 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
High-elevation ecosystems will experience increasing periods of above-average warmth and altered precipitation changes because of climate change. This causes uncertainties for community properties such as productivity and biodiversity. Increasing temperature may increase productivity by increasing growing season length and metabolic rate or decrease productivity by causing drought stress. Competitive outcomes between species may change with altered climatic conditions, causing shifts in community composition. This study investigates the resistance of aboveground biomass and plant community composition of montane and alpine grassland ecosystems to abruptly altered temperature and precipitation conditions. Intact plant-soil communities were translocated downslope spanning an elevational gradient of 2,090 m in the European Alps. We hypothesize that increasing temperature leads to (1) increased aboveground biomass in the absence of precipitation deficits, (2) decreased species richness, and (3) shifts in plant community composition. After one year of exposure to their new environment, aboveground biomass changes appeared to be dependent on precipitation regimes, whereas species richness declined consistently with changed climatic conditions. No deterministic shift in community composition was found. Abrupt changes in climatic conditions can lead to rapid responses of community properties, indicating that these high-elevation communities may have low initial resistance to future heat waves and droughts.
Introduction
Temperature rise because of anthropogenic climate change is expected to be most extreme at high latitudes and elevations (Ceppi et al. Citation2012; Gobiet et al. Citation2014; Pepin et al. Citation2015). Temperature is a fundamental regulator of chemical and biological processes and is likely to both directly and indirectly affect plant community properties (Rustad et al. Citation2001). Properties such as biomass production and species diversity can be tightly linked (Fraser and Pither Citation2015; Hautier et al. Citation2015), and studying their joint responses to rapid environmental shifts can reveal additional properties of communities, such as resistance (Kreyling et al. Citation2017). Increased temperature within a single season can affect these properties in cold biomes by extending growing seasons for plants because of changes in snow regimes (Myneni et al. Citation1997; Inouye and Wielgolaski Citation2003; Laternser and Schneebeli Citation2003; Klein et al. Citation2016; Asam et al. Citation2017). In addition, higher metabolic rates at higher temperatures (Billings and Mooney Citation1968; Lucht et al. Citation2002; Larcher Citation2003) may increase the plant productivity of cold-adapted ecosystems. Despite these potential gains in productivity, plants may simultaneously suffer from even short-term increases in temperature. At more extreme temperatures, this may occur directly via the damage of tissue or overheated photosystems (Larcher Citation2003; Buchner et al. Citation2015). Although, well before such extremes, temperature can indirectly reduce productivity via reduced soil-water availability resulting from increased evapotranspiration and altered precipitation regimes (De Boeck et al. Citation2016). These conflicting drivers may explain why experimental warming studies of grasslands revealed contradicting results on productivity effects. Negative effects of increased temperature on productivity have been found in both in situ warming experiments (De Boeck et al. Citation2008) and translocation experiments (Egli et al. Citation2004); positive productivity effects have also been found in both in situ warming experiments (de Valpine and Harte Citation2001; Wan et al. Citation2005) and translocation experiments (Sebastià Citation2007). Additional studies have found no effect of warming on productivity (Dukes et al. Citation2005) or results that varied temporally or with soil moisture conditions (Harte and Shaw Citation1995; Cantarel, Bloor, and Soussana Citation2013). But to our knowledge it remains unclear which underlying mechanisms prevail and which environmental conditions cause a change in the importance of both conflicting drivers. The stability and resistance of community diversity to changing conditions may conserve ecosystem functions, such as productivity (Hodgson, McDonald, and Hosken Citation2015; Donohue et al. Citation2016). Given this lack of consensus in warming experiments and the risk of increased warm and dry periods in cold-biome ecosystems, continued research on the resistance of communities to abrupt environmental changes is needed.
Resource acquisition and allocation strategies determine species coexistence, as plants compete for limiting resources (Harpole et al. Citation2016). Temperature changes may alter plant-resource availability through multiple pathways, such as increased decomposition rate (Gavazov Citation2010; García-Palacios et al. Citation2013) or the depletion of soil moisture (Schär et al. Citation1999; Jung et al. Citation2010). Increased microbial activity may increase nitrogen availability (Rustad et al. Citation2001; Wang et al. Citation2016), potentially alleviating belowground competition and shifting it toward aboveground competition for light (Hautier, Niklaus, and Hector Citation2009; Borer et al. Citation2014). Such gains or reductions in plant-resource availability may shift competitive outcomes among species of an established community, leading to species loss or community-structure reorganization. An extended growing season may also shift the competition pattern and subsequently the community composition because cold-adapted species might be less adapted to leverage the extended growing season into higher growth and fertility (Wipf Citation2010). This can result because of the sensitivity of increased exposure to frost events as the insulating effect of snow is lost (Wipf, Rixen, and Mulder Citation2006), or by advantaging early emerging species that preempt resource uptake (Mwangi et al. Citation2007).
Thus, in montane and alpine grasslands future warm periods are likely to promote fast adapting, fast growing, high-statured species such as graminoids, which are able to benefit efficiently from the changed resource availability (Bret-Harte et al. Citation2004; Veen et al. Citation2015; Klanderud et al. Citation2017) and therefore outcompete others. Especially graminoids, with their taller stature, were shown to profit from increased temperature and nutrient availability and hence outcompete smaller-statured growth forms for light and space (Theurillat and Guisan Citation2001; Klanderud, Vandvik, and Goldberg Citation2015). In the absence of dispersal, this should lead to deterministic decreases in species richness by outcompeting species unable to adapt to the novel biotic and abiotic environment. These losses may be mitigated or even reversed, however, if novel plant species colonize into an existing community (Engler et al. Citation2009; Alexander, Diez, and Levine Citation2015; Rixen and Wipf Citation2017). Nonetheless, the life cycle and demographic properties of species will cause the processes underlying the colonization of new habitats to lag behind the speed of change (Alexander et al., Citation2017; Dullinger et al. Citation2012).
Here, we investigate community resistance by examining the initial, one-year responses of plant communities to simulated, abrupt changes in temperature and precipitation climatic conditions on montane and alpine grasslands using an experimental downslope translocation of intact plant-soil monoliths in the European Alps. We hypothesize that:
Aboveground biomass increases with increasing temperature in the absence of severe precipitation differences.
In the absence of colonization, increasing temperature leads to a loss of species because of shifts in interspecific competition reflected in shifting functional group dominance and light transmission.
Community composition will shift with increasing temperature and both diverge compared to the original composition (between translocation sites) and show decreased variability relative to its original composition because of deterministic competitive exclusion (within translocation sites).
Materials and methods
Study sites
This study was conducted along an elevation gradient ranging from 350 m to 2,440 m a.s.l. across Germany (DE), Austria (AU), and Switzerland (CH) in the European Alps. Six representative grassland sites ranging from colline to alpine ecosystems were selected for downslope translocation of intact plant-soil monoliths. These six sites were Bayreuth (DE, 350 m a.s.l.), Fendt (DE, 550 m a.s.l.), Graswang (DE, 900 m a.s.l.), Esterberg (DE, 1,300 m a.s.l.), Stubai (AU, 1,850 m a.s.l.), and Furka (CH, 2,440 m a.s.l.). All of the selected grasslands are seminatural, with the exception of alpine Furka, where natural grasslands occur above the tree line. For a description of the environmental site conditions see .
Table 1. Geographic, climatic, and plant sociologic characteristics of the study sites along the elevational gradient in the European Alps from low to high. All vegetation-period specific values are site specific. Data shown were calculated from on-site weather-station data. Long-term data for Esterberg are not available.
Experimental setup
In the summer of 2016, a total of 126 intact plant-soil monoliths were taken from native grasslands by inserting in PVC tubes with a diameter of 30 cm (similar method to Kreyling et al. Citation2017; Wu et al. Citation2012) using a modified jackhammer. This experimental unit size is sufficient to allow for studying the integrative responses to a multitude of community interactions in small statured grasslands (Milbau et al. Citation2007). The sampling depth was variable: At the four low- to mid-elevation sites (DE) monoliths were taken with a depth of 40 cm, but because of shallow soils and increasing stoniness, this depth was reduced to 25 cm for monoliths from Stubai (AU) and Furka (CH). The bottoms of the monolith units were left open to allow for water flow. The monoliths were taken with the turf 1–2 cm below the rim to minimize microclimatic effects while avoiding excess water runoff. The shallow soils of Furka (CH) prevented this, so the monoliths were instead filled from the bottom with local soil to achieve the desired distance between upper rim and turf. After excavation of the monoliths at their site of origin, they were translocated downslope to the recipient sites and dug into the extant vegetation with the rim of the monolith flush to its surrounding. Monoliths were installed in raised beds at the lowest (Bayreuth, DE) and highest (Furka, CH) location. This was done because of high groundwater tables at Bayreuth and to avoid excessive disturbance of the sensitive habitat at Furka. Monoliths of the four German sites, ranging from 1,350 m to 350 m a.s.l., were translocated to each site with a lower elevation than the monolith origin. Monoliths originating from Stubai (AU, 1,850 m a.s.l.) and Furka (CH, 2,440 m a.s.l.) were translocated only to Bayreuth to test the ecological limits of alpine and subalpine grasslands reacting to abrupt changes in environmental conditions. The high sensitivity of these grasslands to disturbance minimized the number of replicates that could be extracted, preventing translocation to intermediate elevations. At all study sites, additional sets of monoliths were translocated within the respective site as control monoliths (see for an overview of replicates from origin and recipient sites, as well as ). The installation of site-specific control monoliths allowed us to control for unintended treatment effects resulting from the extraction and the PVC tubes themselves (e.g., isolation from surrounding soil, which might hamper or inhibit lateral subsurface flows, root damage, etc.).
Table 2. Experimental setup. Number of monoliths translocated from origin (rows) to recipient sites (columns).
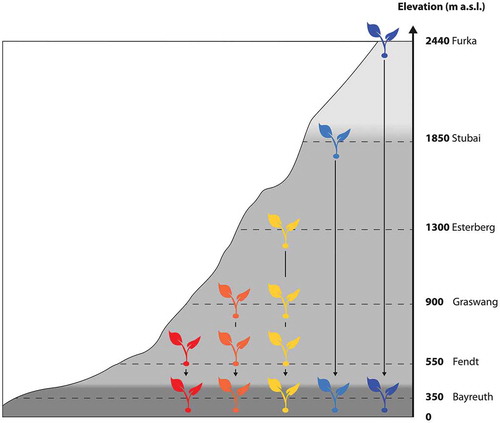
Figure 1. Scheme of experimental setup. Each colored plant represents nine plant-soil monoliths, either translocated as control at the respective origin or to a specific recipient site. Colors of plants represent the investigated temperature gradient, from cold (blue) to warm (red). The grey scale of the mountain represents ecological zones spanned along this elevational gradient, ranging from colline (low elevation) to montane to alpine (high elevation).
Measured environmental and ecological parameters
Within the first ten days after snowmelt in spring 2017, sensors for soil moisture (EcH2O 5-TM, Decagon Devices Inc., USA) were installed horizontal at 5 cm depth together with data loggers (EcH2O Em50, Decagon Devices Inc., USA) using one monolith from each origin at each recipient site. Data were recorded at 15 min intervals to allow fine scale resolution, but were aggregated to daily means for this study. Additionally, on-site weather station data were used to determine precipitation amounts and growing degree days (GDD) as a proxy for thermal time available for plant growth. The GDD was calculated as the sum of the area beneath a sinusoidal curve that was fit to the minimum and maximum daily temperature, with a lower bound of 5°C (Baskerville and Emin Citation1969). This provides a site-specific estimate for the thermal energy available for plants, although it should be noted that with increasing elevation, the thermal microclimate of plants becomes increasingly decoupled from air temperature (Scherrer and Körner Citation2009). Here, we present data for the entire calendar year until the date of harvest as well as for the site-specific growing-season length of 2017. Growing season was defined as the number of days between the first five consecutive days with mean air temperature above 5°C (Zhang et al. Citation2011) and the date of harvest; both dates are site specific.
During the 2017 growing season, aboveground biomass of each monolith was harvested 3 cm above ground level at the time of peak biomass at each site. Aboveground biomass was sorted into single vascular plant species, inventoried to provide species richness per monolith, and then dried at 60°C for 48 h and weighed. In 2016, all monoliths were harvested after translocation at peak season to provide a baseline of aboveground production in subsequent years. Because of harvests in the previous year, litter had not accumulated. Dead or senesced tissue from the 2017 growing season was included in the species-specific biomass. This represented one full year of exposure to the climate of the recipient sites.
The percentage of green cover of each monolith was estimated weekly by a visual survey during the growing season in Bayreuth, always by the same observer (from March 16 to July 10, 2017). This was measured as a proxy of environmental stress, as senesced tissue may indicate either drought stress or water limitation (De Boeck et al. Citation2016).
We quantified light transmission to ground level by measuring the photon flux density of photosynthetically active radiation (PAR) immediately before harvest for all monoliths. We measured PAR above vegetation and at ground level to build a ratio of intercepted light by vegetation structure aboveground. This integrated measure of light transmission was used as a proxy for aboveground competition and shifting resource limitation (from below- to aboveground) in relation to hypothesis 2. We conducted these measurements using a light-ceptometer (AccuPAR LP-80, Decagon Devices Inc., USA) with the calibration set to 30 cm gauge length to capture PAR along the full diameter of the monolith.
Statistical analyses
The treatment effects for all response parameters were calculated as relative changes compared to the control.
with Sample a single translocated monolith of a specific origin and Control the mean of all excavated and reinserted control monoliths of the same specific origin.
Data were checked for normality and homoscedasticity. All analyses were done using R Statistics version 3.3.1 (R Core Team) and the packages “nlme” and “lsmeans.” We tested the effects of translocation on aboveground biomass and species richness, using linear models with the origin of the monolith with either changes in elevation, GDD, or precipitation as predictors. Additionally, we tested for interactions between origin and each environmental variable. The effects of translocation on changes in relative proportion to community aboveground biomass of the plant functional groups graminoids, forbs, and legumes were tested in the same way to detect possible dominance shifts in communities’ plant functional group composition in relation to hypothesis 3. Then, to compare recipient site-specific differences, we used linear mixed-effect models to test whether recipient sites had differing effects on the relative changes in aboveground biomass and total species richness with site of origin as a random factor. We then used a post hoc TukeyHSD to examine each pairwise comparison. To test if the change in aboveground biomass or species richness at a single recipient site was significantly different from zero we used similar linear mixed-effect models without intercept.
To demonstrate the robustness of our monolith approach, we report several additional components of our monolith communities in the supplementary material. First, mean species richness and mean maximum relative abundance (percentage of total aboveground biomass produced by the most abundant species within a monolith) of each origin’s control and translocated monoliths demonstrates that our monoliths were of sufficient size for examining community dynamics. Second, we demonstrate that plant communities were initially indistinguishable following translocation and experimental setup in 2016. We examined the origin-specific effects of translocations on aboveground biomass and richness to different recipient sites from initial data collected in 2016 using ANOVA, followed by a TukeyHSD post hoc test for origins with multiple recipient sites. This comparison was unavailable for the Esterberg site because of recent mowing immediately before monolith extraction.
We tested for mechanisms underlying changes in species richness and aboveground biomass by correlating the relative changes of (1) biomass on light transmission and species richness, (2) light transmission on species richness, and (3) graminoid dominance (relative abundance of graminoids) on species richness, using linear mixed-effect models with a unique origin-recipient site ID nested within the site of origin as random effects.
Because of the observed non-linearity, we used generalized additive models (GAM) to model the observed green cover versus day of the year. GAM was implemented through the “mgcv” package in R using cubic regression splines and cross-validation to select the optimal amount of smoothers for estimating how green cover was predicted by day of the year interacting with monolith origin. Thus, model estimates are composed of six smoothing functions and an intercept value.
Differences in community composition were tested using the modified PERMANOVA approach proposed by Bacaro, Gioria, and Ricotta (Citation2012). Bray-Curtis dissimilarity is an abundance-weighted beta diversity metric and was calculated for pairwise combinations of monoliths. For each combination of a translocation treatment (n = 9) and its control (n = 9) the following pairwise comparisons were made: Each monolith at the control site compared to all others at that site (labeled “within-control”), each monolith at the site of translocation compared to all others at that site (labeled “within-translocation”), and each monolith at the control site compared to each monolith at the translocation site (labeled “between”). To minimize the number of statistical comparisons made, we only tested for differences between the two “within” groups (e.g., does translocation to a given site cause monolith communities to become more or less alike one another than those at the site of origin?) and the “between” group to the “within-control” group (e.g., does translocation cause monolith communities to become more dissimilar than control monolith communities are to each other?). To avoid issues of inherent non-independence that arise as a result of each monolith being used for multiple comparisons, an F statistic was calculated for observed differences between each overall statistical comparison made, followed by 9999 Mantel randomizations of the observed values within the dissimilarity matrix and recomputation of the F statistic. A p value was calculated based on the percentage of randomized F statistics that were larger than the observed F statistic. Because of the multiple comparisons made, we adjusted the 0.05 level of significance using Bonferroni corrections for the number of comparisons made within each origin. This allowed us to be cautious in our interpretation of significant differences, while avoiding being overly conservative in these adjustments.
Results
Aboveground biomass
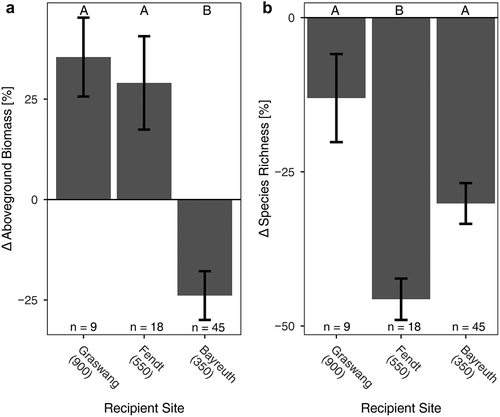
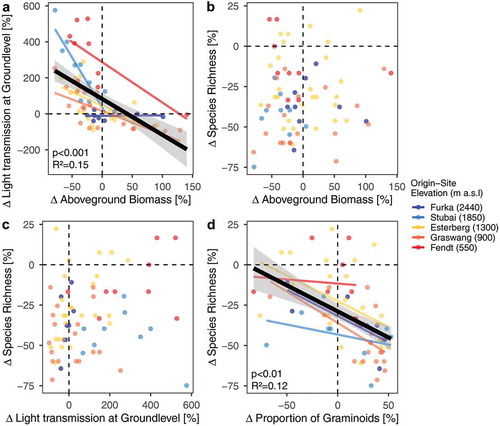
Downslope translocation led to increased thermal time (GDD) for all translocated monoliths, except for the translocation from Fendt (550 m a.s.l.) to Bayreuth (350 m a.s.l.). However, the increased thermal time of Fendt relative to Bayreuth is entirely the result of the five additional days until harvest in Fendt, as the sites were otherwise equivalent. All monoliths that were translocated to the lowest site (Bayreuth, 350 m a.s.l.) experienced a substantial decrease in precipitation (). After a full year of exposure to these new environments, changes in aboveground biomass of the montane and alpine grassland communities did not correlate with elevational distance or thermal time (GDD) changes because of translocation (effect of elevation: p = .17, R2 = 0.05; effect of GDD: p = .7, R2 = 0.04). However, aboveground biomass positively responded to changes in precipitation (effect of precipitation: p < .001, R2 = 0.2; ). Recipient sites of translocated plant communities had different influences on the relative change of aboveground biomass (p < .001; F = 18.51). Aboveground biomass increased for all communities that were translocated to the intermediate recipient sites Graswang (900 m a.s.l.) and Fendt (550 m a.s.l.) on average by 35 percent (p < .01) and 29 percent (p < .01), respectively, even where precipitation was slightly reduced (). However, the overall aboveground biomass decreased for communities that were translocated to the warmest, driest elevation site Bayreuth (350 m a.s.l.) by an average of 24 percent (p < .05; see ). However, the aboveground biomass of communities originating from Fendt (decrease) and Furka (increase) showed no significant change after translocation to the warmest and driest site. Of note is the contrasting pattern of aboveground biomass between the two lowermost recipient sites, which both had reduced precipitation relative to higher sites but to different extents. This indicates a threshold of soil-water limitation. We found no significant effect of change in elevational distance, thermal time (GDD), or precipitation on the relative proportion of the plant functional groups graminoids, forbs, or legumes to community aboveground biomass (see Supplementary Table 1).
Figure 2. (a) Aboveground biomass and (b) Species richness change of plant communities in response to changes in elevation, growing degree days and precipitation resulting from downslope translocation. Significant influence of altered environmental conditions are shown as a black line with grey-shaded 95% confidence intervals. Significance of model factors indicated by asterisk (*** p < .001; ** p < .01; * p < .05; n.s non-significant) and overall model R2 are displayed in the lower right corner of the respective panel. Mean and standard error are displayed in all graphs. For the control monoliths mean and standard error was calculated for all controls grouped. Color code of legend is valid for all panels.
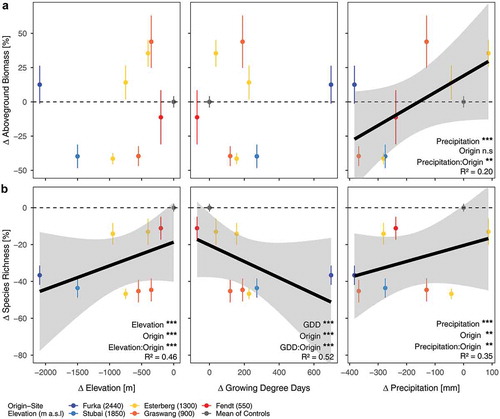
Figure 3. (a) Aboveground biomass and (b) species richness change in plant communities (monoliths) after one year of passive warming by translocation. Relative change for plant communities of all origins translocated downslope to the respective recipient site (m a.s.l. given) grouped together. Replicates at each recipient site given at the bottom of each panel. Mean and standard error are displayed in all graphs. Letters indicate significant differences between recipient sites as results of TukeyHSD post hoc tests conducted after ANOVA p < .001.
Species richness
The species richness of plant communities at all recipient sites consistently decreased with translocation. Thus, after the first year of exposure after downslope translocation, the species richness decline of montane and alpine grasslands was highly correlated with the change in elevation, thermal time, and precipitation (effect of elevation: p < .001, R2 = 0.46; effect of GDD: p < .001, R2 = 0.52; effect of precipitation: p < .001, R2 = 0.35; ). The recipient sites had a significant influence on the relative decrease of species richness (F = 6.6; p < .01). Species richness decreased in monoliths translocated to Graswang (900 m a.s.l.), Fendt (550 m a.s.l.), and Bayreuth (350 m a.s.l.) by a mean relative change of 13 percent (p < .05), 46 percent (p < .001), and 30 percent (p < .001), respectively ().
The mean species richness of the control monoliths ranges from 6.0 species to 20.4 species. While there is variation between origins, we see a significant loss of species in four of five origins at their respective transplanted sites (ranging from a mean species richness of 5.33 to 15.4; Supplementary Figure 1a). Further, the mean maximum relative abundance of control monoliths ranges from 29.1 percent to 48.5 percent, suggesting that single species (and thus individuals) are not dominating the monoliths. For translocated monoliths this generally increased (origin means ranging from 40.6 percent to 68.2 percent), showing a significant increase in two of the five origins (Supplementary Figure 1b).
Green cover
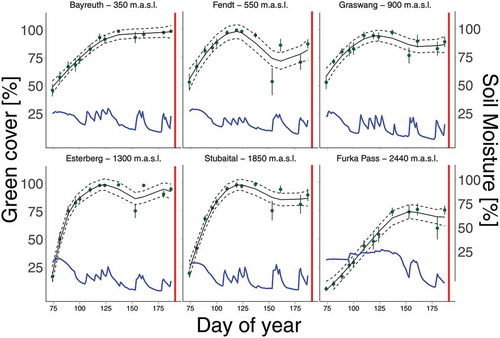
The amount of green cover in plant communities after a full year of exposure to warming at the lowest site (Bayreuth, 350 m a.s.l.) decreased during mid-summer in periods coinciding with low soil moisture measured in the monoliths (). After translocation to the lowest elevation site, the rates of increase and amount of green cover of all plant communities from higher elevations—irrespective of elevational distance translocated—differ significantly from the local control communities of the lowest site (p < .001 for all origins, R2DOY x Origin = 0.77). Plant communities from the lowest elevation site (Bayreuth) showed high resistance to dry periods regarding green cover. In contrast, green cover of plant communities originating from intermediate elevation decreased after an initial, less pronounced dry period of ten days in late May with only one 0.87 mm precipitation event. Notably, plant communities from the highest elevation alpine site (Furka) were generally slower to green up, maintained higher soil-moisture values during the initial dry period, and decreased in green cover only after a second dry period of 17 d without any precipitation in mid-June.
Figure 4. Green cover of translocated plant communities to the lowest elevation site (Bayreuth) showing different speed in greening up and different reaction to low soil-moisture availability. Green cover modeled as GAM shown as solid green line with 95 percent confidence intervals (dashed lines). Blue lines indicate soil moisture for the specific site of origin at the lowest elevation site (350 m a.s.l.). Red line shows harvest date at the recipient site (Bayreuth).
Light transmission and graminoid proportion within plant communities
Light transmission at the ground level of plant communities decreased significantly as aboveground biomass increased (p < .001, R2 = 0.15; see ). Yet, species richness was uncorrelated to both aboveground biomass (p = .23; see ) and light transmission at ground level (p = .35; see ). A significant but weak correlation of decreasing species richness with increasing relative proportion of graminoids to community aboveground biomass was found (p < .01, R2 = 0.12; see ).
Figure 5. Changes in aboveground biomass and species richness showing relationship of relative change compared to the specific controls of (a) aboveground biomass versus light transmission, (b) aboveground biomass versus species richness, (c) light transmission versus species richness, and (d) proportion of graminoids versus species richness. Black lines with grey-shaded 95 percent confidence intervals are the overall model estimate; R2 and p values are given if significant. Colored lines represent site-of-origin model estimates.
Beta diversity
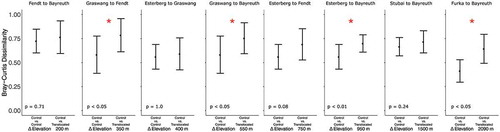
Bray-Curtis dissimilarity (abundance-weighted beta diversity) did not vary significantly between within-site of origin (control) and within-translocated (warmed in respect of GDD) communities for any site. However, dissimilarity values between control and translocated communities did differ significantly for four of eight translocation combinations spanning a range between 200 m and 2,090 m of elevational distance. These significant differences in beta diversity were found for plant communities originating from the highest alpine site (Furka to Bayreuth, 2,090 m elevational distance, p < .001) and from intermediate montane sites (Esterberg to Bayreuth, 950 m elevational distance, p < .01; Graswang to Bayreuth, 550 m elevational distance; and Graswang to Fendt, 350 m elevational distance, both p < .05), with a fifth translocation (Esterberg to Fendt, 750 m elevational distance) showing a marginal significance after Bonferroni adjustments (p = .08). For a visual display of community dissimilarity results see .
Figure 6. Dissimilarity in community composition among plant communities translocated along an elevational gradient from 2,440 m a.s.l. to 350 m a.s.l. Bray-Curtis dissimilarity values among indicated plant community (monolith) groupings for each monolith origin-translocated pairing. Panels are sorted according to elevational distance traveled by plant communities via translocation. Plotted values are means of all possible pairwise values in the indicated grouping with standard deviation error bars. PERMANOVA was used to test “within control dissimilarities” versus “between control/translocated” dissimilarities. Red asterisks indicate the significance between the translocated and control dissimilarities at p < .05 after adjusting for the multiple comparisons made within each origin group, additional p values are given in the lower left corner of each panel.
Initial state after experimental setup in 2016
In 2016, aboveground biomass increased in the monoliths originating from alpine Furka (2,440 m a.s.l.) and montane Graswang (900 m a.s.l.) after translocation to the lowest site, Bayreuth (350 m a.s.l.; p < .05), while all other translocations were nonsignificant (Supplementary Table 2). This suggests that the short-term residence in Bayreuth may have increased biomass shortly after translocation, but this influence was not evident in the 2017. Species richness in 2016 showed only one significant difference within origins, as Graswang (900 m a.s.l.) monoliths translocated to Fendt (550 m a.s.l.) had higher species richness than those in other sites (p < .05; Supplementary Table 2). This result did not persist in 2017, and was in fact inverted. Ultimately, the process of downslope translocation did not appear to negatively affect the monoliths, which may have been indicated by initial loss of species or aboveground biomass.
Discussion
Abrupt warming following downslope translocation of plant-soil monoliths from montane and alpine grasslands revealed rapid changes in productivity and diversity, indicating low resistance. Consistent with our hypothesis, aboveground biomass increased with temperature in mid-elevation sites where the precipitation regime was similar, but decreased at the lowest elevation site where warming was coupled with a strong reduction in precipitation. Downslope translocation consistently led to species loss, again consistent with our hypothesis. However, contrary to our expectations the detected loss of species richness was independent of variations in aboveground biomass and light transmission. This outcome suggests that increased aboveground competition did not drive species loss. Richness decline was significantly stronger with increasing graminoid abundance, although it was still observed at weaker levels in monoliths that showed reduced graminoid abundance. Together with the observed beta-diversity changes between translocation sites, but not within translocation sites, we conclude that community composition shifts were neither highly deterministic nor converging toward similar communities.
Initial aboveground biomass response to abrupt warming
The observed increases in aboveground biomass of grassland communities at intermediate translocation range is consistent with an increase in the length of the growing season because of an earlier snow melt (Inouye and Wielgolaski Citation2003; Laternser and Schneebeli Citation2003; Ernakovich et al. Citation2014; Gobiet et al. Citation2014) and faster metabolic processes (Lucht et al. Citation2002; Larcher Citation2003). The loss in aboveground biomass within plant communities that were translocated to the lowest site (350 m a.s.l.) may be the result of increasing water limitation (Schär et al. Citation1999, Citation2004; Kreyling et al. Citation2017), which is likely stressful for all the translocated plant communities that originated from regions of higher precipitation. The natural drought periods at the end of May (10 d) and in mid-June (17 d) at the lowest site (Bayreuth, 350 m a.s.l.), coupled with high summer temperatures, may have led to increased transpiration demand in the plants and a higher evaporation rate. This led to depleted soil moisture (Seneviratne et al. Citation2010; Quesada et al. Citation2012; Kreyling et al. Citation2016; Wolf et al. Citation2016) as shown by prolonged troughs in the soil-moisture trend lines in Bayreuth for monoliths of all origins. Low soil-moisture availability reduces stomatal conductivity, photosynthetic rate, and indirectly growth and carbon allocation (De Boeck et al. Citation2006, Citation2007; McDowell et al. Citation2008). Of note is the opposing direction of aboveground biomass changes between monoliths translocated to the two lowest elevation sites (350 and 550 m a.s.l.). These sites had similar amounts of thermal time, but only half the amount of precipitation fell at the lowest site (). Together, this suggests that drought stress induced by high temperature and low precipitation at the lowest site drove the observed decrease in aboveground biomass at the lowest site. The slight reduction in precipitation experienced by communities that were translocated to the intermediate recipient site did not result in reduced aboveground biomass. We speculate that a critical threshold of reduced precipitation underlies the observed contrasting pattern of aboveground biomass between the two lowest recipient sites. This is in line with previous studies stating that aboveground biomass is more sensitive to soil water content than temperature under low precipitation regime (Fei et al. Citation2015; Winkler, Chapin, and Kueppers Citation2016). High-elevation plant communities have been shown to be prone to direct tissue damage by overheating after being translocated to lower elevation if transpirational cooling of the plant tissue is impeded because of limited soil-moisture availability (De Boeck et al. Citation2016). All of these drought effects together with the reduction of precipitation explain the observed reduction in aboveground biomass of plant communities from mid-elevations (550–1850 m a.s.l.) at our lowest and warmest site in Bayreuth at 350 m a.s.l. It underpins the importance of jointly considering both temperature and precipitation effects in experiments seeking the ecological impacts of either variable.
The occurrence of drought stress is supported by the rapid reduction in community green cover within plant communities originating from intermediate elevation (900–1,850 m a.s.l.) compared to the local control originating from the lowest elevation (350 m a.s.l.) during natural drought events. Interestingly, alpine plant communities from the highest elevation (2,440 m a.s.l.) show a delayed decrease in green cover starting only during the second natural drought event (mid-June) compared to the ones from mid-elevations (550 m–1850 m a.s.l.) starting during the first natural drought event (late May). Those high-elevation plant communities green up slower, which may be caused by photoperiod regulation of plant growth (Ernakovich et al. Citation2014). The measured soil moisture of the alpine plant communities remained higher than the measured soil moisture in plant communities from intermediate elevations throughout the year at the lowest elevation site. This may indicate that the edaphic properties of this system buffered the community response, although caution is warranted given the lack of replication in soil-moisture measurements. Hence, our results suggest that montane grasslands may be less resistant to periods of water scarcity than grasslands of high, alpine elevation. Alternatively, as long-lived and clonal species inhabit plant communities of high, alpine elevation, they could be particularly slow in response to changing environments (Dullinger et al. Citation2012; Gritsch, Dirnböck, and Dullinger Citation2016).
Initial diversity response to abrupt warming
The consistent loss of plant species with downslope translocation is remarkable. In particular, mid- to high-elevation plant communities experienced significant species losses within one year at all recipient sites relative to the control mesocosms that were extracted and reinserted at their site of origin. However, as the loss of species richness was correlated with the degree of elevational distance, both temperature and precipitation change, our results suggest that the observed species loss is the result of complex interactions between multiple altered environmental drivers changed by translocation. Interestingly, species richness losses were highest at the second lowest site, where thermal time was similar to the lowest site and precipitation was similar to higher elevation sites. This could be an indication of water limitation restraining competitive exclusion at the lowest elevation site and merits further observation in future years. We note that because of the timing of translocations, monoliths are likely to experience limited colonization pressure from the matrix vegetation in this first year of sampling, meaning that future years could see a reversal in this species-loss pattern as the monoliths are colonized. Previous studies conducted at longer temporal scales did not detect a loss of plant species richness throughout time because of either natural global warming (Vittoz et al. Citation2009; Steinbauer et al. Citation2018) or experimental warming (Price and Waser Citation2000). Conversely, short-term experimental studies detect a loss of species richness after experimental warming (Debouk, de Bello, and Sebastià Citation2015; Sebastià, Kirwan, and Connolly Citation2008), which is aligned with the results presented here. This discrepancy between long- and short-term studies might be because of the time lag of colonization (Alexander et al., Citation2017; Dullinger et al. Citation2012). Additionally, observational studies can generally consider larger spatial scales than experimental studies, which inherently incorporates the role of habitat heterogeneity into maintaining diversity. Nonetheless, short-term studies reveal important aspects of community dynamics, such as the biotic conditions that promote resistance to abrupt changes (Kreyling et al. Citation2017). Our study shows a decrease in species richness that was stronger in communities with increased graminoid dominance. Evidence of alpine habitats suggests that shifts in plant-community composition can occur based on abundance shifts toward more thermophile species that outcompete more cold-adapted species (Gottfried et al. Citation2012). This has also been attributed to competitive interaction among various plant functional groups (Theurillat and Guisan Citation2001) and species resource acquisition and allocation strategies (Aerts Citation1999; Diaz et al. Citation2004). With shifting environmental conditions, the relative strengths of community assembly mechanisms and species interaction have been shown to shift as well (Gellesch et al. Citation2013; He, Bertness, and Altieri Citation2013), including temperature-limited ecosystems such as arctic (Klanderud, Vandvik, and Goldberg Citation2015), montane, and alpine habitats (Callaway et al. Citation2002). While in our study competition was not measured per se, the observed loss of species in relation to beta-diversity patterns, light availability shifts, and functional group shifts informs species interaction effects.
Despite the consistent species loss, observed beta-diversity shifts within one year after translocation were less consistent. Most notably, plant communities originating from subalpine Stubai (1,850 m a.s.l.) showed a significant loss of species, but no significant shift in community dissimilarity when comparing translocated communities with their high-elevation controls. Because our dissimilarity metrics are weighted abundances, this suggests a general loss of rare or low-abundance species with a maintenance of the dominant species in plant communities. The opposite was observed for plant communities originating from mid-elevation Esterberg (1,350 m a.s.l.), with no species loss because of translocation to the lowest elevation site, but a strong increase in abundance-weighted dissimilarity compared to the control communities remaining at the site of origin. This pattern is consistent with dominance shifts, where previously subordinate species are able to take advantage of their new environment at the expense of previously dominant species that are reduced in abundance but still persist as part of the population. Overall, this points to a high amount of local site specificity in predicting grassland changes.
While our observed loss of species is consistent with increasing interspecific competition from colder to warmer mountain habitats (Kikvidze et al. Citation2005), we found no evidence suggesting that aboveground competition induced this species loss, as we did not find any significant correlation of light transmission (a proxy for aboveground competition intensity, sensu DeMalach, Zaady, and Kadmon Citation2016) and the loss of species. Increased temperature has been observed to increase decomposition rates and thus increased nitrogen availability (Gavazov Citation2010; García-Palacios et al. Citation2013; Wang et al. Citation2016). An improved soil-nutrient status often leads to increased light competition (Hautier, Niklaus, and Hector Citation2009; Borer et al. Citation2014; DeMalach, Zaady, and Kadmon Citation2016). Although we did not measure any soil parameters directly, we speculate that the lack of evidence of increased aboveground competition indicates a nonsignificance in changes of soil-nutrient competition in our monoliths. Potentially, any increased nitrogen availability with warming is masked by other temperature-regulated mechanisms, such as soil microbial activity or increasing asynchrony between nitrogen availability and plant growth (Ernakovich et al. Citation2014), causing a high level of belowground competition.
The observation of stronger species richness losses in plant communities with increased graminoid dominance is consistent with other studies that found graminoids to be most responsive to warming in cold-adapted habitats (Dormann and Woodin Citation2002; Brooker Citation2006; Winkler, Chapin, and Kueppers Citation2016). Nonetheless, the relative contribution of plant functional groups to aboveground biomass did not show a correlation with the degree of change of either temperature or precipitation. Furthermore, as species richness losses were also observed in plant communities with strong shifts toward forbs, our results of community composition suggest that non-deterministic processes such as priority effects prevail during initial phases of severe environmental change. If the variance of beta diversity within translocated communities converges with warming (i.e., increased similarity among translocated monoliths), this would support the idea of deterministic processes (Chase and Myers Citation2011; Kreyling, Jentsch, and Beierkuhnlein Citation2011; Segre et al. Citation2014). Overall, this suggests that translocated plant communities are not filtered in the initial stages according to a system-wide competitive hierarchy of resident species. Rather, within-monolith priority effects may allow previously subdominant species to take advantage of the more favorable temperature and to outcompete the now subordinate species (Suding et al. Citation2005), indicating a role of community asynchrony (Ma et al. Citation2017). Our results capture only the first-year initial dynamics of the translocated plant communities. As the monoliths continue to be exposed to their new environments, colonization pressure from the matrix vegetation will likely mitigate or even reverse our observed changes in species richness and composition.
The shift in precipitation regime at our lowest site highlights the uncertainty of precipitation regimes in future climate scenarios and how they are coupled to the effects of temperature on productivity and diversity of grasslands (Backhaus et al. Citation2014; Grant et al. Citation2014a, Citation2014b). Our results underline the importance of understanding the interplay of temperature and precipitation (Easterling et al. Citation2000; Schär et al. Citation2004) in driving grassland community dynamics, especially for abrupt climatic changes. These findings highlight that water supplementation at our lowest and driest site may yield additional insights onto the interactive effects of precipitation and temperature and will be an avenue of future research in this study. This study suggests that climate warming may increase aboveground biomass of montane grasslands as long as it is not coupled with a decrease in precipitation or sustained periods of drought. These montane grasslands are likely not adapted to drought stress during the growing season. The consistent loss of species in the first year indicates significant and rapid reordering of competitive outcomes in these communities, which may lead to unpredictable outcomes in the future at larger temporal and spatial scales. Shifts in composition can lead to challenges for conservation as well as for economy. Species loss or homogenization decreases community asynchrony (Gross et al. Citation2014; Hautier et al. Citation2018) and stability (Hautier et al. Citation2015), and the ability of those communities to buffer extreme events is also reduced, causing less secure ecosystem services in future climates (Wilcox et al. Citation2017). As disparities in responses of biomass production and richness to environmental changes in these systems remain, continued observational and experimental studies are warranted, and joint consideration of temperature and precipitation are critical.
Supplemental Material
Download Zip (278.5 KB)Acknowledgments
We thank Christian Körner, Erika Hiltbrunner, and Michael Bahn for giving valuable feedback during the process of writing, and Ralf Hafner for kindly visualizing the experimental setup.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Bernd Josef Berauer
BB, PW, MS, and AJ conceived the ideas; BB, MS, and AJ designed methodology; BB, PW, MAS, MASAK, PE, AH, and JI collected and processed data; BB, PW, and PE analyzed data; BB led the writing of the manuscript; PW, MASAK, PE, AH, JI, MS, MAS, and AJ assisted writing the manuscript. All authors critically contributed to the drafts and gave final approval for publication.
References
- Aerts, R. 1999. Interspecific competition in natural plant communities: Mechanisms, trade-offs and plant-soil feedbacks. Journal of Experimental Botany 50:29–37. doi:10.1093/jxb/50.330.29.
- Alexander, J. M., J. M. Diez, and J. M. Levine. 2015. Novel competitors shape species’ responses to climate change. Nature 525:515–18. doi:10.1038/nature14952.
- Alexander, J. M., L. Chalmandrier, J. Lenoir, T. I. Burgess, F. Essl, S. Haider, C. Kueffer, K. McDougall, A. Milbau, M. A. Nuñez, et al. 2017. Lags in the response of mountain plant communities to climate change. Global Change Biology. doi:10.1111/gcb.13976.
- Asam, S., M. Callegari, L. De Gregorio, A. Jacob, C. Notarnicola, M. Zebisch, M. Matiu, M. Menzel, and G. Fiore. 2017. Spatiotemporal variations of alpine climate, snow cover and phenology. In 2017 9th International Workshop on the Analysis of Multitemporal Remote Sensing Images (Multitemp). doi:10.1109/Multi-Temp.2017.8035222.
- Bacaro, G., M. Gioria, and C. Ricotta. 2012. Testing for differences in beta diversity from plot-to-plot dissimilarities. Ecological Research 27:285–92. doi:10.1007/s11284-011-0899-z.
- Backhaus, S., J. Kreyling, C. Beierkuhnlein, C. Buhk, L. Nagy, D. Thiel, and A. Jentsch. 2014. A transplantation experiment along climatic gradients suggests limitations of experimental warming manipulations. Climate Research 60:63–71. doi:10.3354/cr01219.
- Baskerville, G. L., and P. Emin. 1969. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology 50:514–17. doi:10.2307/1933912.
- Billings, W. D., and H. A. Mooney. 1968. The ecology of arctic and alpine plants. Biological Reviews 43:481–529. doi:10.1111/j.1469-185X.1968.tb00968.x.
- Borer, E. T., E. W. Seabloom, D. S. Gruner, W. S. Harpole, H. Hillebrand, E. M. Lind, P. B. Adler, J. Alberti, T. Michael Anderson, J. D. Bakker, et al. 2014. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–20. doi:10.1038/nature13144.
- Bret‐Harte, M. S., E. A. García, V. M. Sacré, J. R. Whorley, J. L. Wagner, S. C. Lippert, and F. S. Chapin III. 2004. Plant and soil responses to neighbour removal and fertilization in Alaskan tussock tundra. Journal of Ecology 92 (4):635–647.
- Brooker, R. W. 2006. Plant–plant interactions and environmental change. New Phytologist 171:271–84. doi:10.1111/j.1469-8137.2006.01752.x.
- Buchner, O., M. Stoll, M. Karadar, I. Kranner, and G. Neuner. 2015. Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant, Cell & Environment 38:812–26. doi:10.1111/pce.12455.
- Callaway, R. M., R. W. Brooker, P. Choler, Z. Kikvidze, C. J. Lortie, R. Michalet, B. J. Cook, F. I. Pugnaire, B. Newingham, E. T. Aschehoug, et al. 2002. Positive interactions among alpine plants increase with stress. Nature 417:844–48. doi:10.1038/nature00812.
- Cantarel, A. A. M., J. M. G. Bloor, and J. F. Soussana. 2013. Four years of simulated climate change reduces above-ground productivity and alters functional diversity in a grassland ecosystem. Journal of Vegetation Science 24:113–26. doi:10.1111/j.1654-1103.2012.01452.x.
- Ceppi, P., S. C. Scherrer, A. M. Fischer, and C. Appenzeller. 2012. Revisiting Swiss temperature trends 1959-2008. International Journal of Climatology 32:203–13. doi:10.1002/joc.2260.
- Chase, J. M., and J. A. Myers. 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 366:2351–63. doi:10.1098/rstb.2011.0063.
- De Boeck, H. J., C. M. Lemmens, C. Zavalloni, B. Gielen, S. Malchair, M. Carnol, J. Van den Berge, R. Ceulemans, and I. Nijs. 2008. Biomass production in experimental grasslands of different species richness during three years of climate warming. Biogeosciences 5:585–94. doi:10.5194/bgd-4-4605-2007.
- De Boeck, H. J., C. M. H. M. Lemmens, H. Bossuyt, S. Malchair, M. Carnol, R. Merckx, and R. Ceulemans. 2006. How do climate warming and plant species richness affect water use in experimental grasslands? Plant and Soil 288:249–61. doi:10.1007/s11104-006-9112-5.
- De Boeck, H. J., C. M. H. M. Lemmens, S. Vicca, J. Van den Berge, S. Van Dongen, I. A. Janssens, and I. Nijs. 2007. How do climate warming and species richness affect CO 2 fluxes in experimental grasslands? New Phytologist 175:512–22. doi:10.1111/j.1469-8137.2007.02122.x.
- De Boeck, H. J., S. Bassin, M. Verlinden, M. Zeiter, and E. Hiltbrunner. 2016. Simulated heat waves affected alpine grassland only in combination with drought. New Phytologist 209:531–41. doi:10.1111/nph.13601.
- de Valpine, P., and J. Harte. 2001. Plant responses to experimental warming in a montane meadow. Ecology 82:637–48. doi:10.1890/0012-9658(2001)082[0637:PRTEWI]2.0.CO;2.
- Debouk, H., F. de Bello, and M.-T. Sebastià. 2015. Functional trait changes, productivity shifts and vegetation stability in mountain grasslands during a short-term warming. PLoS One 10:e0141899. doi:10.1371/journal.pone.0141899.
- DeMalach, N., E. Zaady, and R. Kadmon. 2016. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecology Letters 1–10. doi:10.1111/ele.12706.
- Diaz, S., J. G. Hodgson, K. Thompson, M. Cabido, J. H. C. Cornelissen, A. Jalili, M. R. Zak, J. P. Grime, F. Zarrinkamar, Y. Asri, et al. 2004. The plant traits that drive ecosystems: Evidence from three continents. Journal of Vegetation Science 15:295–304. doi:10.1658/1100-9233(2004)015[0295:TPTTDE]2.0.CO;2.
- Donohue, I., H. Hillebrand, J. M. Montoya, O. L. Petchey, S. L. Pimm, M. S. Fowler, Q. Yang, A. L. Jackson, M. Lurgi, D. McClean, et al. 2016. Navigating the complexity of ecological stability. Ecology Letters 19:1172–85. doi:10.1111/ele.12648.
- Dormann, C. F., and S. J. Woodin. 2002. Climate change in the Arctic: Using plant functional types in a meta-analysis of field experiments. Functional Ecology 16:4–17. doi:10.1046/j.0269-8463.2001.00596.x.
- Dukes, J. S., N. R. Chiariello, E. E. Cleland, L. A. Moore, M. Rebecca Shaw, S. Thayer, C. B. Field, H. A. Mooney, and M. Loreau. 2005. Responses of grassland production to single and multiple global environmental changes. PLoS Biology 3:e319. doi:10.1371/journal.pbio.0030319.
- Dullinger, S., A. Gattringer, W. Thuiller, D. Moser, N. E. Zimmermann, A. Guisan, K. Hülber, C. Plutzar, M. Leitner, T. Mang, et al. 2012. Extinction debt of high-mountain plants under twenty-first-century climate change. Nature Climate Change 2:619–22. doi:10.1038/nclimate1514.
- Easterling, D. R., J. L. Evans, P. Y. Groisman, T. R. Karl, K. E. Kunkel, and P. Ambenje. 2000. Observed variability and trends in extreme climate events: A brief review. Bulletin of the American Meteorological Society 81:417–25. doi:10.1175/1520-0477(2000)081<0417:OVATIE>2.3.CO;2.
- Egli, M., C. Hitz, P. Fitze, and A. Mirabella. 2004. Experimental determination of climate-change effects on above-ground and below-ground organic matter in alpine grasslands by translocation of soil cores. Journal of Plant Nutrition and Soil Science 167:457–70. doi:10.1002/jpln.200321333.
- Engler, R., C. F. Randin, P. Vittoz, T. Czáka, M. Beniston, N. E. Zimmermann, and A. Guisan. 2009. Predicting future distributions of mountain plants under climate change: Does dispersal capacity matter? Ecography 32:34–45. doi:10.1111/j.1600-0587.2009.05789.x.
- Ernakovich, J. G., K. A. Hopping, A. B. Berdanier, R. T. Simpson, E. J. Kachergis, H. Steltzer, and M. D. Wallenstein. 2014. Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Global Change Biology 20:3256–69. doi:10.1111/gcb.12568.
- Fei, P., X. Manhou, Y. Quangang, Z. Xuhui, W. Tao, and X. Xian. 2015. Different responses of soil respiration and its components to experimental warming with contrasting soil water content. Arctic, Antarctic, and Alpine Research 47:359–68. doi:10.1657/AAAR0014-018.
- Fraser, L. H., and J. Pither. 2015. Worldwide evidence of a unimodal relationship between productivity and plant species richness. Science (New York, N.Y.) 349:287–90. doi:10.1126/science.aaa7974.
- García-Palacios, P., F. T. Maestre, J. Kattge, and D. H. Wall. 2013. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecology Letters 16:1045–53. doi:10.1111/ele.12137.
- Gavazov, K. S. 2010. Dynamics of alpine plant litter decomposition in a changing climate. Plant and Soil 337:19–32. doi:10.1007/s11104-010-0477-0.
- Gellesch, E., R. Hein, A. Jaeschke, C. Beierkuhnlein, and A. Jentsch. 2013. Biotic interactions in the face of climate change. 321–49. Berlin, Heidelberg: Springer. doi:10.1007/978-3-642-30967-0_12.
- Gobiet, A., S. Kotlarski, M. Beniston, G. Heinrich, J. Rajczak, and M. Stoffel. 2014. 21st century climate change in the European Alps—A review. Science of the Total Environment 493:1138–51. doi:10.1016/J.SCITOTENV.2013.07.050.
- Gottfried, M., H. Pauli, A. Futschik, M. Akhalkatsi, P. Barančok, J. L. Benito Alonso, G. Grabherr, J. Dick, B. Erschbamer, M. R. Fernández Calzado, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2:111–15. doi:10.1038/nclimate1329.
- Grant, K., J. Kreyling, L. F. H. Dienstbach, C. Beierkuhnlein, and A. Jentsch. 2014a. Water stress due to increased intra-annual precipitation variability reduced forage yield but raised forage quality of a temperate grassland. Agriculture, Ecosystems and Environment 186:11–22. doi:10.1016/j.agee.2014.01.013.
- Grant, K., J. Kreyling, H. Heilmeier, C. Beierkuhnlein, and A. Jentsch. 2014b. Extreme weather events and plant–plant interactions: Shifts between competition and facilitation among grassland species in the face of drought and heavy rainfall. Ecological Research 29:991–1001. doi:10.1007/s11284-014-1187-5.
- Gritsch, A., T. Dirnböck, and S. Dullinger. 2016. Recent changes in alpine vegetation differ among plant communities. Journal of Vegetation Science 27:1177–86. doi:10.1111/jvs.12447.
- Gross, K., B. J. Cardinale, J. W. Fox, A. Gonzalez, M. Loreau, H. W. Polley, and J. van Ruijven. 2014. Species richness and the temporal stability of biomass production: A new analysis of recent biodiversity experiments. The American Naturalist 183:1–12. doi:10.1086/673915.
- Harpole, W. S., L. L. Sullivan, E. M. Lind, J. Firn, P. B. Adler, E. T. Borer, P. D. Wragg, P. A. Fay, Y. Hautier, H. Hillebrand, et al. 2016. Addition of multiple limiting resources reduces grassland diversity. Nature 537:1–9. doi:10.1038/nature19324.
- Harte, J., and R. Shaw. 1995. Shifting dominance within a montane vegetation community: results of a climate-warming experiment. Science 267:876–80. doi:10.1126/science.267.5199.876.
- Hautier, Y., D. Tilman, F. Isbell, E. W. Seabloom, E. T. Borer, and P. B. Reich. 2015. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348:336–40. doi:10.1126/science.aaa5139.
- Hautier, Y., F. Isbell, E. T. Borer, E. W. Seabloom, W. S. Harpole, E. M. Lind, A. Hector, C. J. Stevens, P. B. Adler, J. Alberti, et al. 2018. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nature Ecology & Evolution 2:50–56. doi:10.1038/s41559-017-0395-0.
- Hautier, Y., P. A. Niklaus, and A. Hector. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science (New York, N.Y.) 324:636–38. doi:10.1126/science.1169640.
- He, Q., M. D. Bertness, and A. H. Altieri. 2013. Global shifts towards positive species interactions with increasing environmental stress. Ecology Letters 16:695–706. doi:10.1111/ele.12080.
- Hodgson, D., J. L. McDonald, and D. J. Hosken. 2015. What do you mean, ‘resilient’? Trends in Ecology & Evolution 30:503–06. doi:10.1016/J.TREE.2015.06.010.
- Inouye, D. W., and F. E. Wielgolaski. 2003. High altitude climates. 195–214. Dordrecht: Springer. doi:10.1007/978-94-007-0632-3_13.
- Jung, M., M. Reichstein, P. Ciais, S. I. Seneviratne, J. Sheffield, M. L. Goulden, K. Zhang, A. Cescatti, J. Chen, R. de Jeu, et al. 2010. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature 467:951–54. doi:10.1038/nature09396.
- Kikvidze, Z., F. I. Pugnaire, R. W. Brooker, P. Choler, C. J. Lortie, R. Michalet, and R. M. Callaway. 2005. Linking patterns and processes in alpine plant communities: A global study. Ecology 86:1395–400. doi:10.1890/04-1926.
- Klanderud, K., E. Meineri, J. Töpper, P. Michel, and V. Vandvik. 2017. Biotic interaction effects on seedling recruitment along bioclimatic gradients: Testing the stress-gradient hypothesis. Journal of Vegetation Science 28:347–56. doi:10.1111/jvs.12495.
- Klanderud, K., V. Vandvik, and D. Goldberg. 2015. The importance of biotic vs. abiotic drivers of local plant community composition along regional bioclimatic gradients. PLoS One 10:1–14. doi:10.1371/journal.pone.0130205.
- Klein, G., Y. Vitasse, C. Rixen, C. Marty, and M. Rebetez. 2016. Shorter snow cover duration since 1970 in the Swiss Alps due to earlier snowmelt more than to later snow onset. Climatic Change 139:637–49. doi:10.1007/s10584-016-1806-y.
- Kreyling, J., A. Jentsch, and C. Beierkuhnlein. 2011. Stochastic trajectories of succession initiated by extreme climatic events. Ecology Letters 14:758–64. doi:10.1111/j.1461-0248.2011.01637.x.
- Kreyling, J., J. Dengler, J. Walter, N. Velev, E. Ugurlu, D. Sopotlieva, A. Jentsch, C. Picon-Cochard, I. Nijs, P. Hernandez, et al. 2017. Species richness effects on grassland recovery from drought depend on community productivity in a multisite experiment. Ecology Letters 20:1405–13. doi:10.1111/ele.12848.
- Kreyling, J., M. A. S. Arfin Khan, F. Sultana, W. Babel, C. Beierkuhnlein, T. Foken, J. Walter, and A. Jentsch. 2016. Drought effects in climate change manipulation experiments: Quantifying the influence of ambient weather conditions and rain-out shelter artifacts. Ecosystems 1–15. doi:10.1007/s10021-016-0025-8.
- Larcher, W. 2003. Physiological plant ecology: ecophysiology and stress physiology of functional groups. Berlin: Springer.
- Laternser, M., and M. Schneebeli. 2003. Long-term snow climate trends of the Swiss Alps (1931-99). International Journal of Climatology 23:733–50. doi:10.1002/joc.912.
- Lucht, W., I. C. Prentice, R. B. Myneni, S. Sitch, P. Friedlingstein, W. Cramer, P. Bousquet, W. Buermann, and B. Smith. 2002. Climatic control of the high-latitude vegetation greening trend and Pinatubo effect. Science (New York, N.Y.) 296:1687–89. doi:10.1126/science.1071828.
- Ma, Z., H. Liu, Z. Mi, Z. Zhang, Y. Wang, W. Xu, and J.-S. He. 2017. Climate warming reduces the temporal stability of plant community biomass production. Nature Communications 8:15378. doi:10.1038/ncomms15378.
- McDowell, N., W. T. Pockman, C. D. Allen, D. D. Breshears, N. Cobb, T. Kolb, E. A. Yepez, J. Sperry, A. West, D. G. Williams, et al. 2008. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist 178:719–39. doi:10.1111/j.1469-8137.2008.02436.x.
- Milbau, A., D. Reheul, B. De Cauwer, and I. Nijs. 2007. Factors determining plant–neighbour interactions on different spatial scales in young species-rich grassland communities. Ecological Research 22:242–47. doi:10.1007/s11284-006-0018-8.
- Mwangi, P., M. Schmitz, C. Scherber, C. Roscher, J. Schumacher, M. Scherer-Lorenzen, and B. Schmid. 2007. Niche pre-emption increases with species richness in experimental plant communities. Journal of Ecology 95:65–78. doi:10.1111/j.1365-2745.2006.01189.x.
- Myneni, R. B., C. D. Keeling, C. J. Tucker, G. Asrar, and R. R. Nemani. 1997. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. doi:10.1038/386698a0.
- Pepin, N., R. S. Bradley, H. F. Diaz, M. Baraer, E. B. Caceres, N. Forsythe, H. Fowler, G. Greenwood, M. Z. Hashmi, X. D. Liu, et al. 2015. Elevation-dependent warming in mountain regions of the world. Nature Climate Change. doi:10.1038/nclimate2563.
- Price, M. V., and N. M. Waser. 2000. Responses of subalpine meadow vegetation to four years of experimental warming. Ecological Applications 10:811–23. doi:10.1890/1051-0761(2000)010[0811:ROSMVT]2.0.CO;2.
- Quesada, B., R. Vautard, P. Yiou, M. Hirschi, and S. I. Seneviratne. 2012. Asymmetric European summer heat predictability from wet and dry southern winters and springs. Nature Climate Change 2:736–41. doi:10.1038/nclimate1536.
- Rixen, C., and S. Wipf. 2017. Non-equilibrium in alpine plant assemblages: Shifts in Europe’s summit floras. 285–303. Cham: Springer. doi:10.1007/978-3-319-55982-7_12.
- Rustad, L. E., J. L. Campbell, G. M. Marion, R. J. Norby, M. J. Mitchell, A. E. Hartley, R. Wright, and J. Gurevitch. 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–62. doi:10.1007/s004420000544.
- Schär, C., D. Lüthi, U. Beyerle, and E. Heise. 1999. The soil–precipitation feedback: A process study with a regional climate model. Journal of Climate 12:722–41. doi:10.1175/1520-0442(1999)012<0722:TSPFAP>2.0.CO;2.
- Schär, C., P. L. Vidale, D. Lüthi, C. Frei, C. Häberli, M. A. Liniger, and C. Appenzeller. 2004. The role of increasing temperature variability in European summer heatwaves. Nature 427:332–36. doi:10.1038/nature02300.
- Scherrer, D., and C. Körner. 2009. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Global Change Biology 16:2602–13. doi:10.1111/j.1365-2486.2009.02122.x.
- Sebastià, M. T. 2007. Plant guilds drive biomass response to global warming and water availability in subalpine grassland. Journal of Applied Ecology 44:158–67. doi:10.1111/j.1365-2664.2006.01232.x.
- Sebastià, M.-T., L. Kirwan, and J. Connolly. 2008. Strong shifts in plant diversity and vegetation composition in grassland shortly after climatic change. Journal of Vegetation Science 19:299–306. doi:10.3170/2008-8-18356.
- Segre, H., R. Ron, N. De Malach, Z. Henkin, M. Mandel, and R. Kadmon. 2014. Competitive exclusion, beta diversity, and deterministic vs. stochastic drivers of community assembly. Ecology Letters 17:1400–08. doi:10.1111/ELE.12343.
- Seneviratne, S. I., T. Corti, E. L. Davin, M. Hirschi, E. B. Jaeger, I. Lehner, and A. J. Teuling. 2010. Investigating soil moisture–climate interactions in a changing climate: A review. Earth-Science Reviews 99:125–61. doi:10.1016/J.EARSCIREV.2010.02.004.
- Steinbauer, M. J., J.-A. Grytnes, G. Jurasinski, A. Kulonen, J. Lenoir, H. Pauli, S. Wipf, M. Winkler, M. Bardy-Durchhalter, E. Barni, et al. 2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556:231–34. doi:10.1038/s41586-018-0005-6.
- Suding, K. N., S. L. Collins, L. Gough, C. Clark, E. E. Cleland, K. L. Gross, and S. Pennings. 2005. Functional-and abundance-based mechanisms explain diversity loss due to N fertilization. http://www.pnas.org/content/pnas/102/12/4387.full.pdf.
- Theurillat, J.-P., and A. Guisan. 2001. Potential impact of climate change on vegetation in the European Alps: A review. Climatic Change 50:77–109. doi:10.1023/A:1010632015572.
- Veen, G. F., (Ciska), M. K. Sundqvist, D. Metcalfe, and S. D. Wilson. 2015. Above-ground and below-ground plant responses to fertilization in two subarctic ecosystems. Arctic, Antarctic, and Alpine Research 47:693–702. doi:10.1657/AAAR0014-085.
- Vittoz, P., C. Randin, A. Dutoit, F. Bonnet, and O. Hegg. 2009. Low impact of climate change on subalpine grasslands in the Swiss Northern Alps. Global Change Biology 15:209–20. doi:10.1111/j.1365-2486.2008.01707.x.
- Wan, S., D. Hui, L. Wallace, and Y. Luo. 2005. Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Global Biogeochemical Cycles 19:1–13. doi:10.1029/2004GB002315.
- Wang, C., Z. Chen, S. Unteregelsbacher, H. Lu, S. Gschwendtner, R. Gasche, M. Dannenmann, R. Vernimmen, and F. Siegert. 2016. Climate change amplifies gross nitrogen turnover in montane grasslands of Central Europe in both summer and winter seasons. Global Change Biology 22:2963–78. doi:10.1111/gcb.13353.
- Wilcox, K. R., A. T. Tredennick, S. E. Koerner, E. Grman, L. M. Hallett, M. L. Avolio, Y. Zhang, G. R. Houseman, F. Isbell, D. S. Johnson, et al. 2017. Asynchrony among local communities stabilises ecosystem function of metacommunities. Ecology Letters 20:1534–45. doi:10.1111/ele.12861.
- Winkler, D. E., K. J. Chapin, and L. M. Kueppers. 2016. Soil moisture mediates alpine life form and community productivity responses to warming. Ecology 97:1555–65. doi:10.1890/15-1197.1.
- Wipf, S. 2010. Phenology, growth, and fecundity of eight subarctic tundra species in response to snowmelt manipulations. Plant Ecology 207:53–66. doi:10.1007/s11258-009-9653-9.
- Wipf, S., C. Rixen, and C. P. H. Mulder. 2006. Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Global Change Biology 12:1496–506. doi:10.1111/j.1365-2486.2006.01185.x.
- Wolf, S., T. F. Keenan, J. B. Fisher, D. D. Baldocchi, A. R. Desai, A. D. Richardson, I. T. van der Laan-Luijkx, B. E. Law, M. E. Litvak, N. A. Brunsell, et al. 2016. Warm spring reduced carbon cycle impact of the 2012 US summer drought. Proceedings of the National Academy of Sciences of the United States of America 113:5880–85. doi:10.1073/pnas.1519620113.
- Wu, Z., P. Dijkstra, G. W. Koch, and B. A. Hungate. 2012. Biogeochemical and ecological feedbacks in grassland responses to warming. Nature Climate Change 2:458–61. doi:10.1038/nclimate1486.
- Zhang, X., L. Alexander, G. C. Hegerl, P. Jones, A. K. Tank, T. C. Peterson, B. Trewin, and F. W. Zwiers. 2011. Indices for monitoring changes in extremes based on daily temperature and precipitation data. Wiley Interdisciplinary Reviews: Climate Change 2:851–70. doi:10.1002/wcc.147.
