslope) to the penitente field have significantly higher 18S and 16S OTU richness (alpha diversity) than ice (clear and red) communities (ANOVA p < .001, F = 63; p < .001, F = 66.24, respectively; , ). Analysis of both 18S and 16S beta diversity showed clustering of communities based on habitat type (ice vs. soil; ADONIS p < .001, R2 = 0.63; p < .001, R2 = 0.46, respectively; , ). The test for homogeneity of dispersion was not significant for both 18S and 16S communities (PERMDISP p > .3, F = 1.28; p > .2, F = 1.54, respectively), showing that the main factor driving community clustering was the difference between habitat types rather than within habitat types.
Figure 2. Alpha rarefaction curves based on the number of operational taxonomic units (OTUs) observed for penitente ice and soils within and below penitentes based on a 97 percent similarity clustering: (a) eukaryotes and (b) bacteria. Soil within and below penitentes had two times the number of eukaryotic OTUs and about four times the number of bacterial OTUs compared to the penitente ice (18S ANOVA p < .001, F = 63; 16S ANOVA p < .001, F = 66.24). Data shown are means ± s.e. (soil within penitentes n = 5; soil below penitentes n = 4; red ice n = 5; clear ice n = 2). Note that the scale of the y-axis changes between the panels.
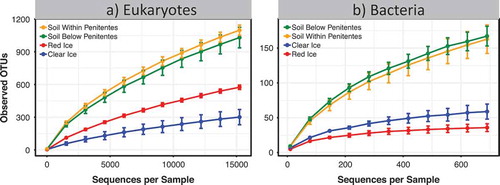
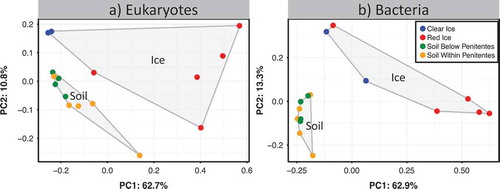
Figure 3. Cluster diagram-based PCoA plot using weighted Unifrac of eukaryotes (a) and bacteria (b). Both eukaryotic and bacterial soil communities and ice communities are significantly different from each other (18S ADONIS p < .001, R2 = 0.63; 16S ADONIS p < .001, R2 = 0.46). Polygons surround total soil and total ice communities in both figures.
Eukaryotic taxonomic composition
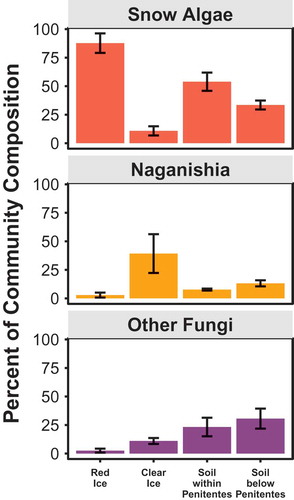
Red ice samples were dominated by algal sequences with three OTUs accounting for approximately 85 percent of total relative abundance (). The most abundant algal OTU in red ice is a 100 percent match for the snow algae Chlamydomonas nivalis (), and its closest environmental matches were from a variety of globally distributed glacial environments, including Mt. Kilimanjaro (KX771811, 100% identity; Vimercati, Darcy, and Schmidt Citation2019), snowpack in the Swiss Alps (KT184441, 100% identity), red snow from Svalbard (JQ790560, 100% identity), Antarctica (GU117587, 100% identity; Remias et al. Citation2010), Alaska (LC371441, 100% identity; Segawa et al. Citation2018), Greenland (AB902971, 98.6% identity), and the Pamir region of Tajikistan (AB903025, 100% identity). OTUs affiliated with C. nivalis are also found in soil samples within and below penitentes, albeit at lower abundance (32% and 5%, respectively). The second most abundant 18S OTU in red ice is distantly related to the genus Chloromonas (only 96% identity to Chloromonas fukushimae AB906342; Matsuzaki, Hara, and Nozaki Citation2014), suggesting it belongs to a previously uncharacterized new species or genus because most Chloromonas species share more than 99 percent identity in the hypervariable V3-V4 region of the 18S rRNA gene (Remias et al. Citation2018). The closest uncultured matches include glacial sequences from Antarctica (AB903009, 94% identity), the Tibetan plateau (DQ104086, 93% identity; Zhang et al. Citation2009), and Alaska (LC371418, 92% identity; Segawa et al. Citation2018). This novel algal OTU was also found in soils within and below penitentes, with a relative abundance of 16 percent and 20 percent, respectively.
Table 1. Sequences count, GenBank ID of the closest blast hit, identity percent, and tentative taxonomic affiliation of most abundant phylotypes retrieved in red and clear penitentes ice samples: top= eukaryotes; bottom = bacteria.
Figure 4. Relative abundance of the main eukaryotic phylotypes as a percentage of total sequences retrieved from penitente ice and soil samples based on polymerase chain reaction (PCR) amplifications of the 18S rRNA gene using Euk1391F/EukBr primer sets. Snow algae make up most of the diversity in red ice samples and they are abundant in soil samples within and just downhill from the penitente field.
The third most abundant algal OTU in red ice samples is only distantly related to Chloromonas sp. Hakkoda-1, with only 95 percent identity (LC012710, Matsuzaki et al. Citation2015), also suggesting that it belongs to an undescribed new species. The closest environmental matches for this phylotype are from Pyrenean glacial ice (JX456233, 95% identity; García-Descalzo et al. Citation2013) and red snow in Antarctica (AB903008, 92% identity). This OTU is also found in soils within and below penitentes, with a relative abundance of 6 percent and 8 percent, respectively. Microscopic examination of red ice samples also revealed the presence of cysts resembling those of many snow algae ().
Figure 5. Photomicrograph (1000x magnification) of a spore of a Chlamydomonas sp. from red penitente ice.

The 18S community composition of clear ice samples is significantly different from the community of the red ice and is dominated by a basidiomycete yeast related to Naganishia friedmannii (AB032630, 100% identity; ) and an OTU with a 99 percent match for Coleoptera (JX844984; Robertson et al. Citation2013). Another relatively abundant fungal OTU is a 100 percent match to a Dothideomycete isolated from the aerosol of a low-elevation vegetated region (MG569511).
Bacterial taxonomic composition
The most abundant 16S OTU (54% relative abundance) within red ice samples is unclassified and its closest match in the NCBI database is only at 77 percent identity (KR846626). This unclassified OTU was followed in decreasing abundance by OTUs within the Pseudomonadales, Actinomycetales, Burkholderiales, and Rhodospirillales orders. In contrast, the most abundant OTUs in clear ice samples are mainly Cyanobacteria (Oscillatoriales and Nostocales orders; ), Bacteroidetes (Saprospirales), Proteobacteria (Rhizobiales, Burkholderiales, and Xanthomonadales), Firmicutes, Acidobacteria, and Actinobacteria (Actinomycetales). The dominant cyanobacterium was a 100 percent match for a Microcoleus sp. (Oscillatoriales) from a soil biocrust from Spain (MF527140; Muñoz-Martín et al. Citation2019) and the second most abundant cyanobacterium was in the Nostocales and is a 100 percent match with sequences from other cold environments, such as the high Andes (GQ306061; Schmidt et al. Citation2009), Antarctic soil crusts (MF468245), a Chilean glacier foreland (JQ776471; Raggio et al. Citation2012), and soils from the high Himalayas (HQ189094; Schmidt et al. Citation2011).
Soils within and below penitente ice structures have closely overlapping communities dominated by OTUs within the Acidobacteria, Actinobacteria (Actinomycetales), Bacteroidetes (Saprospirales), Verrucomicrobia (Chthoniobacterales), and Chloroflexi (Ktedonobacteria). The most abundant Acidobacteria phylotype is also 100 percent identical to sequences detected in perennially cold environments, such as the top of Mt. Kilimanjaro (KX771519; Vimercati, Darcy, and Schmidt Citation2019) and soil biocrusts from Antarctica (MF468273).
Discussion
Our analysis represents the first description of microbial life in ice structures known as nieves penitentes. These structures, commonly found in the driest parts of the central and southern Andes, are of particular interest because they can be an intermittent source of meltwater to tephra in one of the most extreme dry environments on Earth (Lynch et al. Citation2012; Solon et al. Citation2018). Penitentes are formed during the metamorphosis of snow beds (areas where windblown snow accumulates) into rows of evenly spaced ice spires (). As such, they represent a unique and previously unstudied environment for microbial life.
Our study revealed for the first time that snow algal communities similar to those in many other snow or ice environments are present in the ice of nieves penitentes, at least in the penitente field at 5,277 m a.s.l. on Volcán Llullaillaco. The most abundant 18S OTUs from red patches and in soils associated with the penitente field are snow algae with closest matches to the genera Chlamydomonas and Chloromonas. Although there have been no previous reports of snow algae in penitente ice, there have been descriptions of snow algae in snow and on glaciers in the Andes (Takeuchi and Kohshima Citation2004; Nedbalová and Sklenár Citation2008). In addition, Solon et al. (Citation2018) previously reported the presence of snow algae in the tephra soils within penitente fields on Volcán Llullaillaco and nearby Volcán Socompa. Conversely, no snow algae (or any other algae) or cyanobacteria have been found in numerous surveys of dry tephra soils that make up most of the barren landscape at elevations above 5,100 m a.s.l. on both Llullaillaco and Socompa (Costello et al. Citation2009; Lynch et al. Citation2012; Solon et al. Citation2018). We therefore assume that the snow algae from the penitentes are the source of algae found in the soils within and near the penitentes. That is, snow algae have complex life cycles that include residence in soil for part of the year (Remias Citation2012) and their putative life cycle includes the formation of thick-walled cysts, making them resistant to high irradiance, dryness, and freezing (Hoham and Duval Citation2001; Holzinger and Lütz Citation2006; Komárek and Nedbalová Citation2007; Remias et al. Citation2010). These and other attributes discussed further on would likely allow them to survive in tephra associated with penitentes and on the ice surface of penitentes themselves. In addition, cysts of snow algae such as C. nivalis can be photosynthetically active (Remias, Lütz-Meindl, and Lütz Citation2005; Remias et al. Citation2010), but it is not clear if they can photosynthesize outside of the snow environment in dry soils.
It is also important that the snow algae found on Volcán Llullaillaco are red in color. The red color is likely the result of a suite of pigments, including carotenoids (Müller et al. Citation1998; Remias, Lütz-Meindl, and Lütz Citation2005), which shield the photosynthetic machinery from over-illumination (Remias Citation2012) and act as a pool of antioxidative compounds (Grossman, Lohr, and Im Citation2004). These pigments dissipate excess energy as heat and red pigments radiate higher amounts of heat compared to other colors (Dial, Ganey, and Skiles Citation2018). Interestingly, increased carotenoid production has also been linked by previous studies to freeze-thaw tolerance (Dieser, Greenwood, and Foreman Citation2010). This may be especially relevant to the harsh environments of Llullaillaco, where extreme thermal fluctuations occur every day of the year at elevations above 5,000 m a.s.l. (Lynch et al. Citation2012; Vimercati et al. Citation2016). Red-snow algae also tend to inhabit drier, more nutrient-poor environments compared to algae of other colors (Lutz et al. Citation2014). Red snow is also indicative of a more mature carotenoid-rich resting stage, compared to green motile trophic stages (Lutz et al. Citation2014). We did not observe any green patches in the penitente field but that could be because we visited the site for only several hours: there may be green motile stages present at this site earlier in the development of penitentes. We also could not find any colored patches in penitente structures above 5,400 m a.s.l. on either Llullaillaco or Socompa: higher elevations are colder and drier, possibly establishing an upper elevation limit to snow algae distribution (Dial et al. Citation2016), but more intensive surveys would be needed to verify this hypothesis. Interestingly, sequences of algae such as Mesotaenium berggrenii and Ancylonema sp., which are known to live on bare glacial surfaces (Remias, Holzinger, and Lütz Citation2009), including ice fields in South America (Takeuchi and Kohshima Citation2004), were not found in the present study. This may be because these algae lack a motile stage in their life cycle and therefore are not able to migrate into transitory structures such as penitentes.
Our finding of microbial communities dominated by red-pigmented algae also brings up the intriguing question of whether these communities may be contributing to the formation of penitentes. Models incorporating physical processes have provided insight into penitente formation (Betterton Citation2001; Bergeron, Berger, and Betterton Citation2006; Claudin et al. Citation2015), however, organic biomass, particularly snow algae, may play a role in penitente formation, as has been suggested for the formation of other snow and ice structures (Takeuchi, Kohshima, and Seko Citation2001; Lutz et al. Citation2014; Ganey et al. Citation2017). Snow and glacier algae can influence physical glacial surface processes by changing glacial albedo much like dust deposits (Takeuchi Citation2009). That is, snow algae can reduce surface albedo with reductions up to 80 percent and increase the melting of surrounding ice (Lutz et al. Citation2014; Dial, Ganey, and Skiles Citation2018; Ryan et al. Citation2018). But it is unlikely that snow algae can migrate through ice and therefore it is more likely that they move up through the snowpack as the penitentes morph from snow to ice. Obviously more work is needed to determine at what point in the formation of penitentes the snow algae are present. It will take detailed field observations to understand the temporal dynamics of algal migration and penitente formation.
Eukaryotic community
The closest environmental matches of the most abundant algal OTU in red ice from penitentes and in soils between penitentes were related to snow algal phylotypes globally distributed in the cryosphere, including arctic, Antarctic, and alpine ecosystems. This finding lends further support to the concept of the global distribution of red-snow algae ecotypes throughout the cold terrestrial biosphere. A number of studies using next-generation sequencing based on both ITS and 18S rRNA genes have elucidated the geographic distribution patterns of red-snow algae in polar regions, showing that genera such as Chlamydomonas are cosmopolitan. For example, algal blooms causing red snow in polar regions comprise mainly cosmopolitan phylotypes (Lutz et al. Citation2016; Segawa et al. Citation2018). Propagule distribution can explain their global distribution in the cryosphere; Brown, Ungerer, and Jumpponen (Citation2016) suggested that red-snow algae may be readily dispersed across large distances because their size (~15 μm in diameter; Remias, Lütz-Meindl, and Lütz Citation2005) is compatible with long-range aerial transport. Future studies combining both morphological data and sequencing of the ITS2 marker gene will be necessary to fully describe algal phylotypes found in penitentes. It has been shown that 18S rRNA gene amplicons do not adequately identify taxa on the species level in several taxonomic groups (Xiao et al. Citation2014).
Red-snow algae decrease in relative abundance in soil compared to red ice, especially in soils further from penitentes, giving way to organisms such as the basidiomycete yeast Naganishia in the soils below penitentes (13% of sequences). However, Naganishia is not as dominant as it is in nearby extremely dry tephra, where it can make up 90 percent of the eukaryotic community (Lynch et al. Citation2012; Solon et al. Citation2018). Chytrids were also detected in soils within and below the penitente field, as noted for other moist high-elevation soils (Freeman et al. Citation2009). Chytrids are also found in association with snow algae (Naff, Darcy, and Schmidt Citation2013; Brown, Olson, and Jumpponen Citation2015), but we did not detect any chytrids in our penitente ice samples.
Clear ice samples host a very different community than that seen in red ice and soil samples, with the two most abundant OTUs being matches for Naganishia and Coleoptera. Naganishia may be active within the penitentes in areas not dominated by algal blooms; alternatively, their high abundance may be a reflection of wind-transported cells from the tephra soils surrounding the penitente field. The second most abundant OTU, matching Coleoptera, likely reflects aeolian deposition of dead organic matter from lower elevation environments, as has been shown for other low biomass, high-elevation environments, including tephra from a penitente field on Volcán Socompa (Solon et al. Citation2018), and clear glacial ice in the Dry Valleys of Antarctica (Sommers et al. Citation2019).
Bacterial community
Our molecular analysis revealed that cyanobacteria within the Nostocales and Oscillatoriales orders comprise 28 percent of the total bacterial community in clear ice and they are also detected in soils surrounding penitentes; however, at extremely low relative abundances (0.6–1.4%). This is the first report of cyanobacterial sequences in any high-elevation habitat investigated on Llullaillaco (cf. Lynch et al. Citation2012; Solon et al. Citation2018). Alternatively, cyanobacterial sequences were not retrieved in red ice patches. This may indicate that penitentes provide enough water for cyanobacterial communities to develop, but snow algae may outcompete cyanobacteria as primary producers in these environments because of their ability to colonize snow and ice thanks to their flagellated states, which are absent in cyanobacteria. It is known that cyanobacteria are relatively rare in melting snowfields and other non-stable cryosystems, possibly because they are unable to keep pace with constant losses in meltwater (Vincent Citation2000; Quesada and Vincent Citation2012). A broader-scale study of penitente fields will be necessary to further investigate the relative role of competition and priority effects in structuring the community of primary producers in penitente ice. Interestingly, the bacterial OTU with the highest relative abundance (54%) was unclassified according to GreenGenes and its closest match on NCBI was only at 77 percent identity. This may suggest that penitentes harbor novel bacterial diversity; however, a long-read sequencing approach will be needed to allow fine-scale phylogeny of these communities.
Astrobiological implications
Recent studies of high-elevation volcanic soils (tephra) of the Atacama region have made a strong case that this environment is perhaps the best earthly analog for surface and near-surface soils on Mars (Pulschen et al. Citation2015; Schmidt et al. Citation2018; Solon et al. Citation2018). This is because high-elevation (>5,000 m a.s.l.) areas of the Atacama are subjected to the highest levels of UV radiation (Cabrol et al. Citation2014), the most extreme daily freeze-thaw cycles (Lynch et al. Citation2012), and one of the driest climates on Earth (Costello et al. Citation2009). This combination of extreme conditions has selected for some of the simplest microbial communities yet studied in a surface soil ecosystem on Earth (Lynch et al. Citation2012; Solon et al. Citation2018). Despite these extreme conditions, the study reported here shows for the first time that penitentes harbor snow algae communities, at least up to an elevation of 5,277 m a.s.l. on Volcán Llullaillaco. This finding, along with the presence of complex microbial communities in soils affected by penitente meltwater, indicate that penitentes are oases of life in the otherwise barren expanses of high-elevation tephra on Volcán Llullaillaco. These findings are of importance to the field of astrobiology because they add to our understanding of the cold-dry limits to life on Earth and because penitente-like formations have recently been discovered on Pluto (Moores et al. Citation2017) and perhaps on Europa (Hobley, Moore, and Howard Citation2013).
Conclusions and future studies
We employed high-throughput sequencing to characterize cryophilic communities dwelling in penitente ice structures and adjacent soils on Volcán Llullaillaco. Penitente ice supports the only known photosynthetic communities (mainly algae) in this harsh high-elevation environment. This work differs from previous work that showed the presence of algae in soils associated with penitentes (Solon et al. Citation2018) in two distinct ways: by showing that (1) snow algae are present in the penitentes themselves and (2) some of these algae are related to known snow algae. This first report of snow algae occurring in penitente ice opens the door to future work that will address the altitudinal limits of these communities. There are also intriguing unanswered questions relating to whether snow algae are contributing to the formation of penitentes or whether they just migrate into them while they are forming. Finally, the microbiomes of penitentes and the surrounding harsh environment deserve further study as extraterrestrial analog systems. All of these unanswered questions will be a challenge to address given the remoteness and harshness of the stark landscapes in which penitentes occur.
Acknowledgments
We thank Corporación Nacional Forestal (CONAF) for permission to collect samples in Llullaillaco National Park; P. Sowell, G. Zimmerman, T. Bowen, and M. Perez for help in the field; and C. Bueno de Mesquita for help with data analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46.
- Arroyo, M. T. K., F. A. Squeo, J. J. Armesto, and C. Villagran. 1988. Effects of aridity on plant diversity in the northern Chilean Andes—Results of a natural experiment. Annals of the Missouri Botanic Garden 75:55–78. doi:10.2307/2399466.
- Bergeron, V., C. Berger, and M. D. Betterton. 2006. Controlled irradiative formation of penitentes. Physical Review Letters 96 (9):098502. doi:10.1103/PhysRevLett.96.098502.
- Betterton, M. D. 2001. Theory of structure formation in snowfields motivated by penitentes, suncups, and dirt cones. Physical Review E 63 (5):056129. doi:10.1103/PhysRevE.63.056129.
- Brown, S. P., B. J. Olson, and A. Jumpponen. 2015. Fungi and algae co-occur in snow: An issue of shared habitat or algal facilitation of heterotrophs? Arctic, Antarctic, and Alpine Research 47 (4):729–49. doi:10.1657/AAAR0014-071.
- Brown, S. P., M. C. Ungerer, and A. Jumpponen. 2016. A community of clones: Snow algae are diverse communities of spatially structured clones. International Journal of Plant Sciences 177 (5):432–39. doi:10.1086/686019.
- Cabrol, N. A., U. Feister, D. Häder, H. Piazena, E. A. Grin, and A. Klein. 2014. Record solar UV irradiance in the tropical Andes. Frontiers in Environmental Science 2:19. doi:10.3389/fenvs.2014.00019.
- Caporaso, J. G., K. Bittinger, F. D. Bushman, T. Z. DeSantis, G. L. Andersen, and R. Knight. 2010a. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 26 (2):266–77. doi:10.1093/bioinformatics/btp636.
- Caporaso, J. G., J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Pena, J. K. Goodrich, J. I. Gordon, et al. 2010b. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7 (5):335–36. doi:10.1038/nmeth.f.303.
- Caporaso, J. G., C. L. Lauber, W. A. Walters, D. Berg-Lyons, J. Huntley, N. Fierer, S. M. Owens, J. Betley, L. Fraser, M. Bauer, et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal 6(8):1621. doi:10.1038/ismej.2012.11.
- Claudin, P., H. Jarry, G. Vignoles, M. Plapp, and B. Andreotti. 2015. Physical processes causing the formation of penitentes. Physical Review E 92 (3):033015. doi:10.1103/PhysRevE.92.033015.
- Corripio, J. C., and R. S. Purves. 2005. Surface energy balance of high altitude glaciers in the central Andes: The effect of snow penitentes. In Climate and hydrology of mountain areas, ed. C. de Jong, D. N. Collins, and R. Ranzi, 15–28. Chichester: Wiley.
- Costello, E. K., S. R. P. Halloy, S. C. Reed, P. Sowell, and S. K. Schmidt. 2009. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa Volcano, Puna de Atacama, Andes. Applied and Environmental Microbiology 75:735–47. doi:10.1128/AEM.01469-08.
- Darcy, J. L., and S. K. Schmidt. 2016. Nutrient limitation of microbial phototrophs on a debris-covered glacier. Soil Biology and Biochemistry 95:156–63. doi:10.1016/j.soilbio.2015.12.019.
- Darwin, C. 1839. Journal of researches into the geology and natural history of various countries visited by H.M.S Beagle. London: Henry Colburn Publishers.
- DeSantis, T. Z., P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G. L. Andersen. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology 72 (7):5069–72. doi:10.1128/AEM.03006-05.
- Dial, R. J., G. Q. Ganey, and S. M. Skiles. 2018. What color should glacier algae be? An ecological role for red carbon in the cryosphere. FEMS Microbiology Ecology 94 (3):fiy007. doi:10.1093/femsec/fiy007.
- Dial, R. J., T. Scott Smeltz, P. F. Sullivan, C. L. Rinas, K. Timm, J. E. Geck, S. Carl Tobin, T. S. Golden, and E. C. Berg. 2016. Shrubline but not treeline advance matches climate velocity in montane ecosystems of south‐central Alaska. Global Change Biology 22 (5):1841–56. doi:10.1111/gcb.13207.
- Dieser, M., M. Greenwood, and C. M. Foreman. 2010. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arctic, Antarctic, and Alpine Research 42 (4):396–405. doi:10.1657/1938-4246-42.4.396.
- Edgar, R. C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 (19):2460–61. doi:10.1093/bioinformatics/btq461.
- Freeman, K. R., A. P. Martin, D. Karki, R. C. Lynch, M. S. Mitter, A. F. Meyer, J. E. Longcore, D. R. Simmons, and S. K. Schmidt 2009. Evidence that chytrids dominate fungal communities in high-elevation soils. Proceedings of the National Academy of Sciences 106:18315–18320.
- Ganey, G. Q., M. G. Loso, A. B. Burgess, and R. J. Dial. 2017. The role of microbes in snowmelt and radiative forcing on an Alaskan icefield. Nature Geoscience 10 (10):754. doi:10.1038/ngeo3027.
- García-Descalzo, L., E. García-López, M. Postigo, F. Baquero, A. Alcazar, and C. Cid. 2013. Eukaryotic microorganisms in cold environments: Examples from Pyrenean glaciers. Frontiers in Microbiology 4:55. doi:10.3389/fmicb.2013.00077.
- Grossman, A. R., M. Lohr, and C. S. Im. 2004. Chlamydomonas reinhardtii in the landscape of pigments. Annual Review of Genetics 38:119–73. doi:10.1146/annurev.genet.38.072902.092328.
- Hobley, D. E. J., J. M. Moore, and A. D. Howard. 2013. How rough is the surface of Europa at lander scale? 44th Lunar and Planetary Science Conference, March 18–22, The Woodlands, Texas.
- Hoham, R. W., and B. Duval. 2001. Microbial ecology of snow and freshwater ice with emphasis on snow algae. In Snow Ecology, ed. H. G. Johns, J. W. Pomeroy, D. A. Walker, and R. S. Hoham, 186–203. New York: Cambridge University Press.
- Holzinger, A., and C. Lütz. 2006. Algae and UV irradiation: Effects on ultrastructure and related metabolic functions. Micron 37 (3):190–207. doi:10.1016/j.micron.2005.10.015.
- Komárek, J., and L. Nedbalová. 2007. Green cryosestic algae. In Algae and Cyanobacteria in Extreme Environments. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol. 11, ed. J. Seckbach, 321–342. Dordrecht: Springer.
- Lliboitry, L. 1954. The origin of penitentes. Journal of Glaciology 2 (15):331–38. doi:10.1017/S0022143000025181.
- Lozupone, C., and R. Knight. 2005. UniFrac: A new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology 71 (12):8228–35. doi:10.1128/AEM.71.12.8228-8235.2005.
- Lozupone, C. A., M. Hamady, S. T. Kelley, and R. Knight. 2007. Quantitative and qualitative ß diversity measures lead to different insights into factors that structure microbial communities. Applied and Environmental Microbiology 73 (5):1576–85. doi:10.1128/AEM.01996-06.
- Lutz, S., A. M. Anesio, S. E. Jorge Villar, and L. G. Benning. 2014. Variations of algal communities cause darkening of a Greenland glacier. FEMS Microbiology Ecology 89 (2):402–14. doi:10.1111/1574-6941.12351.
- Lutz, S., A. M. Anesio, R. Raiswell, A. Edwards, R. J. Newton, F. Gill, and L. G. Benning. 2016. The biogeography of red snow microbiomes and their role in melting arctic glaciers. Nature Communications 7:11968. doi:10.1038/ncomms11968.
- Lynch, R. C., A. J. King, M. E. Farías, P. Sowell, C. Vitry, and S. K. Schmidt. 2012. The potential for microbial life in the highest‐elevation (> 6000 masl) mineral soils of the Atacama region. Journal of Geophysical Research: Biogeosciences 117 (G2). doi:10.1029/2012JG001961.
- Matsuzaki, R., Y. Hara, and H. Nozaki. 2014. A taxonomic study of snow Chloromonas species (Volvocales, Chlorophyceae) based on light and electron microscopy and molecular analysis of cultured material. Phycologia 53 (3):293–304. doi:10.2216/14-3.1.
- Matsuzaki, R., H. Kawai-Toyooka, Y. Hara, and H. Nozaki. 2015. Revisiting the taxonomic significance of aplanozygote morphologies of two cosmopolitan snow species of the genus Chloromonas (Volvocales, Chlorophyceae). Phycologia 54 (5):491–502. doi:10.2216/15-33.1.
- Matthes, F. E. 1934. Ablation of snow‐fields at high altitudes by radiant solar heat. EOS Transactions American Geophysical Union 15 (2):380–85. doi:10.1029/TR015i002p00380.
- Moores, J. E., C. L. Smith, A. D. Toigo, and S. D. Guzewich. 2017. Penitentes as the origin of the bladed terrain of Tartarus Dorsa on Pluto. Nature 541:188–90. doi:10.1038/nature20779.
- Müller, T., W. Bleiß, C. D. Martin, S. Rogaschewski, and G. Fuhr. 1998. Snow algae from northwest Svalbard: Their identification, distribution, pigment and nutrient content. Polar Biology 20 (1):14–32. doi:10.1007/s003000050272.
- Muñoz‐Martín, M. Á., I. Becerra‐Absalón, E. Perona, L. Fernández‐Valbuena, F. Garcia‐Pichel, and P. Mateo. 2019. Cyanobacterial biocrust diversity in Mediterranean ecosystems along a latitudinal and climatic gradient. New Phytologist 221 (1):123–41. doi:10.1111/nph.15355.
- Naff, C. S., J. L. Darcy, and S. K. Schmidt. 2013. Phylogeny and biogeography of an uncultured clade of snow chytrids. Environmental Microbiology 15 (10):2672–80. doi:10.1111/1462-2920.12116.
- Nedbalová, L., and P. Sklenár. 2008. New records of snow algae from the Andes of Ecuador. Arnaldoa 15:17–20.
- Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, H. Wagner, et al. 2013. Package ‘vegan’. Community Ecology Package, Version 2:9.
- Price, M. N., P. S. Dehal, and A. P. Arkin. 2009. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution 26 (7):1641–50. doi:10.1093/molbev/msp077.
- Pulschen, A. A., F. Rodrigues, R. T. D. Duarte, G. G. Araujo, I. F. Santiago, I. G. Paulino-Lima, C. A. Rosa, M. J. Kato, V. H. Pellizari, and D. Galante. 2015. UV-resistant yeasts isolated from a high-altitude volcanic area on the Atacama Desert as eukaryotic models for astrobiology. MicrobiologyOpen 4 (4):574–88. doi:10.1002/mbo3.264.
- Quast, C., E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies, and F. O. Glöckner. 2012. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research 41 (D1):D590–D596. doi:10.1093/nar/gks1219.
- Quesada, A., and W. F. Vincent. 2012. Cyanobacteria in the cryosphere: Snow, ice and extreme cold. In Ecology of Cyanobacteria II, ed. B. Whitton, 387–399. Dordrecht: Springer.
- Raggio, J., T. G. A. Green, P. D. Crittenden, A. Pintado, M. Vivas, S. Pérez-Ortega, A. De Los Ríos, and L. G. Sancho. 2012. Comparative ecophysiology of three Placopsis species, pioneer lichens in recently exposed Chilean glacial forelands. Symbiosis 56 (2):55–66. doi:10.1007/s13199-012-0159-1.
- Remias, D. 2012. Cell structure and physiology of alpine snow and ice algae. In Plants in Alpine Regions, ed. C. Lütz, 175–85. Vienna: Springer.
- Remias, D., A. Holzinger, and C. Lütz. 2009. Physiology, ultrastructure and habitat of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European Alps. Phycologia 48:302–12. doi:10.2216/08-13.1.
- Remias, D., U. Karsten, C. Lütz, and T. Leya. 2010. Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma 243:73–86. doi:10.1007/s00709-010-0123-y.
- Remias, D., U. Lütz-Meindl, and C. Lütz. 2005. Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. European Journal of Phycology 40 (3):259–68. doi:10.1080/09670260500202148.
- Remias, D., L. Procházková, A. Holzinger, and L. Nedbalová. 2018. Ecology, cytology and phylogeny of the snow alga Scotiella cryophila K-1 (Chlamydomonadales, Chlorophyta) from the Austrian Alps. Phycologia 57 (5):581–92. doi:10.2216/18-45.1.
- Robertson, J. A., A. Ślipiński, K. Hiatt, K. B. Miller, M. F. Whiting, and J. V. Mchugh. 2013. Molecules, morphology and minute hooded beetles: A phylogenetic study with implications for the evolution and classification of Corylophidae (Coleoptera: Cucujoidea). Systematic Entomology 38 (1):209–32. doi:10.1111/sen.2013.38.issue-1.
- Ryan, J. C., A. Hubbard, M. Stibal, T. D. Irvine-Fynn, J. Cook, L. C. Smith, K. Cameron, and J. Box. 2018. Dark zone of the Greenland Ice Sheet controlled by distributed biologically-active impurities. Nature Communications 9 (1):1065. doi:10.1038/s41467-018-03353-2.
- Schmidt, S. K., E. M. S. Gendron, K. Vincent, A. J. Solon, P. Sommers, Z. R. Schubert, L. Vimercati, D. L. Porazinska, J. L. Darcy, and P. Sowell. 2018. Life at extreme elevations on Atacama volcanoes: The closest thing to Mars on Earth? Antonie Van Leeuwenhoek 111 (8):1389–401. doi:10.1007/s10482-018-1066-0.
- Schmidt, S. K., R. C. Lynch, A. J. King, D. Karki, M. S. Robeson, L. Nagy, M. W. Williams, M. S. Mitter, and K. R. Freeman. 2011. Phylogeography of microbial phototrophs in the dry valleys of the high Himalayas and Antarctica. Proceedings of the Royal Society B 278:702–08. doi:10.1098/rspb.2010.1254.
- Schmidt, S. K., D. R. Nemergut, A. E. Miller, K. R. Freeman, A. J. King, and A. Seimon. 2009. Microbial activity and diversity during extreme freeze–thaw cycles in periglacial soils, 5400 m elevation, Cordillera Vilcanota, Perú. Extremophiles 13 (5):807–16. doi:10.1007/s00792-009-0268-9.
- Segawa, T., R. Matsuzaki, N. Takeuchi, A. Akiyoshi, F. Navarro, S. Sugiyama, T. Yonezawa, and H. Mori. 2018. Bipolar dispersal of red-snow algae. Nature Communications 9 (1):3094. doi:10.1038/s41467-018-05521-w.
- Solon, A. J., L. Vimercati, J. L. Darcy, P. Arán, D. Porazinska, C. Dorador, M. E. Farías, and S. K. Schmidt. 2018. Microbial communities of high-elevation fumaroles, penitentes, and dry tephra “soils” of the Puna de Atacama volcanic zone. Microbial Ecology 76 (2):340–51. doi:10.1007/s00248-017-1129-1.
- Sommers, P., J. L. Darcy, D. L. Porazinska, E. M. S. Gendron, A. G. Fountain, F. Zamora, K. Vincent, K. M. Cawley, A. Solon, L. Vimercati, et al. 2019. Comparison of microbial communities in the sediments and water columns of frozen cryoconite holes in the McMurdo Dry Valleys, Antarctica. Frontiers in Microbiology. doi:10.3389/fmicb.2019.00065.
- Takeuchi, N. 2009. Temporal and spatial variations in spectral reflectance and characteristics of surface dust on Gulkana Glacier, Alaska Range. Journal of Glaciology 55 (192):701–09. doi:10.3189/002214309789470914.
- Takeuchi, N., and S. Kohshima. 2004. A snow algal community on a Patagonian glacier, Tyndall Glacier in the Southern Patagonia Icefield. Arctic, Antarctic, and Alpine Research 36 (1):92–99. doi:10.1657/1523-0430(2004)036[0092:ASACOT]2.0.CO;2.
- Takeuchi, N., S. Kohshima, and K. Seko. 2001. Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a Himalayan glacier: A granular algal mat growing on the glacier. Arctic, Antarctic, and Alpine Research 33 (2):115–22. doi:10.1080/15230430.2001.12003413.
- Vimercati, L., J. L. Darcy, and S. K. Schmidt. 2019. The disappearing periglacial ecosystem atop Mt. Kilimanjaro supports both cosmopolitan and endemic microbial communities. Scientific Reports. In press.
- Vimercati, L., S. Hamsher, Z. Schubert, and S. K. Schmidt. 2016. Growth of a high-elevation Cryptococcus sp. during extreme freeze-thaw cycles. Extremophiles 20 (5):579–88. doi:10.1007/s00792-016-0844-8.
- Vincent, W. F. 2000. Cyanobacterial dominance in the polar regions. In The ecology of cyanobacteria, ed. B. A. Whitton and M. Potts, 321–440. Dordrecht: Springer.
- Xiao, X., H. Sogge, K. Lagesen, A. Tooming-Klunderud, K. S. Jakobsen, and T. Rohrlack. 2014. Use of high throughput sequencing and light microscopy show contrasting results in a study of phytoplankton occurrence in a freshwater environment. PloS One 9 (8):e106510. doi:10.1371/journal.pone.0106510.
- Zhang, X., X. Ma, N. Wang, and T. Yao. 2009. New subgroup of Bacteroidetes and diverse microorganisms in Tibetan plateau glacial ice provide a biological record of environmental conditions. FEMS Microbiology Ecology 67 (1):21–29. doi:10.1111/j.1574-6941.2008.00604.x.
