ABSTRACT
A warming climate has been shown to drive thermophilization—shifts in species abundance toward those adapted to warm and dry conditions. The community dynamics shaping this process have been proposed to vary between temperature-limited alpine plant communities and those that are both temperature and moisture limited. In nine sites across the xeric alpine zone in the White Mountains, California, USA, we experimentally increased summertime temperature and precipitation for three seasons and quantified community responses with a climatic niche analysis. We asked if thermophilization occurred in response to experimental heating, and if this effect was ameliorated by experimental watering. Under experimentally warmer conditions, we found no change in the mean community-weighted climatic niche (CCN); however, thermophilization of this community was observed based on a shift in the seventy-fifth percentile of the CCN and an increase in the proportional abundance of the hottest, driest adapted species. In addition, total vegetation abundance increased and species richness decreased with heating. Experimental watering did not ameliorate these effects of heating. Together, these results suggest that warming in arid alpine areas may result in less diverse plant communities dominated by hot, dry associated species, although short-term responses may be limited because of community lags.
Introduction
Mountain systems provide important early indicators of plant community responses to changing climate (Körner Citation2003; Grabherr, Gottfried, and Pauli Citation2010; Rixen and Wipf Citation2017; Steinbauer et al. Citation2018); climate warming is more pronounced at higher elevations (Pepin et al. Citation2015), and exposure to climate change is exacerbated in xeric mountain ranges because of shifts from snow-dominated to rain-dominated precipitation (McCullough et al. Citation2016). Alpine species, often cool-adapted and long-lived, are particularly sensitive to temperature change mediated either directly via their physiology and demography or indirectly via competitive exclusion by faster growing species that recruit from lower elevations (Cranston et al. Citation2015; Rumpf et al. Citation2017; Graae et al. Citation2018). Moreover, the physical isolation of mountain ranges creates large geographic barriers to species movement at regional scales (Dirnböck, Essle, and Rabitsch Citation2011). However, the topographic complexity of mountains creates a variety of potentially suitable microrefugia that may allow alpine species to persist at local scales (Scherrer and Körner Citation2011; Spasojevic et al. Citation2013; Winkler et al. Citation2016b; Graae et al. Citation2018). Understanding how the changing climate drives community shifts across these isolated, topographically heterogeneous landscapes can facilitate predictions of the potential vulnerability of these socio-ecologically important ecosystems (Pecl et al. Citation2017).
Observational and experimental studies of alpine species reveal distributional and community shifts in response to warming conditions (Walther, Beißner, and Burga Citation2005; Gottfried et al. Citation2012; Morueta-Holme et al. Citation2015; Suding et al. Citation2015; Lesica and Crone Citation2016; Winkler, Chapin, and Kueppers Citation2016a). Recent work using resurveys of mountaintops spanning more than 100 years has linked accelerating increases in alpine plant diversity to increases in temperature (Steinbauer et al. Citation2018). The newly colonized species in this study exhibit traits associated with lower altitudes, suggesting that species moving upslope are driving this pattern of increasing diversity (Steinbauer et al. Citation2018). A warming climate has been specifically shown to drive thermophilization shifts in species abundance toward those adapted to warm and dry conditions (Bertrand et al. Citation2011; Gottfried et al. Citation2012; Savage and Vellend Citation2015; Vanneste et al. Citation2017). This process is shaped by the loss of or decrease in the abundance of cryophilic species, and a gain or increase in abundance of thermophilic species (Gottfried et al. Citation2012). Although fine-scale microclimatic gradients driven by topography may buffer species loss in response to warmer conditions (Scherrer and Körner Citation2011; Lenoir et al. Citation2013; Patsiou, Conti, and Zimmermann Citation2014), community shifts are likely to occur as competition and facilitation dynamics play out across fine-scale gradients of climate and substrate type (Alexander et al. Citation2018; Kulonen et al. Citation2018). Further, competitive exclusion by low elevation-associated species invading alpine vegetation may more strongly shape community responses to a warming climate than the change in climate itself (Alexander, Diez, and Levine Citation2015).
Interactions between temperature and precipitation also influence these plant communities’ responses to changing climatic conditions (Rapacciuolo et al. Citation2014; Harsch and Hille Ris Lambers Citation2016). Trends in distributional shifts of alpine plants in response to recent warming in water-limited Mediterranean mountains differ from shifts in temperature-limited boreal-temperate mountains (Pauli et al. Citation2012). The impact of warming on alpine community dynamics has also been shown to be dependent on soil-moisture availability experimentally, with the potential for positive effects on abundance and productivity under wetter conditions and negative effects under drier conditions (Elmendorf et al. Citation2012; Winkler, Chapin, and Kueppers Citation2016a). Changes in snow-melt timing because of the interactive effects of snow persistence on temperature exposure (i.e., snow protection from winter cold) and soil-moisture availability also have variable species-specific effects on alpine plants (Wipf, Stoeckli, and Bebi Citation2009). Overall, studies of alpine plant responses to climate change have predominantly focused on temperature-limited systems (Körner Citation2003; Gottfried et al. Citation2012). Mountain ranges in semi-arid regions, such as western North America, where temperature and moisture both play strong roles in the determination of the distribution of alpine communities, require more research on community responses to changing climate (Isard Citation1986; Cavieres et al. Citation2006; Lesica and Crone Citation2016; Winkler, Chapin, and Kueppers Citation2016a). Spatially replicated tests of the independent and interactive effects of temperature and moisture will elucidate how alpine plant communities may shift across the complex topography of a single mountain range.
We performed such a test in the White Mountains, United States, which have experienced marked change in water balance with twentieth-century climate change (Rundel, Gibson, and Sharifi Citation2008; Rapacciuolo et al. Citation2014). Climatic water deficit (CWD), an integrative measure of the relationship between temperature and precipitation availability, has increased by approximately 20 mm (hotter, drier conditions) in this region since mid-century (Flint et al. Citation2013; Rapacciuolo et al. Citation2014). The alpine zone in the White Mountains is unusually xeric and depauperate in plant diversity relative to alpine areas worldwide (Körner Citation2003). Historical resurveys indicate that there have already been shifts in the alpine communities in this mountain range with a changing climate (Kopp and Cleland Citation2014). Between 1961 and 2010 there was an upward shift of the subalpine shrub Artemisia rothrockii and a decrease in the abundance of multiple alpine cushion plants at the subalpine–alpine ecotone attributed to a concurrent decrease in mean annual precipitation and an increase in summer minimum temperature (Kopp and Cleland Citation2014).
In this study, we simultaneously manipulated both temperature and moisture availability and quantified the alpine plant community responses in nine locations across the alpine zone of this xeric mountain range. We asked whether thermophilization of the communities occurs in response to experimental heating, and if this effect is ameliorated by experimental watering. Because of potential community lags shaped by the physical and biological characteristics of the alpine zone (rugged topography, long-lived species, limited dispersal; Alexander et al. Citation2018), we hypothesize that thermophilization will be primarily driven by the shifts in species proportional abundances rather than species immigration or local extinction. Specifically, with warming we expect to observe an increase in the abundance of species with higher climatic niche values—more hot, dry adapted—relative to ambient aggregate community climatic niches. In addition, we hypothesize that experimental watering will reduce the thermophilization of the community with warming by limiting the loss of cool, wet adapted species. Overall, only with both warmer and drier conditions do we expect to see shifts in the alpine community climatic niche across this mountain range.
Methods
Study site and design
This study was conducted across the White Mountains, a narrow, steep range that spans the California–Nevada border in the western United States. The alpine zone in the White Mountains extends from 3,500 m to 4,344 m (White Mountain peak). The rain shadow of the nearby Sierra Nevada leads to a reduction in precipitation delivered to the White Mountains, particularly for wintertime snow (Lloyd and Mitchell Citation1973; Rundel, Gibson, and Sharifi Citation2008). Sitting at an intersection between multiple floristic regions, the White Mountain alpine flora originates from the Sierra Nevada, Great Basin/Mojave desert, and Rocky Mountain floras (Billings Citation1978; Morefield Citation1992) and is predominantly made up of long-lived, slow-growing perennials, including chamaephytes, cushion plants, herbaceous dicots, and graminoids (Spira Citation1987; Rundel, Gibson, and Sharifi Citation2008; Kopp and Cleland Citation2014). Many common alpine species of the White Mountains can also be found in the nearby Sierra Nevada range, as well as the surrounding lower montane and cold desert landscapes (Rundel, Gibson, and Sharifi Citation2008).
The alpine plant community was surveyed in nine sites that span the alpine zone in the White Mountains during the 2015, 2016, and 2017 summer growing seasons. These study years ranged from drought years (2015) to high snowpack years (2017) in the White Mountains and the annual environmental variability varied widely across sites (Oldfather and Ackerly Citation2018). Because lithology has a large effect on plant community composition in the White Mountains (Van de Ven, Weiss, and Ernst Citation2007), our study targeted a single rock type (granite) to focus on the spatial effects of temperature and soil moisture. Restricting our sites to this rock type precluded covering the highest elevation plant communities in the White Mountains. Additionally, this study was part of a larger project examining the demography of a target species (Ivesia lycopodioides var. scandularis; Oldfather and Ackerly Citation2018); as a result, the communities are those found in locations where this species occurs, and tend to be on somewhat deeper soils and flatter slopes compared to the landscape overall (Pollak Citation1997).
In each of these nine sites, we manipulated climatic conditions with a full factorial experiment augmenting growing-season temperature and precipitation for three summers (2015, 2016, 2017; see for details). Summertime temperature was increased with passive warming chambers installed each season within a week of snow melt-out, and removed after the end of each growing season to avoid winter damage. The chambers were constructed of double-walled polycarbonate and were hexagonal with a height of 31 cm and a width of 41 cm at the base, narrowing to an opening with a width of 28 cm at the top. Summer precipitation was manipulated by the evenly spaced addition of one liter of water applied five times per plot throughout the summer growing season (11.1 mm of rain-equivalent per storm event culminating in approximately 56 mm addition during the course of the entire summer; a 55 percent increase in historical mean summertime precipitation). Overall, the experimental design resulted in from two to four independent replicates of the four experimental states (ambient, heated, watered, heated and watered) in each of the nine sites.
Figure 1. Schematic of study design across the elevational range of alpine habitat in the White Mountains, CA, USA. Sites (white diamonds) are numbered in ascending order from low to high elevation. In each of the sites, 30 × 30 cm plots were randomly assigned to one of four treatments (ambient, heated, watered, heated and watered) with a replication of two plots per experimental treatment (and a replication of four plots for the ambient treatment in all sites, except site 2 and site 3, which also had a replication of two plots for the ambient treatment). The heating treatment was performed using passive warming chambers (shown in the top right in sites 9, 5, and 1). Species abundance was quantified in each plot in 2015, 2016, and 2017 as the frequency of presence in each cell (gridded cells shown in the bottom right).
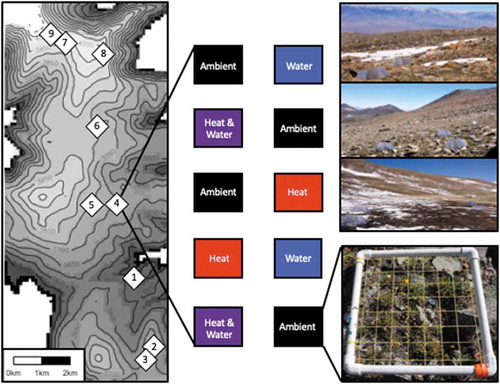
Between August 1 and 15 in each of three years (2015–2017) we quantified the presence and frequency of all alpine plants in eight to ten 30 × 30 cm plots within each of the nine sites. Each square plot was divided into forty-nine 4 × 4 cm sub-squares with a 1 cm buffer on all sides of the plot. The frequency of each species was counted as the number of cells in which live tissue was present in a plot and served as our metric for species abundance. In each plot, we also performed an ocular estimate of percent cover of live vegetation, rock, scree, bare ground, and litter.
The impacts of the treatments and the ambient environmental conditions of the sites were assessed with iButton Thermochrons (Maxim, San Jose, CA, USA; installed 2 cm below the soil surface in each plot and sampling every four hours) and manual measurements of soil moisture with a Hydrosense TDR (Campbell Scientific, Logan, USA) conducted at intervals of two days after application of additional water within a timespan of two days for all sites (resulting in a minimum of five sampling periods for each growing season). For each plot, accumulated degree days were calculated as the sum of mean daily soil temperatures for days above 0°C within the summer growing season (May–August), summertime soil moisture was calculated as the mean soil moisture across all sampling events, and days of snow cover was calculated as the number of days between October and June with less than 0.5°C diel variability in soil temperature (Harte and Shaw Citation1995). Number of snow-covered days was used to quantify the variation in snowpack dynamics in this site because limited wintertime precipitation in this mountain range drives long-time periods during the winter without snow (exposing plants to subfreezing temperature) and multiple snow melt-out events during the early growing season.
Climatic niche analysis
We quantified thermophilization as a shift in a community-weighted climatic niche (CCN) for each site. A CCN is an abundance-weighted metric of a community (similar to community-weighted mean traits) derived from the component species’ niches, and can be based on any climate variable or indicator variable of interest (Gottfried et al. Citation2012; De Frenne et al. Citation2013). The weighting gives abundant species a larger effect on the community’s CCN. We based the CCN on climatic water deficit (CWD), an important climatic determinant of vegetation distributions that takes into account the influence of both temperature and soil moisture, as well as their combined seasonality (Stephenson Citation1990). CWD is an integrative measure of the sum of energy availability (potential evapotranspiration) in excess of water supply (precipitation), with higher values indicating hotter, drier conditions, and lower values indicating cooler, wetter conditions (Stephenson Citation1990; Flint et al. Citation2013).
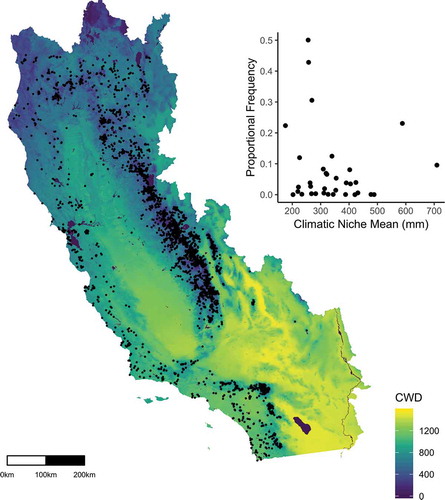
The niches of the component species were calculated based on their current regional distributions across California (Lee-Yaw et al. Citation2016). Specimen localities of all species present in our study were compiled from a recently published extract from the Consortium of California Herbaria specimen database filtered for taxonomic and geographic accuracy (Baldwin et al. Citation2017; ). Using a 270 m raster of average CWD (1981–2010) for California, we extracted CWD values for each specimen locality (Flint et al. Citation2013). The species-specific climatic niche was calculated as the average of the extracted CWD value across all localities for each species. The mean and the twenty-fifth and seventy-fifth percentile CCN for each plot in each site was calculated using these species-specific climatic niches and weighted by the frequency of each species in each plot.
Figure 2. Map of climatic water deficit (CWD; mm) for California with points representing the locality data for all study species used to calculate the species climatic niche means. In subset, the relationship between the proportional frequency averaged across all sites for each species and the species climatic niche mean.
We first examined how the CCN values across our study sites related to the elevational and climatic gradients to test if our climatic niche metric based on species regional distributions was representative of species local distributions. For this analysis we used linear mixed-effects models, using only data from non-manipulated (ambient) plots. For the elevation gradient, the CCN of each ambient plot was regressed against the elevation (linear and quadratic) with random effects of year and plot. For the climatic gradients, the ambient CNN was regressed against the site-specific summertime accumulated degree days, summertime soil moisture, and days of persistent snowpack averaged across years, with random effects of year and plot.
To assess thermophilization with the experimental warming and watering, we compared the mean, twenty-fifth, and seventy-fifth percentiles of the CCN in experimental plots relative to ambient plots within each site. Shifts in the mean CCN represent mean changes in the community climatic niche and shifts in the upper and lower percentiles represent changes in the presence or abundance of the hottest and coolest adapted species, respectively (De Frenne et al. Citation2013). We built linear mixed-effects models with CCN (mean and percentiles) as the response variable with fixed additive and multiplicative effects of the experimental manipulations and random effects of site, plot, and year.
We examined whether the proportional abundance of each species was dependent on the species (i) having a lower or higher climatic niche relative to the aggregated ambient CCN for each site (j) (∆niche (i,j) = specific-species climatic nichei – CCNj). Negative ∆niche values indicate that a species has a low climatic niche (more cool, wet adapted) relative to the abundant species in that site, and positive ∆niche values indicate that a species has a high climatic niche (more hot, dry adapted) relative to the abundant species in that site. This analysis of treatment effect on proportional abundance was performed within each year (as opposed to a temporal analyses) to examine whether there was an overall community response to the manipulations in both drought (2015) and high snowpack years (2017). To test whether the experimental manipulations interacted with ∆niche to shape species’ proportional abundance we built a linear mixed-effects model with species-specific abundance as a response variable, fixed effects of ∆niche, heating, watering, and their three-way interaction, and random effects of site, plot, year, and species.
Finally, we examined changes in species richness and vegetation percent cover in response to the climate manipulations with separate linear mixed-effects models, including the fixed effects of heating and watering, as well as their interaction, and the random effects of site and year. We explored whether species climatic niche means were predictive of species presence under each treatment using a generalized linear mixed-effects model (Binomial error structure) with presence/absence as the response variable, fixed effects of the species-specific climatic niche mean, heating, watering, and their three-way interaction, and random effects of site, plot, and year.
All modeling was performed in R 3.4.3 with the lme4 package (Bates et al. Citation2015; R Core Team Citation2018). The significance of each parameter from all models was estimated using Type II Wald χ2 tests and the differences between treatment groups was determined by contrast tests with Tukey adjustments to account for multiple comparisons. The variance explained (pseudo-R2) for the mixed-effects modeling was determined using the MuMin package (Nakagawa and Schielzeth Citation2013).
Results
Climatic conditions across sites and treatments
The nine study sites spanned elevational (3,494–4,028 m) as well as temperature, soil moisture, and snow persistence gradients. Under ambient conditions and averaged across all years accumulated degree days ranged from 732°C to 1,289°C, mean summertime soil moisture ranged from 9 percent to 33 percent, and mean numbers of days with snow cover ranged from 62 to 171. The open-top chambers significantly increased the daily minimum temperatures by 0.8°C (χ2 = 54.78, df = 1, p < .001) and the accumulated degree days by 53°C (approximately 5 percent increase relative to ambient conditions) (χ2 = 11.006, df = 1, p = .009). The heating treatment did not have a measurable drying effect (χ2 = 328, df = 1, p = 0.214). There was also no measurable effect of the watering treatment on the soil moisture for any of the sites (χ2 = 141, df = 1, p = .792; see the “Discussion”).
Climatic niche analysis
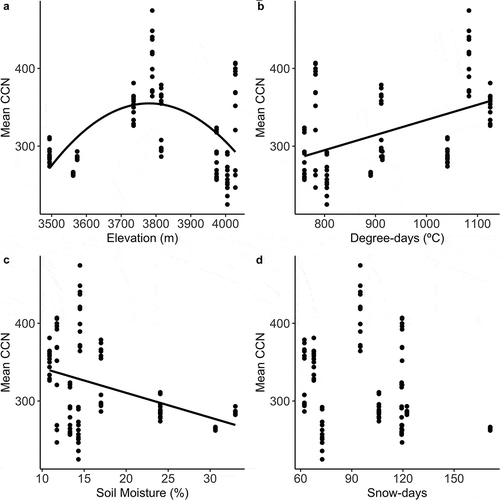
Climatic niche values for the species found across these sites ranged widely (CWD = 176 mm–710 mm) and the most abundant species were widely distributed across the range of CWD species means (, ). The mean CCN across all sites ranged from 196 mm to 498 mm, the CCN twenty-fifth percentile ranged from 167 mm to 308 mm, and the CCN seventy-fifth percentile ranged from 216 mm to 764 mm. Variation in mean CCN was explained by both the elevation (marginal R2 = 0.25, conditional R2 = 0.94) and the climatic gradients (marginal R2 = 0.39, conditional R2 = 0.94). CCN increased with accumulated degree days (βDegree-days = 0.246, p = .001) and decreased with available soil moisture (βSoil-moisture = − 4.624, p = .003; ). In contrast, the mean CCN did not vary predictably across the snow persistence gradient (βSnow-days = 0.474, p = .174; ). Across the geographic gradient (elevation), the mean ambient CCN was highest at mid-elevations (βElevation = 7.571, p = .001; βElevation2 = − 0.001, p = .001; ).
Table 1. Study species with the number of sites that the species were present in, mean frequency within a plot for sites in which the species were present, and species-specific climatic niche means (CNM) based on the species regional distribution.
Figure 3. Mean ambient community-weighted climatic niche (CCN) across plots in relation to the (a) elevational and (b–d) climatic gradients. Note that climate values were averaged across the ambient plots, so they are shared by all plots within a site. Lines represent significant model fits.
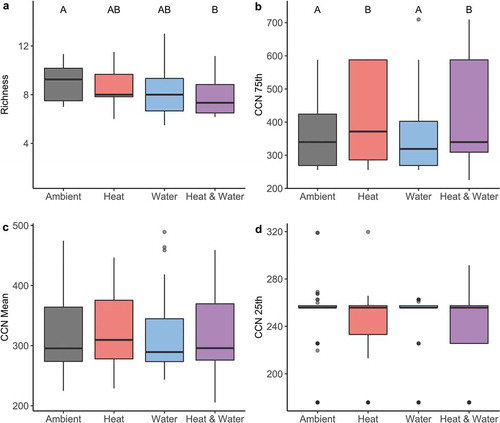
The CCN seventy-fifth percentile was greater under experimental heating (marginal R2 = 0.02, conditional R2 = 0.86; βheat = 39.978, p = .013; ). When averaged across the watering treatments, there was a significant difference between the ambient and heated plots across all sites (pambient-heat = 0.014); under ambient conditions the mean seventy-fifth percentile CCN was 366 mm and under heated conditions the mean seventy-fifth percentile was 413 mm (an increase of 12.8 percent). This greater upper percentile of the CCN indicates that heating drove an increase in the presence and/or abundance of the species adapted to the hottest, driest conditions—evidence of thermophilization. This effect was not mediated by the watering treatment (χ2water = 0.246, df = 1, p = .620; χ2heat*water = 0.164, df = 1, p = .645). Also, despite a general trend of the mean CCN being greater with heating and an amelioration of this effect with watering (), the experimental manipulations had no significant effect on the mean CCN (χ2heat = 0.930, df = 1, p = .335; χ2water = 0.0001, df = 1, p = .995; χ2heat*water = 0.086, df = 1, p = .769) or the twenty-fifth percentile CCN (χ2heat = 0.866, df = 1, p = .352; χ2water = 0.310, df = 1, p = .578; χ2heat*water = 0.985, df = 1, p = .321; ). CCN dynamics across years are shown in Supplementary Figure 1b–d.
Figure 4. (a) Species richness deceased with experimental heating and watering. (b) The CCN seventy-fifth percentile significantly increased with the experimental heating. Letters indicate significantly different groups. (c) Mean CCN responses to climate manipulations show a non-significant trend of higher mean CCN with heating, and a lessening of this effect when heating is in combination with experimental watering. There are no significant differences between any of the treatment groups. (d) The CCN twenty-fifth percentile had no significant response to the treatments.
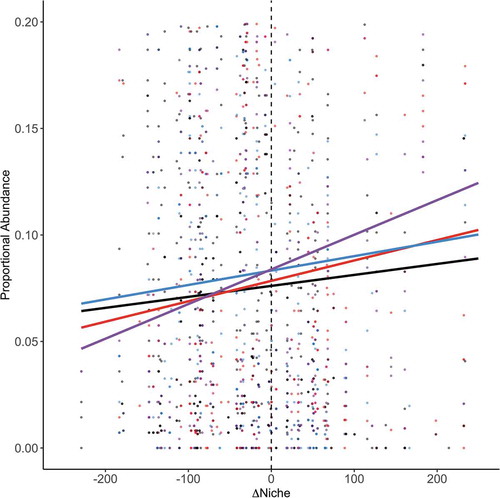
Both the proportional abundance of hot, dry adapted species relative to their surrounding community (+∆niche) was higher and the proportional abundance for species that were cool, wet adapted relative to their surrounding community was lower under experimental heating (−∆niche; marginal R2 = 0.01, conditional R2 = 0.33; βheat*∆niche = 4.426e-05, p = .040; ). There were no additional significant additive or interaction effects of ∆niche, heating, or watering on proportional abundance (χ2∆niche = 1.237, df = 1, p = .266; χ2heat = 0.170, df = 1, p = .680; χ2water = 1.970, df = 1, p = .160; χ2heat*water = 0.025, df = 1, p = .873; χ2water*∆niche = 1.349, df = 1, p = .245; χ2heat*water*∆niche = 0.627, df = 1, p = .428).
Figure 5. The proportional abundance of species relative to the difference between the species climatic niche mean and the aggregated ambient site CCN mean (∆niche). Each point represents a species by site by year combination. The lines represent model fits for each treatment group: ambient (black), heating (red), watering (blue), heating and watering (purple).
Richness was lower under both heating and watering (marginal R2 = 0.03, conditional R2 = 0.48; βheat = − 0.467, p = .013; βwater = − 0.523, p = .006; βheat*water = − 0.162, p = .710; ). Richness dynamics between treatments across years are shown in Supplemental . Ambient conditions had a mean of nine species per plot and richness was lower by approximately one species under the heating and watering treatments (μambient = 8.986, μheat = 8.518, μwater = 8.463, μheat*water = 7.833, df = 244, pambient-heat*water = 0.001). However, interactions between the species-specific climatic niche means and the treatments were not predictive of species presence or absence within a plot (χ2species.cnm = 2.565, df = 1, p = .109; χ2heat = 0.242, df = 1, p = .623; χ2water = 0.213, df = 1, p = .644; χ2heat*water = 0.190, df = 1, p = .663; χ2heat* species.cnm = 0.007, df = 1, p = .935; χ2water* species.cnm = 0.025, df = 1, p = .874; χ2heat*water* species.cnm = 1.342, df = 1, p = .246). Mean vegetation cover was 54 percent across all sites and significantly higher (57%) with experimental heating (βheat = 2.681, p = .027). The percent cover of vegetation was not impacted by experimental watering (χ2water = 0.016, df = 1, p = .898; χ2heat*water = 0.940, df = 1, p = .332).
Discussion
We examined the effects of experimental warming and watering on xeric alpine plant communities across a mountain range. Under warmer climatic conditions, thermophilization of this community was supported by two lines of evidence: (1) an increase in the seventy-fifth percentile CCN, indicating an increase of hot, dry adapted species within each site (), and (2) a positive interaction between experimental heating and ∆niche, demonstrating that species with higher climatic niche values relative to their community increased in proportional abundance and species with lower climatic niche values relative to their community decreased in proportional abundance (). Despite CCNs varying across both temperature and soil moisture gradients, experimental watering did not ameliorate the effect of heating in the climatic niche analysis. Concordant with evidence of thermophilization, we also found that overall abundance increased and species richness decreased with experimental heating. Together, these results indicate that with warmer conditions the White Mountain’s alpine zone will be made up of less diverse plant communities dominated by species associated with hotter, drier conditions.
Consistent with other high-elevation alpine systems, most species in this alpine community were regionally associated with cool, wet conditions (Körner Citation2003). Although our sites span a limited elevation gradient, we still saw a large variation in both the climatic niche means of the species present and in the aggregated ambient CCNs. This variation was the result of climatic gradients; CCN increased with accumulated degree days and decreased with soil moisture (). These significant relationships indicate that our climatic niche metric based on species regional distributions is representative of species local distributions across fine-scale climatic gradients (Scherrer and Körner Citation2011). The method by which we quantified our species-specific climatic niche assumed that there is no ecotypic variation in the climatic niche of species, although a subset of the study species had broad ranges (), and that there can be large physical barriers to dispersal between populations in mountainous regions (Dirnböck, Essle, and Rabitsch Citation2011). Additionally, our use of climate data and botanical specimen locations at relatively coarse resolutions to calculate species climate niche means disregards the importance of fine-scale climate heterogeneity for species distributions (Bramer et al. Citation2018). However, the clear relationships between the regional-based CCN metrics and our field-measured local temperature and moisture gradients both supports the use of this CCN metric that encompasses both temperature and moisture availability and strengthens our conclusions concerning positive shifts in the CCN with experimental heating.
The topography of this mountain range resulted in cool, dry conditions at high elevations; warm, dry conditions at mid-elevations; and warm, wet conditions at low elevations across our study sites (Oldfather and Ackerly Citation2018). This decoupling of temperature and moisture gradients across the elevation gradient resulted in the highest CCN values occurring at mid-elevations, where the combined influence of temperature and soil moisture was strongest (). Different mountain life forms are differentially sensitive to temperature and moisture limitations (Winkler, Chapin, and Kueppers Citation2016a; Bueno de Mesquita et al. Citation2018). Therefore, although low and high elevation communities had different taxonomic members, the community members at mid-elevations had similar CCNs because of a potential switch in limitation (temperature to soil moisture or vice versa). With these spatially-decoupled dueling limitations, we predict that thermophilization in this system may be driven by mid-elevation vegetation invading both the upper and lower alpine ecotones communities, homogenizing the overall alpine community in response to changing climate (Tingley et al. Citation2012; Suding et al. Citation2015). This potential pattern would contrast with thermophilization being driven solely by lower-elevation communities moving uphill, as observed in other alpine systems (Rixen and Wipf Citation2017; Rumpf et al. Citation2017). This combined effect of temperature and soil limitation may be exacerbated by switches from snow-dominated to rain-dominated systems in xeric, high-elevation systems, which leads to increasing climate-change exposure at the highest elevation (McCullough et al. Citation2016).
In this temperature- and soil moisture–limited alpine system, we show that minimally warmer conditions may drive a community shift toward relatively hotter, drier adapted species within a short time frame. This work is in agreement with others who found an increase in hot-adapted species, but a lesser subsequent decrease in cool-adapted species (Gottfried et al. Citation2012; Alexander et al. Citation2018; Kulonen et al. Citation2018). We observed no response to the experimental heating in the mean CCN or for the coolest, wettest adapted species (twenty-fifth percentile CCN; ). This lack of response of the mean and lower percentile, but a shift in the hottest, driest adapted species (seventy-fifth percentile CCN) parallels the observation of Kelly and Goulden (Citation2008), who found that with thirty years of recent climate change the distribution of species along an elevation gradient became upwardly skewed because of shifts in species abundance. These results may also testify to the resistance of alpine plant communities where community inertia resists changing climate conditions. Alpine plants are long lived, have extensive carbohydrate storage, and are adapted to dramatic fluctuation in environmental conditions (Körner Citation2003; Graae et al. Citation2018). Consequently, these alpine species may be less likely to be locally extirpated by the direct effect of changing climate on their physiology and demography, but more likely to be displaced by competition with faster-growing species associated with hotter climatic conditions (Lenoir et al. Citation2010; Alexander, Diez, and Levine Citation2015; Kopp and Cleland Citation2015; Rumpf et al. Citation2017). This lack of response of cool-adapted plant species has been termed extinction debt, and is hypothesized to be driven by lags in both population and community dynamics (Dullinger et al. Citation2012; Hylander and Ehrlén Citation2013). Loss of cool-adapted alpine endemics through species interactions rather than by direct effects is predicted to be a slower process because of lags in dispersal, establishment, and local extinction of alpine species (Rixen and Wipf Citation2017; Alexander et al. Citation2018). These lags could lead to disequilibrium dynamics where species are currently distributed in areas with unsuitable climate, but are predicted to be extirpated in the future (Körner Citation2003; Svenning and Sandel Citation2013).
The flora in an arid alpine system may be especially vulnerable to climate change (Engler et al. Citation2011). In contrast to Steinbauer et al. (Citation2018), who found an increase in richness, we found that richness decreased with experimental heating (). This pattern of decreased richness with warming conditions in more water-limited systems was also found in Mediterranean mountains and on the driest topographic aspects of single mountaintops (Pauli et al. Citation2012; Winkler et al. Citation2016b). We did not find that this pattern of lower species richness under experimental heating was the result of the loss of cool, wet adapted species. However, species consistently (across all sites and years) absent from experimentally heated plots (Erigeron clokeyi, Townsendia condensate, Boechera lemmonii, Solidago multiradiata) were primary cool, wet adapted, with climatic niche means below the average across all species and low frequencies (). Erigeron clokeyi, which has a species-specific climatic niche approximately 80 mm higher than the species average, is an exception to this pattern. One potential caveat of the observation of decreased richness with warming is that the chamber itself may physically limit dispersal into the experimentally heated plots; however, we designed the chamber so that the open top was the approximate width of the community plot to reduce effects on dispersal limitation.
In line with previous work, we found an increase in overall vegetation cover with warming (infilling) (Rumpf et al. Citation2017). Warming conditions may have allowed for more growth of the abundant species initially by reducing temperature limitation (Gilman et al. Citation2010), although the impacts of low soil moisture on growth would remain (Winkler, Chapin, and Kueppers Citation2016a). The increase in overall abundance may be driven by an encroachment of competitors that will eventually drive the loss of cool, wet adapted species (Graae et al. Citation2011; Lembrechts et al. Citation2018; Steinbauer et al. Citation2018). Although high climate heterogeneity in alpine zones may allow species to more easily track their suitable climatic conditions, these fine-scale climate gradients may also reduce the amount of suitable habitat for each species, as well as facilitate uphill movement of potential competitors, increasing competitive pressures (Scherrer and Körner Citation2011; Graae et al. Citation2018; Kulonen et al. Citation2018). Alternatively, the influence of positive interactions are thought to have a large effect in sparse, xeric habitats such as our study system (Michalet et al. Citation2014; Alexander et al. Citation2018). Increases in abundance may increase the possibility for facilitative interactions between or within species, reducing additional species loss (Callaway et al. Citation2002; Cavieres et al. Citation2014).
Although previous work found that alpine community responses to warming conditions is mediated by water availability (Engler et al. Citation2011; Elmendorf et al. Citation2012; Winkler, Chapin, and Kueppers Citation2016a), we observed minimal influences of the watering treatment, both additively and in interaction with the experimental heating. Although we manipulated summertime precipitation we did not modify wintertime precipitation (e.g., snow), an important driver of community composition in many alpine systems (Stanton, Rejmánek, and Galen Citation1994; Körner Citation2003; Jonas et al. Citation2008; Mark et al. Citation2015). The patterning of snowpack across the landscape has a strong influence on soil-moisture availability throughout the growing season (Isard Citation1986; Jonas et al. Citation2008; Litaor, Williams, and Seastedt Citation2008). Our lack of a quantifiable change in soil moisture with the watering treatment or heating treatment (because of soil drying) indicates that snowpack persistence had a larger effect on the soil-moisture gradient and the plant community composition than our manipulations (or potentially summertime monsoonal precipitations inputs; Wipf, Stoeckli, and Bebi Citation2009). Last, the low water-holding capacity of granitic soils and the reduced amount of organic soil in alpine systems may also have reduced the residence time of any experimental precipitation inputs, limiting their impact on community dynamics (Wenk and Dawson Citation2007; Kulonen et al. Citation2018).
Understanding how species and communities shift locally and regionally poses a great challenge as we manage for resilience in the face of a changing climate (Pecl et al. Citation2017). As predicted, we observed thermophilization of the alpine plant communities in the White Mountains with experimental warming, adding to the short list of examples of thermophilization in xeric mountain ranges (Pauli et al. Citation2012). These community-weighted climatic niche responses to heating of less than 1°C, paired with an overall reduction in richness and an increase in vegetation cover, indicate that the alpine plant community in more xeric alpine systems may be highly sensitive to changing climatic conditions. However, the lack of a change in the mean community climatic niche, in contrast to the observed change in only the hottest, driest adapted species, suggests that during the short term these community shifts may be strongly influenced by community lags. Finally, this study supports other work that predicted larger community change and diversity loss with climate change in mountains under water-limited scenarios (Engler et al. Citation2011; Rixen and Wipf Citation2017).
Supplemental Material
Download Zip (342 KB)Acknowledgments
We would like to thank Dr. Jonathon Lenoir, Dr. Sonja Wipf, Dr. Todd Dawson, and one anonymous reviewer for their insightful comments on this manuscript. This research was supported by the White Mountain Research Center, UC Natural Reserve System, and the U.S. National Science Foundation Graduate Research Fellowship Grant DGE-1106400 (to M.F.O.).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alexander, J. M., I. Burgess, F. Essl, S. Haider, C. Kueffer, K. Mcdougall, A. Milbau, W. Rabitsch, L. J. Rew, and N. J. Sanders. 2018. Lags in the response of mountain plant communities to climate change. Global Change Biology 24:1–17. doi:10.1111/gcb.13976.
- Alexander, J. M., J. M. Diez, and J. M. Levine. 2015. Novel competitors shape species’ responses to climate change. Nature 525:515–18. doi:10.1038/525S9a.
- Baldwin, B. G., A. H. Thornhill, W. A. Freyman, D. D. Ackerly, M. M. Kling, N. Morueta-Holme, and B. D. Mishler. 2017. Species richness and endemism in the native flora. American Journal of Botany 104:487–501. doi:10.3732/ajb.1600326.
- Bates, D., M. Maechler, B. Bolker, and S. Walker. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67 (1):1–48. doi:10.18637/jss.v067.i01.
- Bertrand, R., J. Lenoir, C. Piedallu, G. R. Dillon, P. De Ruffray, C. Vidal, J. C. Pierrat, and J. C. Gégout. 2011. Changes in plant community composition lag behind climate warming in lowland forests. Nature 479:517–20. doi:10.1038/nature10548.
- Billings, W. D. 1978. Alpine phytogeography across the Great Basin. Great Basin Naturalist Memoirs 2:105–17.
- Bramer, I., B. J. Anderson, J. Bennie, A. J. Bladon, P. De Frenne, D. Hemming, R. A. Hill, M. R. Kearney, C. Körner, A. H. Korstjens, et al. 2018. Advances in monitoring and modelling climate at ecologically relevant scales. Advances in Ecological Research 58:101–61.
- Bueno de Mesquita, C. P., L. S. Tillmann, C. D. Bernard, K. C. Rosemond, N. P. Molotch, and K. N. Suding. 2018. Topographic heterogeneity explains patterns of vegetation response to climate change (1972–2008) across a mountain landscape, Niwot Ridge, Colorado. Arctic, Antarctic, and Alpine Research 50:1–16. doi:10.1080/15230430.2018.1504492.
- Callaway, R. M., R. W. Brooker, P. Choler, Z. Kikvidze, F. I. Pugnaire, B. Newingham, and E. T. Aschehoug. 2002. Positive interactions among alpine plants increase with stress. Nature 417:844–48. doi:10.1038/nature00812.
- Cavieres, L. A., E. I. Badano, A. Sierra-Almeida, S. Gómez-González, and M. A. Molina-Montenegro. 2006. Positive interactions between alpine plant species and the nurse cushion plant Laretia acaulis do not increase with elevation in the Andes of central Chile. New Phytologist 169:59–69. doi:10.1111/j.1469-8137.2005.01573.x.
- Cavieres, L. A., R. W. Brooker, B. J. Butterfield, B. J. Cook, Z. Kikvidze, C. J. Lortie, R. Michalet, F. I. Pugnaire, C. Schöb, S. Xiao, et al. 2014. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecology Letters 17:193–202. doi:10.1111/ele.12217.
- Cranston, B. H., A. Monks, P. A. Whigham, and K. J. M. Dickinson. 2015. Variation and response to experimental warming in a New Zealand cushion plant species. Austral Ecology 40:642–50. doi:10.1111/aec.12231.
- De Frenne, P., F. Rodríguez-Sánchez, D. A. Coomes, L. Baeten, G. Verstraeten, M. Vellend, M. Bernhardt-Römermann, C. D. Brown, J. Brunet, J. Cornelis, et al. 2013. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences 110:18561–65. doi:10.1073/pnas.1311190110.
- Dirnböck, T., F. Essle, and W. Rabitsch. 2011. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Global Change Biology 17:990–96. doi:10.1111/j.1365-2486.2010.02266.x.
- Dullinger, S., A. Gattringer, W. Thuiller, D. Moser, N. E. Zimmermann, A. Guisan, W. Willner, C. Plutzar, M. Leitner, T. Mang, et al. 2012. Extinction debt of high-mountain plants under twenty-first-century climate change. Nature Climate Change 2:619–22. doi:10.1038/nclimate1514.
- Elmendorf, S. C., G. H. R. Henry, R. D. Hollister, R. G. Bjork, A. D. Bjorkman, T. V. Callaghan, L. S. Collier, E. J. Cooper, J. H. C. Cornelissen, T. A. Day, et al. 2012. Global assessment of experimental climate warming on tundra vegetation : Heterogeneity over space and time. Ecology Letters 15:164–75. doi:10.1111/j.1461-0248.2012.01764.x.
- Engler, R., C. F. Randin, W. Thuiller, S. Dullinger, N. E. Zimmermann, M. B. Araújo, P. B. Pearman, G. Le Lay, C. Piedallu, C. H. Albert, et al. 2011. 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology 17:2330–41. doi:10.1111/j.1365-2486.2010.02393.x.
- Flint, L. E., A. L. Flint, J. H. Thorne, and R. Boynton. 2013. Fine-scale hydrologic modeling for regional landscape applications: The California Basin Characterization Model development and performance. Ecologial Processes 2:1–21.
- Gilman, S. E., M. C. Urban, J. Tewksbury, G. W. Gilchrist, and R. D. Holt. 2010. A framework for community interactions under climate change. Trends in Ecology & Evolution 25:325–31. doi:10.1016/j.tree.2010.03.002.
- Gottfried, M., H. Pauli, A. Futschik, M. Akhalkatsi, P. Barančok, B. Alonso, J. Luis, G. Coldea, J. Dick, B. Erschbamer, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2:111–15. doi:10.1038/nclimate1329.
- Graae, B. J., R. Ejrnæs, S. I. Lang, E. Meineri, P. T. Ibarra, and H. H. Bruun. 2011. Strong microsite control of seedling recruitment in tundra. Oecologia 166:565–76. doi:10.1007/s00442-010-1878-8.
- Graae, B. J., V. Vandvik, W. S. Armbruster, W. L. Eiserhardt, J. C. Svenning, K. Hylander, J. Ehrlén, J. D. M. Speed, K. Klanderud, K. A. Bråthen, et al. 2018. Stay or go - how topographic complexity influences alpine plant population and community responses to climate change. Perspectives in Plant Ecology, Evolution and Systematics 30:41–50. doi:10.1016/j.ppees.2017.09.008.
- Grabherr, G., M. Gottfried, and H. Pauli. 2010. Climate change impacts in alpine environments. Geography Compass 4:1133–53. doi:10.1111/geco.2010.4.issue-8.
- Harsch, M. A., and J. Hille Ris Lambers. 2016. Climate warming and seasonal precipitation change interact to limit species distribution shifts across western North America. PLoS One 11:1–17. doi:10.1371/journal.pone.0159184.
- Harte, J., and R. Shaw. 1995. Shifting dominance within a montane vegetation community: Results of a climate-warming experiment. Science 267:876–80. doi:10.1126/science.267.5199.876.
- Hylander, K., and J. Ehrlén. 2013. The mechanisms causing extinction debts. Trends in Ecology and Evolution 28:341–46. doi:10.1016/j.tree.2013.01.010.
- Isard, S. A. 1986. Factors influencing soil moisture and plant community. Arctic, Antarctic, and Alpine Research 18:83–96. doi:10.2307/1551216.
- Jonas, T., C. Rixen, M. Sturm, and V. Stoeckli. 2008. How alpine plant growth is linked to snow cover and climate variability. Journal of Geophysical Research: Biogeosciences 113:1–10. doi:10.1029/2007JG000680.
- Kelly, A. E., and M. L. Goulden. 2008. Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences 105:11823–26. doi:10.1073/pnas.0802891105.
- Kopp, C. W., and E. E. Cleland. 2014. Shifts in plant species elevational range limits and abundances observed over nearly five decades in a western North America mountain range. Journal of Vegetation Science 25:135–46. doi:10.1111/jvs.12072.
- Kopp, C. W., and E. E. Cleland. 2015. A range-expanding shrub species alters plant phenological response to experimental warming. PLoS One 10:1–9. doi:10.1371/journal.pone.0139029.
- Körner, C. 2003. Alpine plant life: Functional plant ecology of high mountain ecosystems. Heidelberg: Springer.
- Kulonen, A., R. A. Imboden, C. Rixen, S. B. Maier, and S. Wipf. 2018. Enough space in a warmer world? Microhabitat diversity and small-scale distribution of alpine plants on mountain summits. Diversity and Distributions 24:252–61. doi:10.1111/ddi.12673.
- Lee-Yaw, J. A., H. M. Kharouba, M. Bontrager, C. Mahony, A. M. Csergo, A. M. E. Noreen, Q. Li, R. Schuster, and A. L. Angert. 2016. A synthesis of transplant experiments and ecological niche models suggests that range limits are often niche limits. Ecology Letters 19:710–22. doi:10.1111/ele.12604.
- Lembrechts, J. J., J. Lenoir, M. A. Nu, C. Geron, G. Buss, A. Milbau, and I. Nijs. 2018. Microclimate variability in alpine ecosystems as stepping stones for non-native plant establishment above their current elevational limit. Ecography 40:1–9.
- Lenoir, J., B. J. Graae, P. A. Aarrestad, I. G. Alsos, W. S. Armbruster, G. Austrheim, C. Bergendorff, H. J. B. Birks, K. A. Bråthen, J. Brunet, et al. 2013. Local temperatures inferred from plant communities suggest strong spatial buffering of climate warming across Northern Europe. Global Change Biology 19:1470–81. doi:10.1111/gcb.12129.
- Lenoir, J., J.-C. Gégout, A. Guisan, P. Vittoz, T. Wohlgemuth, N. E. Zimmermann, S. Dullinger, H. Pauli, W. Willner, and J.-C. Svenning. 2010. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 33:295–303. doi:10.1111/j.1600-0587.2010.06279.x.
- Lesica, P., and E. E. Crone. 2016. Arctic and boreal plant species decline at their southern range limits in the Rocky Mountains. Ecology Letters 20:166–74. doi:10.1111/ele.12718.
- Litaor, M. I., M. Williams, and T. R. Seastedt. 2008. Topographic controls on snow distribution, soil moisture, and species diversity of herbaceous alpine vegetation, Netwot Ridge, Colorado. Journal of Geophysical Research: Biogeosciences 113:1–10. doi:10.1029/2007JG000419.
- Lloyd, R., and R. Mitchell. 1973. A Flora of the White Mountains, California and Nevada. Berkeley: University of California Press.
- Mark, A. F., A. C. Korsten, D. U. Guevara, K. J. M. Dickinson, P. Michel, S. R. P. Halloy, J. M. Lord, S. E. Venn, W. John, P. A. Whigham, et al. 2015. Ecological responses to 52 years of experimental snow manipulation in high-alpine cushionfield, old man range, south-central New Zealand. Arctic, Antarctic, and Alpine Research 47:751–72. doi:10.1657/AAAR0014-098.
- McCullough, I. M., F. W. Davis, J. R. Dingman, L. E. Flint, A. L. Flint, J. M. Serra-Diaz, A. D. Syphard, M. A. Moritz, L. Hannah, and J. Franklin. 2016. High and dry: High elevations disproportionately exposed to regional climate change in Mediterranean-climate landscapes. Landscape Ecology 31:1063–75. doi:10.1007/s10980-015-0318-x.
- Michalet, R., C. Schöb, C. J. Lortie, R. W. Brooker, and R. M. Callaway. 2014. Partitioning net interactions among plants along altitudinal gradients to study community responses to climate change. Functional Ecology 28:75–86. doi:10.1111/1365-2435.12136.
- Morefield, J. D. 1992. Spatial and ecologic segregation of phytogeographic elements in the White Mountains of California and Nevada. Journal of Biogeography 19:33–50. doi:10.2307/2845618.
- Morueta-Holme, N., K. Engemann, P. Sandoval-Acuña, J. D. Jonas, R. M. Segnitz, and J.-C. Svenning. 2015. Strong upslope shifts in Chimborazo’s vegetation over two centuries since Humboldt. Proceedings of the National Academy of Sciences 112:12741–45. doi:10.1073/pnas.1509938112.
- Nakagawa, S., and H. Schielzeth. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4:133–42. doi:10.1111/j.2041-210x.2012.00261.x.
- Oldfather, M. F., and D. D. Ackerly. 2018. Microclimate and demography interact to shape stable population dynamics across the range of an alpine plant. New Phytologist 222:193–205. doi:10.1111/nph.15565.
- Patsiou, T. S., E. Conti, and N. E. Zimmermann. 2014. Topoclimatic microrefugia explain the persistence of a rare endemic plant in the Alps during the last 21 millennia. Global Change Biology 20 (7):2286–300. doi:10.1111/gcb.12515.
- Pauli, H., M. Gottfried, S. Dullinger, O. Abdaladze, J. Luis, B. Alonso, G. Coldea, J. Dick, B. Erschbamer, R. Calzado, et al. 2012. Recent plant diversity changes on Europe’s mountain summits. Science 336:353–55. doi:10.1126/science.1219033.
- Pecl, G., M. Araújo, J. Bell, J. Blanchard, T. Bonebrake, I. Chen, T. Clark, R. Colwell, F. Danielsen, B. Evengård, et al. 2017. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355:1–9. doi:10.1126/science.aai9214.
- Pepin, N., S. Bradley, H. Diaz, E. B. Baraer, N. Caceres, H. Forsythe, G. Fowler, M. Z. Greenwood, X. Hashmi, J. R. Liu, et al. 2015. Elevation-dependent warming in mountain regions of the world. Nature Climate Change 5:424–30. doi:10.1038/nclimate2563.
- Pollak, O. 1997. Morphlogy and dynamics of alpine populations of Ivesia lycopodioides ssp.scandularis from the White Mountains of California. University of California White Mountain Research Station Symposium, Bishop, California, USA.
- R Core Team. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/.
- Rapacciuolo, G., S. P. Maher, A. C. Schneider, T. T. Hammond, M. D. Jabis, R. E. Walsh, K. J. Iknayan, G. K. Walden, M. F. Oldfather, D. D. Ackerly, et al. 2014. Beyond a warming fingerprint: Individualistic biogeographic responses to heterogeneous climate change in California. Global Change Biology 20:2841–55. doi:10.1111/gcb.12638.
- Rixen, C., and S. Wipf. 2017. Non-equilibrium in alpine plant assemblages: Shifts in Europe’s summit floras. In High mountain conservation in a changing world. Advances in global change research, ed. J. Catalan, J. Ninot, and M. Aniz, vol. 62. Cham: Springer.
- Rumpf, S. B., K. Hülber, G. Klonner, D. Moser, M. Schütz, and J. Wessely. 2017. Range dynamics of mountain plants decrease with elevation. Proceedings of the National Academy of Sciences 115:1848–53. doi:10.1073/pnas.1713936115.
- Rundel, P., A. C. Gibson, and M. R. Sharifi. 2008. The alpine flora of the White Mountains, California. Madroño 55:202–15. doi:10.3120/0024-9637-55.3.202.
- Savage, J., and M. Vellend. 2015. Elevational shifts, biotic homogenization and time lags in vegetation change during 40 years of climate warming. Ecography 38:546–55. doi:10.1111/ecog.2015.v38.i6.
- Scherrer, D., and C. Körner. 2011. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. Journal of Biogeography 38:406–16. doi:10.1111/jbi.2011.38.issue-2.
- Spasojevic, M. J., W. D. Bowman, H. C. Humphries, T. R. Seastedt, and K. N. Suding. 2013. Changes in alpine vegetation over 21 years: Are patterns across a heterogeneous landscape consistent with predictions? Ecosphere 4 (9):117. doi:10.1890/ES13-00133.1.
- Spira, T. 1987. Alpine annual plants species in the White Mountains of Eastern California. Madroño 34:315–23.
- Stanton, A. M. L., M. Rejmánek, and C. Galen. 1994. Changes in vegetation and soil fertility along a predictable snowmelt gradient in the Mosquito Range, Colorado, U.S.A. Arctic and Alpine Research 26:364–74. doi:10.2307/1551798.
- Steinbauer, M. J., J.-A. Grytnes, G. Jurasinski, A. Kulonen, J. Lenoir, H. Pauli, C. Rixen, M. Winkler, M. Bardy-Durchhalter, E. Barni, et al. 2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556:231–34. doi:10.1038/s41586-018-0005-6.
- Stephenson, N. L. 1990. Climatic control of vegetation distribution: The role of the water balance. The American Naturalist 135:649–70. doi:10.1086/285067.
- Suding, K. N., E. C. Farrer, A. J. King, L. Kueppers, and M. J. Spasojevic. 2015. Vegetation change at high elevation: Scale dependence and interactive effects on Niwot Ridge. Plant Ecology & Diversity 8 (5–6):713–25.
- Svenning, J.-C., and B. Sandel. 2013. Disequilibrium vegetation dynamics under future climate change. American Journal of Botany 100:1–21. doi:10.3732/ajb.1300145.
- Tingley, M. W., M. S. Koo, C. Moritz, A. C. Rush, and S. R. Beissinger. 2012. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Global Change Biology 18:3279–90. doi:10.1111/gcb.2012.18.issue-11.
- Van de Ven, C. M., S. B. Weiss, and W. G. Ernst. 2007. Plant species distributions under present conditions and forecasted for warmer climates in an arid mountain range. Earth Interactions 11:1–33. doi:10.1175/EI205.1.
- Vanneste, T., O. Michelsen, B. J. Graae, M. O. Kyrkjeeide, H. Holien, K. Hassel, S. Lindmo, R. E. Kapás, and P. De Frenne. 2017. Impact of climate change on alpine vegetation of mountain summits in Norway. Ecological Research 32:579–93. doi:10.1007/s11284-017-1472-1.
- Walther, G.-R., S. Beißner, and C. Burga. 2005. Trends in the upward shift of alpine plants author. Journal of Vegetation Science 16:541–48. doi:10.1111/jvs.2005.16.issue-5.
- Wenk, E. H., and T. E. Dawson. 2007. Interspecific differences in seed germination, establishment, and early growth in relation to preferred soil type in an alpine community. Arctic, Antarctic, and Alpine Research 39:165–76. doi:10.1657/1523-0430(2007)39[165:IDISGE]2.0.CO;2.
- Winkler, D., K. Chapin, and L. Kueppers. 2016a. Soil moisture mediates alpine life form and community productivity responses to warming. Ecology 97:1553–63. doi:10.1890/15-1197.1.
- Winkler, M., A. Lamprecht, K. Steinbauer, K. Hulber, J.-P. Theurillat, F. Breiner, P. Choler, S. Ertl, A. Giron, G. Rossi, et al. 2016b. The rich sides of mountain summits – A pan-European view on aspect preferences of alpine plants. Journal of Biogeography 43:2261–73. doi:10.1111/jbi.12835.
- Wipf, S., V. Stoeckli, and P. Bebi. 2009. Winter climate change in alpine tundra: Plant responses to changes in snow depth and snowmelt timing. Climatic Change 94:105–21. doi:10.1007/s10584-009-9546-x.
