ABSTRACT
Herbivores can play an important role in determining arctic ecosystem function with effects determined in part by herbivore identity. We examined the impact of long-term (twenty-two years) small and large mammal herbivore exclusion in two arctic plant communities in northern Alaska: dry heath (DH) and moist acidic tundra (MAT). Our aims were to examine how herbivore exclusion influences (1) plant communities and (2) soil nutrient pools and microbial processes. Though herbivore absence increased moss and decreased evergreen shrub cover in MAT, there were few other significant effects on vegetation in either community. We also observed no influence of exclusion on most soil properties. However, in DH, phosphatase activity was greater in areas where small mammals alone were present, suggesting that they are altering phosphorus (P) availability, perhaps through herbivores’ influence on the plant community and subsequently on competition for P with the microbial community. We conclude that herbivore impacts in the Arctic are dependent on both the plant community and herbivore identity (size). We show the importance of understanding the roles of herbivores in the Arctic and contribute to a growing number of herbivore studies in a biome likely to experience future changes in herbivore communities and ecosystem function.
Introduction
Herbivores can have strong influences on ecosystem properties and processes, with their impact depending on the identity of the herbivores present. Communities of larger-bodied herbivores have been described as having larger impacts than communities of smaller-bodied herbivores (Cumming and Cumming Citation2003), although a number of studies have shown important effects of small herbivores on ecosystem properties (Howe and Brown Citation1999; Bakker et al. Citation2004; Johnson et al. Citation2011) and even effects of similar sized to those of large mammals on nitrogen (N) cycling (Clark et al. Citation2005). Additionally, herbivore identity as either a migrant or resident species may be an important control on the impacts of herbivores on ecosystems, especially in the Arctic. Migratory species such as caribou (Rangifer tarandus) may use areas at high densities for a short period of time; in contrast, resident species such as voles (Microtus spp.) and lemmings (Lemmus spp.) have relatively small home ranges and are present and active year-round. Some resident herbivore species may also go through large population increases and crashes (Batzli et al. Citation1980; Ims, Yoccoz, and Killengreen Citation2011), with these species being more important at local scales during times of high abundance.
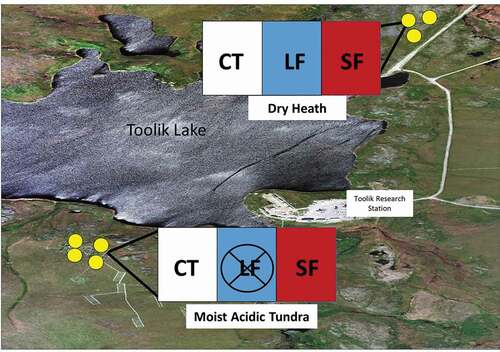
Figure 1. Overview of study area located in two types of arctic tundra near Toolik Lake, Alaska. Yellow dots represent locations of experimental herbivore exclosures, with three fencing blocks in DH and four in MAT. Each block included a CT (no heribvores excluded), an SF (large and small heribvores excluded), and an LF (large herbivores only excluded). The fencing block designs are inlayed
Arctic herbivores can influence ecosystems by altering plant communities and soil processes. Small and large arctic herbivores may influence vegetation abundance and cover (Johnson et al. Citation2011; Cahoon et al. Citation2012), plant biomass (Olofsson, Tommervik, and Callaghan Citation2012), light limitation (Borer et al. Citation2014), plant nutrient levels (Jefferies, Klein, and Shaver Citation1994; Tuomi et al. Citation2019), photosynthetic potential (Li et al. Citation2018), and productivity and plant senescence (Chew Citation1974; Batzli Citation1978; Mosbacher et al. Citation2018). In addition to influencing vegetation, herbivores can have both direct and indirect effects on soil processes. Arctic mammalian herbivores can redistribute soil (Tikhomirov Citation1959; McKendrick et al. Citation1980) and may influence carbon (C) and nutrient cycling by bringing material from lower soil layers to the soil surface (Ballova et al. Citation2019) where it can be accessed by microbes and plants. Herbivores can also influence soil properties and nutrient cycling by producing feces and urine (Clark et al. Citation2005), influencing the composition of the litter pool (Wardle, Bonner, and Barker Citation2002), altering soil temperatures (van der Wal, van Lieshout, and Loonen Citation2001; Borer et al. Citation2014), and affecting soil pore space and soil moisture (van Klink et al. Citation2015). These interactions between herbivores and arctic ecosystem functions have the potential to influence this ecosystem over long time periods.
Long-term herbivore exclosures have provided insights into the chronic impact of herbivores on arctic ecosystems. For example, in one of the longest runnning exclosure experiments on the north coast of Alaska, data showed higher graminoid abundance and lower lichen abundance in control sites compared to herbivore exclusion sites after fifty years of lemming exclusion (Johnson et al. Citation2011), implying that there may be a positive relationship between herbivore activity and plant biomass. Though most research examining arctic herbivores has observed decreases in plant biomass due to herbivory (Moen and Oksanen Citation1998; Olofsson, Moen, and Oksanen Citation2002; Post and Pedersen Citation2008; Olofsson et al. Citation2009), other studies have shown increases (Johnson et al. Citation2011) or no difference (Olofsson, Moen, and Oksanen Citation2002) in plant biomass, suggesting that the effects of herbivores may vary by vegetation community. Differences in effects of herbivory may in part be due to differences in vegetation communities (Moen and Oksanen Citation1998) or the length of time the experiment has been running and possible transient effects of herbivory (Tilman Citation1988; Mallen-Cooper, Nakagawa, and Eldridge Citation2019). Long-term exclosures in the Arctic have also shown a relationship between herbivores and landscape level ecosystem functions such as albedo, methane (CH4) flux, ecosystem respiration, net ecosystem exchange, and C storage (Cahoon et al. Citation2012; Vaisanen et al. Citation2014; Lara et al. Citation2016; Ylanne and Stark Citation2019). These exclosure experiments have been informative about herbivore–ecosystem interactions, but most experiments excluded either all herbivores or a specific size class of herbivores and did not examine the potential differential impacts between herbivore guilds (e.g., ungulates vs. rodents; although see Pastor and Naiman Citation1992; Grellmann Citation2002; Olofsson et al. Citation2009).
Herbivore exclosures constructed in the 1990s at the Arctic Long-Term Ecological Research (ARC-LTER) site near Toolik Lake, Alaska, provide an opportunity to examine the long-term impacts of herbivores and begin to understand how different herbivore guilds influence ecosystem structure. These exclosures have increased the understanding of the interaction between herbivores and vegetation communities (Gough, Ramsey, and Johnson Citation2007; Gough et al. Citation2008) and soil food webs (Gough et al. Citation2012). Though these exclosures were monitored for the past twenty years, the impact of herbivore exclusion on soil biogeochemical and physical processes remains unexamined. Furthermore, most studies have focused on the influence of herbivory on arctic vegetation and ecosystem-level processes, and fewer studies have assessed the long-term impacts of arctic herbivores on soil nutrient pools and microbial processes (although see Stark and Grellmann Citation2002; Olofsson, Stark, and Oksanen Citation2004; Sitters et al. Citation2017, Citation2019; Stark et al. Citation2019). This has led to the need to have a better understanding of the role of herbivores in systems with slow nutrient cycling. Our goal was to examine how the vegetation community and soil nutrient pools respond to long-term reduced herbivore activity in two arctic plant communities. The specific questions we aimed to answer were as follows:
What are the long-term impacts of reduced mammal activity on vegetation community structure and soil nutrient pools in two arctic plant communities?
How does the guild of mammalian herbivores (rodent vs. caribou) affect vegetation community and soil processes?
Materials and methods
Study site
We conducted this study in long-term herbivore exclosures located in moist acidic tundra (MAT) and dry heath (DH) tundra at the ARC-LTER site near Toolik Lake, Alaska, during the summer of 2017. The ARC-LTER is located north of the Brooks Range along the Dalton Highway (68°37′40″ N, 149°35′41″ W). The MAT experimental site is located along the southern side of Toolik Lake at an elevation of 755 m, and the DH site is located along the northeastern side of the lake at an elevation of 720 m. Vegetation at the MAT site is equally represented by evergreen shrubs (Rhododendron palustre, Vaccinium vitis-idaea), deciduous shrubs (Betula nana, Rubus chamaemorus), and graminoids (Eriophorum vaginatum, Carex bigelowii) with abundant Sphagnum mosses (Gough, Ramsey, and Johnson Citation2007; Gough et al. Citation2012; McLaren, Buckeridge et al. Citation2017), while the DH site is dominated by evergreen shrubs (Loiseuleuria procumbens, Rhododendron palustre, Empetrum nigirum, V. vitis-idaea) and lichens (Gough, Wookey, and Shaver Citation2002; Gough et al. Citation2012). For a complete list of plant species in both plant communities, please see our online data (data accessibility section). Both sites are underlain by continuous permafrost (Shaver et al. Citation2014). At Toolik Field Station (<1 km from either experimental site), air temperatures range from −57.6 to 28.2°C (mean annual air temperature = −6.8), soil surface temperatures range from −25.7 to 33.0°C (mean annual soil temperature = −0.6), and yearly mean precipitation is 256.7 mm (Environmental Data Center Team Citation2017). Local mammalian dominant resident herbivores include singing voles (Microtus miurus) and tundra voles (M. oeconomus), with additional herbivores including collared lemmings (Dicrostonyx groenlandicus), red-backed voles (Clethrionomys rutilis), arctic ground squirrels (Spermophilus parryii), and migratory caribou (Batzli and Henttonen Citation1990; Gough et al. Citation2008). Plant species important for local small mammal herbivores include tussock cotton grass (Eriophorum vaginatum) for tundra voles and willows (Salix spp.) for singing voles (Batzli and Lesieutre Citation1991). Lichens, shrubs, and tussock cotton grass are important local forage species for caribou (Walsh et al. Citation1997; Walker et al. Citation2001; Joly, Jandt, and Klein Citation2009).
Experimental herbivore exclosures were established within each vegetation community in 1996 () within an existing experimental layout consisting of three (DH) or four (MAT) blocks of 5 × 20 m plots separated by 2-m walkways. At each block there is a fencing plot (5 × 20 m each), with a series of 5 × 5 m fences and control sites. Each block consists of a large herbivore exclosure (LF, 15.2 × 15.2 cm mesh), a large and small herbivore exclosure (SF, 1.3 cm × 1.3 cm mesh), and a control (CT, no fencing) plot (Gough, Ramsey, and Johnson Citation2007). Each exclosure is approximately 2 m tall to prevent herbivores from feeding over the exclosures and has approximately 10 cm of the exclosures buried into the soil to prevent small mammals from burrowing under the exclosures. Although all three treatments are present at both sites, the LF plots in the MAT were not sampled for this study.
Vegetation community
In late July 2017, we assessed the vegetation community within each experimental plot at the DH and MAT sites. We used 1 × 1 m quadrats to quantify the percentage cover of vascular and nonvascular plants, bare ground, and plant litter in eight contiguous replicates within each plot. Vascular plants were identified to species level and mosses and lichens were grouped across species. For analysis we determined proportional cover by summing the percentage cover of all plants and then calculating the relative abundance of each group to standardize across plots. Most analyses were conducted on plant growth forms (graminoids, evergreen shrubs, deciduous shrubs, forbs, lichens, mosses) rather than individual species.
Soil analysis
We collected soil samples from each treatment in the DH on 22 July 2017 and MAT on 24 July 2017. For the DH, we collected three randomly located samples per plot; using a serrated bread knife we collected 10 × 10 cm columns of the organic horizon to a depth of 5 cm. For the DH, the mineral layer was shallow (~5 cm depth), so we only sampled the upper organic layer of soil (top 5 cm of the organic layer). Additionally, because the mineral layer had numerous rocks and would thus require large volumes of soil sampled for analysis, in this community we did not sample this layer in order to minimize destructive impacts in these long-term exclosures. In the MAT, three 10 × 10 cm columns of soils were cut from each plot to a depth of approximately 30 cm or to the depth of the active layer (i.e., frozen soil was not sampled), whichever was less. We separated each column of MAT soil into the upper organic layer (top 5 cm, as above), the lower organic layer (the remaining depth of the organic column), and 5 cm of the mineral layer (when accessible) in the field. We separated the top 5 cm from the rest of the organic layer to enable comparison between the two ecosystem types and also to follow sampling protocols from other previously published studies at these sites (Mack et al. Citation2004; McLaren and Buckeridge Citation2019).
For each soil column and depth, we dried a subsample of each core (approximately 5 cm3) at 50°C for 48 hours to assess bulk density and gravimetric water content. When volume could not be accurately assessed, only gravimetric water content measurements were taken. Subsequently, we individually homogenized each soil sample by hand, removing all large roots (>1 mm diameter), partitioned samples for analysis within two days of collection, and then froze samples before shipping to the University of Texas at El Paso, where they were stored at −20°C until analysis.
We analyzed soil samples for total percentage C (%C) and total percentage N (%N), inorganic nutrients (NH4+, NO3−, PO43−); organic nutrients (extractable organic C [EOC], extractable total N [ETN], extractable organic P [EOP]); microbial biomass C, N, P; and extracellular enzyme activity using the following methods.
We dried, ground, and processed soil subsamples for percentage C and percentage N contents using a dry combustion C and N analyzer (ElementarPyroCube). To determine soil inorganic nutrients, we thawed and extracted frozen subsamples (5 g) in 25 ml of 0.5 M K2SO4 for 2 hours, filtered through glass filter paper, and analyzed extractant using colorimetric microplate assays (BioTek Synergy HT microplate reader, BioTek Instruments Inc., Winooski, Vermont). NH4+-N (NH4+) was determined using a modified Berlethot assay (Rhine et al. Citation1998), NO3−-N (NO3−) using a modified Griess assay (Doane and Horwath Citation2003), and PO43−-P (PO43−) using a malachite green assay (D’Angelo, Crutchfield, and Vandiviere Citation2001).
EOC was determined colorimetrically after an Mn(III) reduction assay (Bartlett and Ross Citation1988). ETN and EOP were determined using a modified alkaline persulfate digestion using a 1:1 ratio of oxidizing reagent to sample and autoclaved for 40 minutes at 121°C. (Lajtha et al. Citation1999) followed by analysis for NO3− and PO43− respectively as above. To determine microbial biomass C, N, and P, we conducted the above assays on samples using a direct chloroform addition modification of the fumigation–extraction method (Brookes et al. Citation1985; Voroney, Brooks, and Beyaert Citation2006), where 5 g of thawed soil was incubated for 24 hours with 2 ml of ethanol-free chloroform, followed by extraction in 25 ml of 0.5 M K2SO4. We calculated microbial biomass for C, N, and P (MBC, MBN, and MBP) by subtracting ETN, EOP, or EOC respectively of nonfumigated samples from that of fumigated samples. No correction factor was applied for incomplete CHCl3 release or sorption of P because these values are not known for K2SO4 extraction for these two ecosystems.
Extracellular enzyme (exoenzyme) activity was assessed for ten exoenzymes involved in the microbial acquisition of C, N, and P: C-aquiring enzymes (β-glucosidase, β-cellobiosidase, β-xylosidase, α-glucosidase), N-acquiring enzymes (N-acetyl-glucosaminidase, leucine amino peptidase)) and P-acquiring enzymes (phosphatase, phosphodiesterase), as well as the oxidative enzymes phenol oxidase and peroxidase. One gram of soil was blended with a sodium acetate buffer to reflect natural soil conditions (pH = 5) and pipetted onto ninety-six-well plates with eight replicates per soil. Substrate tagged with fluorescing 4-methylum-belliferone (MUB) or 7-amido-4-methyl coumarin (MC; leucine amino peptidase only) was added to soil slurrys. Samples were incubated at 20°C and enzyme activity (fluorescence) measured every 30 minutes for 3.5 hours following methods adapted from Saiya-Cork, Sinsabaugh, and Zak (Citation2002) and McLaren, Buckeridge, et al. (Citation2017). For each substrate, we measured the background fluorescence of soils and substrate and the quenching of MUB or MC by soils and used standard curves of MUB or MC to calculate the rate of substrate hydrolyzed. Florescence was measured at 365 mm excitation and 450 nm emission using a BioTek Synergy HT microplate reader (BioTek Instruments Inc.). Oxidative enzyme analysis was performed using an l-3,4-dihydroxyphenylalanine (l-DOPA) substrate for phenol oxidase and peroxidase. Color absorbance was measured at 460 nm using a reader after 24 hours of incubation.
Statistical methods
We performed statistical analyses with the program R (R Core Team Citation2018) with a cutoff of p < .05 for inferring statistical significance. In all analyses, sites were analyzed separately. In DH, there were three treatment blocks with three types of fencing treatments (CT, LF, SF), whereas in MAT there were four blocks and two fencing treatments (CT, SF). A block factor was included to reflect the field experimental design.
To assess changes in plant communities, we used the package vegan (Oksanen et al. Citation2019) to calculate Shannon diversity indices for each treatment at each site; Pielou’s evenness was then calculated from the diversity values. Differences in diversity, evenness, and species richness between treatments in each vegetation community were examined using analysis of variance (ANOVA) or t-tests as appropriate. To determine whether there was an effect of exclosures on percentage cover, we used a blocked multivariate analysis of variance (MANOVA) with Pillai’s trace test statistic and an experimental block as our blocking factor for each site. In the MANOVA, we used percentage cover of each plant growth form (graminoid, evergreen shrub, deciduous shrub, forb, lichen, moss, bare ground) as the dependent variable and exclosure treatment (CT, SF, LF) as the independent variable.
Differences in soil variables between treatments and soil depth were determined using ANOVA or t-tests as appropriate, with nutrient concentrations, microbial biomass, and enzyme activity as response variables and exclosure type and soil depth as independent variables. When data could not be normalized, Kruskal-Wallis and Wilcoxon tests were used. For soil response variables, vegetation communities were analyzed separately, with soil depth only analyzed in MAT and guild identity (SF and LF treatments) only analyzed in DH.
Results
Vegetation
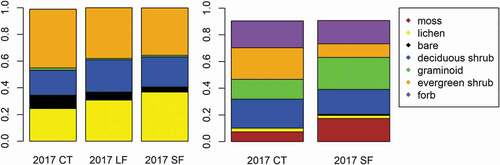
In DH tundra, we observed changes in the vegetation structure due to herbivory presence and the identity of the herbivore present in the system. Shannon diversity indices varied between the fencing treatments (F2,6 = 5.19, p = .05). Tukey’s post hoc tests showed that diversity in the large herbivore only exclosure (LF) trended lower than controls (CT; p = .08) and all herbivore exclosures (SF; p = .07), though there was no difference in diversity between SF and CT (p > .10; ). Similar to diversity, evenness values varied among treatments (F2,6 = 9.98, p = .01), with LF again being lower than CT (p = .02) and SF (p = .02; ). There were no differences among treatments for species richness (F2,6 = 2.68, p = .15; ). Although the mean abundance of some plant groups appeared to differ across fencing treatments, particularly lichens, which had lower abundance in CT than SF and LF (), the MANOVA found no significant effect of fencing on the plant community overall (F2,6 = 0.97, p = .54) or for any individual growth forms (p > .05).
Table 1. Mean and standard error for species diversity, species richness, and evenness of plant communities in 2017 from an herbivore exclosure experiment in two different tundra types: DH and MAT
In the MAT, in contrast with DH, we found no difference in Shannon diversity between the SF treatment and the CT (t4.86 = 1.28, p = .18). Furthermore, we found no differences in evenness (t3.95 = −0.24, p = .82) or richness (t5.46 = 1.70, p = .15) between treatments (). Again, we found no significant effect of fencing on plant community composition overall (F1,6 = 0.97, p = .28; ). However, when analyzing each growth form independently, we found that herbivore exclusion significantly increased moss cover (F2,6 = 13.00, p = .01) and reduced evergreen shrub cover (F1,6 = 10.00, p = .01). There was also a trend toward greater graminoid cover inside the SF treatment (F1,6 = 2.70, p = .15).
Soil nutrient pools
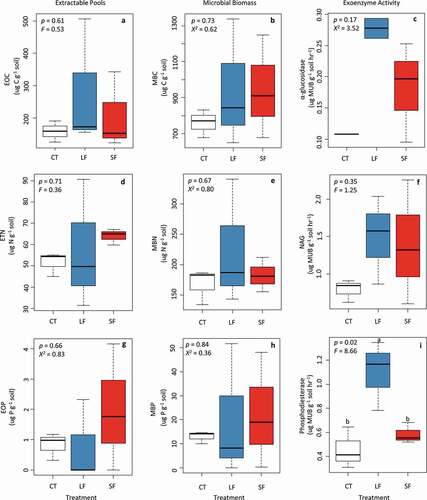
Generally, there were significant differences in soil variables between sites, with MAT having higher CNP pool concentrations and enzyme activities than DH (Supplemental Table 1). There were no significant responses to long-term herbivore exclusion for percentage C and N, inorganic N and P pools, extractable CNP pools, or microbial biomass CNP pools in either vegetation community (, Supplemental Table 2, , and Supplemental Figures 1–3). Small mammal activity did not affect soil exoenzyme activity in the MAT (Supplementary Table 3, Supplemental Figure 5). In the MAT, most soil variables differed by depth (p < .01; Supplementary Table 2, Supplemental Figures 6–7) but not by treatment (p = .05). In the DH, there were few effects of herbivore exclusion on exoenzyme activity (Supplemental Figure 4, Supplemental Table 3). We did observe that areas that were only accessed by small mammal herbivores (LF) had significantly higher phosphodiesterase activity (F2,6 = 8.66, p = .02) than areas that could be accessed by both large and small herbivores and herbivore-free exclosures ().
Table 2. Impact of herbivores on soil variables after twenty years of exclusion. ANOVA and Kruskal-Wallis summary results from comparisons of soil variables between exclosures in DH and MAT
Figure 3. Box plots showing the impact of herbivores on (a), (d), (g) extractable organic nutrients, (b), (e), (h) microbial biomass, and (c), (f), (i) potential exoenzyme activity in soils collected in July 2017 from an herbivore exclosure experiment in DH (n = 3) tundra at the ARC-LTER located at Toolik, Alaska
Discussion
Overall, we found few effects of long-term reductions in herbivore activity on vegetation in two arctic plant communities. Although the effects were not large, herbivore presence did alter each plant community differently. In the MAT, mosses were more abundant and evergreen shrubs were less abundant in areas where herbivores were excluded compared to controls, similar to effects found after a shorter period of herbivore exclusion in these same plots (Gough, Ramsey, and Johnson Citation2007; Gough et al. Citation2012). Herbivore activity in MAT negatively affected graminoids; thus, when herbivores were removed, graminoid cover increased and evergreen shrubs may have experienced greater competition, resulting in a decline in evergreen relative abundance. Though Gough, Ramsey, and Johnson (Citation2007) showed that herbivory became more important under fertilized conditions, our data show that even without fertilization, herbivory can play a role in regulating some forage species. The increase in mosses with reduced caribou and vole activity has also been seen in other studies (Rydgren et al. Citation2007) and is likely due to voles using mosses as winter forage (Batzli and Lesieutre Citation1991) and potentially disturbing the mosses through trampling (van der Wal, van Lieshout, and Loonen Citation2001) and creating runways. Such increases in moss cover may also negatively influence evergreen shrub establishment and growth (Holmgren et al. Citation2015) and may additionally partially explain the reduced evergreen shrub cover we observed in the MAT. Even though we did not observe exclusion effects on soil properties in the MAT, changes in moss cover due to herbivory may influence system properties such as nutrient availability (Olofsson et al. Citation2009; Bueno et al. Citation2016) and soil temperatures (Gornall et al. Citation2011) in the future.
Our results from the DH sites show that the influence of herbivores on this plant community is different from that in the MAT, by decreasing plant diversity and evenness when caribou alone are excluded, and that interactions between types of herbivores may be important. Although there were no statistical differences in plant growth form abundance among treatments, likely due to low replication at the block level and therefore low statistical power, there was greater lichen cover in large mammal exclusion (LF) plots (37 ± 7 percent) compared to areas where all herbivores access (CT) plots (24 ± 3 percent) in the DH, which corresponds with increases in lichen cover with caribou exclusion found in other studies (Olofsson, Stark, and Oksanen Citation2004; Gough et al. Citation2008; Pajunen, Virtanen, and Roininen Citation2008). However, this observed increase was not present when small mammals were also excluded (SF plots, 30 ± 9 percent). Though this increase in lichen cover was not observed in a European heath community when herbivores were excluded (Grellmann Citation2002), it suggests that there may be an interaction between the activity of different herbivore guilds; lichen cover may increase due to the absence of caribou, but small mammal activity or foraging on vascular plants could also potentially alleviate competition pressure on lichens by reducing vascular plant abundance.
In addition to differing effects on the vegetation communities, we found that herbivore guilds may impact soils differently. In the DH site, we found higher phosphodiesterase activity in areas where large, but not small, mammals were excluded, compared to control sites (all herbivores present) and all herbivore exclusion. An increase in phosphatases suggests that microbes are experiencing P limitation, and the trend of increases in PO43− in the large herbivore exclusion (LF) treatments (Supplemental Figure 2) further suggests that this increase in phosphatases may be increasing P availability in the soil. Thus, increases in phosphatases in the DH indicate that large mammals may be regulating P availability but only when small mammals are not present. In contrast to Sitters et al. (Citation2019), which found that heavy reindeer grazing created more P-limited conditions, our results support the opposite trend—that reduced caribou activity creates P-limited conditions. Our observed increase in lichens in the LF treatments may partially explain increased phosphatases. Because P can be limiting to lichens (Hurri, Makkonen, and Hyvärinen Citation2007) and lichens can produce their own phosphatases (Hogan et al. Citation2010), increases in lichen abundance may directly result in increases in phosphatase activity. Alternatively, changes in the vegetation community may alter competition for P in the microbial community and, in turn, influence phosphatase production.
Although the purpose of this article was to compare herbivore impacts between each plant community, and we thus focus on shallow soils, we did observe generally higher carbon and nutrient concentrations in the organic soils than mineral soils in the MAT (Supplemental and 3). The impacts of herbivores (urine, feces, litter inputs) are likely concentrated in the upper portion of soil layers and then cycle between the organic layers. This is supported by the few differences we observed between the organic layers. Though the mineral layer differed from the organic layers, we found few responses in the mineral layer due to herbivore treatments, suggesting that the impacts of herbivores may be immediate and not persist over long time periods. Though we did not sample at depth in the DH, we expect that we would see similar deep soil responses to herbivores as in the MAT.
Our sampling is part of an ongoing effort examining how herbivores influence ecosystem dynamics in these long-term exclosures (Gough, Ramsey, and Johnson Citation2007; Gough et al. Citation2008, Citation2012). Our data show little change from previous samplings, which also show relatively few changes over time (Gough and Johnson Citation2018), and provide valuable additional time points for this experiment and the examination of ecosystem functions in a changing arctic environment. Interestingly, in a study design similar to ours in another heath community but also including fertilization (Stark and Grellmann Citation2002), slower nutrient cycling under grazing after seven years of exclusion was reported. Their research found that excluding herbivores influenced MBC and microbial respiration, but MBN was only affected by exclusion plus fertilization (Stark and Grellmann Citation2002). The fact that we did not detect any changes in soil nutrient pools twenty-one years after treatment began (Supplemental Table 3) indicates that influences of herbivores may be transient. Many of the variables we examined (e.g., available nutrients) show strong variation seasonally (McLaren, Darrouzet-Nardi, et al. Citation2018) and between years (Edwards and Jefferies Citation2013), and with a single sampling it is possible that we missed transient effects that occurred during other parts of the growing season or during other years. Alternatively, studies have found that herbivore impacts may increase through time (Mallen-Cooper, Nakagawa, and Eldridge Citation2019) and, due to slow ecosystem processes in the Arctic, it may take greater than twenty years to see effects. Because the impacts of herbivores in the Arctic can persist for greater than 150 years (Egelkraut et al. Citation2018), their impacts are likely to change over time (Mallen-Cooper, Nakagawa, and Eldridge Citation2019), and more work is needed to track the legacy level effects of herbivory within arctic ecosystems.
The level and intensity of herbivore activity may also influence arctic ecosystem processes. Some studies have shown that heavy grazing can increase N cycling and primary productivity and moderate grazing may decrease these properties (Zamin and Grogan Citation2013), whereas others have shown the opposite (Pastor and Naiman Citation1992). Regardless, changes in herbivore density may have lasting effects on ecosystem functions and may be especially important with species with cyclic population densities such as voles. In the year of this study, and the years immediately preceding it, vole abundance was low (Maguire and Rowe Citation2017; Rowe and Steketee Citation2019, unpublished data), and vole densities inside the exclosures may have not differed greatly from that outside the exclosures. In addition to low densities of voles, the activities of these herbivores are particularly localized (e.g., latrine sites) and thus our randomized soil sampling may have missed sites where herbivore activities do affect soil nutrient cycling. Although we found few effects during a potential low phase of the local vole population cycle, the effect of voles on ecosystem functions during the high point in their population cycle has been documented in other ecosystems (Olofsson, Tommervik, and Callaghan Citation2012), suggesting that the effects of voles in this ecosystem are density dependent. Potential suppression in arctic herbivore population cycles (Ims, Henden, and Killengreen Citation2008) may thus alter the role of herbivores in arctic systems in the future.
Here we described the influence of herbivores on vegetation and soil function in two arctic plant communities after twenty years of herbivore exclusion. We found that herbivory pressure altered moss cover and evergreen shrub abundance in the MAT and influenced P-acquiring enzyme activity in the DH. We provide evidence of differing impacts between different herbivore guilds for both vegetation and soil properties. Although other studies found stronger effects of herbivores under increased nutrient or warmed conditions, our data, collected under ambient conditions, may provide a baseline with which to examine the impacts of herbivores in a changing arctic environment. Future changes in arctic systems may alter herbivore populations and communities as well as their influences on ecosystem ecology. Though our replicate numbers were low, we believe that our results are representative of the potential impacts of herbivores in these two Alaskan arctic plant communities. However, the impacts of herbivores on these processes are likely to vary among the major regional vegetation types, and we recommend that further studies incorporate additional systems to better elucidate the impacts of herbivores in the Arctic as a whole. Future work should also examine how potential changes in herbivore population dynamics and species assemblages of herbivores may influence ecosystem functions.
Data Accessibility
Data from this project are available on the Arctic Data Center. McLaren et al. (Citation2019), Soil biogeochemical variables collected on the Arctic LTER experimental plots in moist acidic and dry heath tundra, Arctic LTER Toolik Field Station, Alaska, 2017 (https://doi.10.6073/pasta/5a5cbb785bde48522bde7b87c65d3c13). Gough (Citation2019a), Relative percent cover of plant species for years 2012–2017 in the Arctic Long-Term Ecological Research (ARC-LTER) 1989 moist acidic tundra (MAT89) experimental plots, Toolik Field Station, Alaska. Environmental Data Initiative (https://doi.10.6073/pasta/f31def760db3f8e6cfee5fee07cc693e). Gough (Citation2019b), Relative percent cover of plant species for years 2013 2014 2016 2017 in LTER dry heath tundra experimental plots established in 1989, Arctic LTER Toolik, Field Station Alaska. Environmental Data Initiative (https://doi.10.6073/pasta/25d3f0db55e9df6f99fc3e9596433090).
Supplemental Material
Download Zip (1.7 MB)Acknowledgments
We thank Toolik Field Station and CH2MHill Polar Services for their logistical support of this project. Our research would not have been possible without the aid of undergraduate research assistants; we especially recognize Ruby An, Jacqueline Alfaro, Luis Del Val, Daniel Morrow, and O.J. Barrera.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bakker, E. S., H. Olff, M. Boekhoff, J. M. Gleichman, and F. Berendse. 2004. Impact of herbivores on nitrogen cycling: Contrasting effects of small and large species. Oecologia 138:91–101. doi:https://doi.org/10.1007/s00442-003-1402-5.
- Ballova, Z., L. Pekarik, V. Pis, and J. Sibik. 2019. How much do ecosystem engineers contribute to landscape evolution? A case study on Tatra marmots. Catena 182:104121. doi:https://doi.org/10.1016/j.catena.2019.104121.
- Bartlett, R. J., and D. S. Ross. 1988. Colorimetric determination of oxidizable carbon in acid soil solutions. Soil Science Society of America Journal 52:1191–92. doi:https://doi.org/10.2136/sssaj1988.03615995005200040055x.
- Batzli, G. O. 1978. The role of herbivores in mineral cycling. Environmental Chemistry and Processes, Department of Energy Series 45:95–112.
- Batzli, G. O., and C. Lesieutre. 1991. The influence of high quality food on habitat use by arctic microtine rodents. Oikos 60:299–306. doi:https://doi.org/10.2307/3545071.
- Batzli, G. O., and H. Henttonen. 1990. Demography and resource use by microtine rodents near Toolik Lake, Alaska, U.S.A. Arctic and Alpine Research 22:51–64. doi:https://doi.org/10.2307/1551720.
- Batzli, G. O., R. G. White, S. F. MacLean, F. A. Pitelka, and B. D. Collier. 1980. The herbivore-based trophic system. In An arctic ecosystem: The coastal tundra at Barrow, Alaska, ed. J. Brown, P. C. Miller, L. L. Tieszen, and F. L. Bunnell, 335–410. Stroudsburg, Pennsylvania: Dowden, Hutchinson & Ross, Inc.
- Borer, E., E. Seabloom, D. Gruner, W. Harpoole, H. Hillebrand, E. Lind, P. Adler, J. Alberti, T. Anderson, J. Bakker, et al. 2014. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–20. doi:https://doi.org/10.1038/nature13144.
- Brookes, P. C., A. Landman, G. Pruden, and D. S. Jenkinson. 1985. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nigtrogen in soil. Soil Biology and Biogeochemistry 17:837–42. doi:https://doi.org/10.1016/0038-0717(85)90144-0.
- Bueno, C. G., S. N. Williamson, I. C. Barrio, Á. Helgadóttir, and D. S. Hik. 2016. Moss mediates the influence of shrub species on soil properties and processes in alpine tundra. PloS One 11:e0164143. doi:https://doi.org/10.1371/journal.pone.0164143.
- Cahoon, S. M. P., P. F. Sullivan, E. Post, and J. M. Welker. 2012. Large herbivores limit CO2 uptake and suppress carbon cycle responses to warming in West Greenland. Global Change Biology 18:469–79. doi:https://doi.org/10.1111/j.1365-2486.2011.02528.x.
- Chew, R. M. 1974. Consumers as regulators of ecosystems: An alternative to energetics. Ohio Journal of Science 74:359–70.
- Clark, J. E., E. C. Hellgren, J. L. Parsons, E. E. Jorgensen, D. M. Engle, and D. M. Leslie. 2005. Nitrogen outputs from fecal and urine deposition of small mammals: Implications for nitrogen cycling. Oecologia 144:447–55. doi:https://doi.org/10.1007/s00442-005-0004-9.
- Cumming, D. H., and G. S. Cumming. 2003. Ungulate community structures and ecological processes: Body size, hoof area and trampling in African savannas. Oecologia 134:560–68. doi:https://doi.org/10.1007/s00442-002-1149-4.
- D’Angelo, E., J. Crutchfield, and M. Vandiviere. 2001. Rapid, sensitive, microscale determination of phosphate in water and soil. Journal of Environmental Quality 30:2206–09. doi:https://doi.org/10.2134/jeq2001.2206.
- Doane, T. A., and W. R. Horwath. 2003. Spectrophotometric determination of nitrate with a single reagent. Analytical Letters 36:2713–22. doi:https://doi.org/10.1081/AL-120024647.
- Edwards, K. A., and R. L. Jefferies. 2013. Inter-annual and seasonal dynamics of soil microbial biomass and nutrients in wet and dry low-Arctic sedge meadows. Soil Biology and Biochemistry 57:83–90. doi:https://doi.org/10.1016/j.soilbio.2012.07.018.
- Egelkraut, D., K.-Å. Aronsson, A. Allard, M. Åkerholm, S. Stark, and J. Olofsson. 2018. Multiple feedbacks contribute to a centennial legacy of reindeer on tundra vegetation. Ecosystems 21:1545–63. doi:https://doi.org/10.1007/s10021-018-0239-z.
- Environmental Data Center Team. 2017. Meteorological monitoring program at Toolik, Alaska, 99775. Fairbanks: Toolik Field Station, Institute of Arctic Biology, University of Alaska Fairbanks.
- Gornall, J. L., S. J. Woodin, I. S. Jónsdóttir, and R. van der Wal. 2011. Balancing positive and negative plant interactions: How mosses structure vascular plant communities. Oecologia 166:769–82. doi:https://doi.org/10.1007/s00442-011-1911-6.
- Gough, L. 2019a. Relative percent cover of plant species for years 2012-2017 in the Arctic long-term ecological research (ARC-LTER) 1989 moist acidic tundra (MAT89) experimental plots, toolik field station, Alaska. ver 1. Environmental Data Initiative. doi:https://doi.org/10.6073/pasta/f31def760db3f8e6cfee5fee07cc693e.
- Gough, L. 2019b. Relative percent cover of plant species for years 2013 2014 2016 2017 in LTER dry heath tundra experimental plots established in 1989, Arctic LTER toolik, field station Alaska ver 1. Environmental Data Initiative.
- Gough, L., and D. R. Johnson. 2018. Mammalian herbivory exacerbates plant community responses to long-term increased soil nutrients in two Alaskan tundra plant communities. Arctic Science 4:153–66. doi:https://doi.org/10.1139/as-2017-0025.
- Gough, L., E. A. Ramsey, and D. R. Johnson. 2007. Plant-herbivore interactions in Alaskan arctic tundra change with soil nutrient availability. Oikos 116:407–18. doi:https://doi.org/10.1111/oik.2007.116.issue-3.
- Gough, L., J. C. Moore, G. R. Shaver, R. T. Simpson, and D. R. Johnson. 2012. Above- and belowground responses of arctic tundra ecosystems to altered soil nutrients and mammalian herbivory. Ecology 93:1683–94. doi:https://doi.org/10.1890/11-1631.1.
- Gough, L., K. Shrestha, D. R. Johnson, and B. Moon. 2008. Long-term mammalian herbivory and nutrient addition alter lichen community structure in Alaskan dry heath tundra. Arctic, Antarctic, and Alpine Research 40:65–73. doi:https://doi.org/10.1657/1523-0430(06-087)[GOUGH]2.0.CO;2.
- Gough, L., P. A. Wookey, and G. R. Shaver. 2002. Dry heath arctic tundra responses to long-term nutrient and light manipulation. Arctic, Antarctic, and Alpine Research 34:211–18. doi:https://doi.org/10.1080/15230430.2002.12003486.
- Grellmann, D. 2002. Plant responses to fertilization and exclusion of grazers on an arctic tundra heath. Oikos 98:190–204. doi:https://doi.org/10.1034/j.1600-0706.2002.980202.x.
- Hogan, E. J., G. Minnullina, L. J. Sheppard, I. D. Leith, and P. D. Crittenden. 2010. Response of phosphomonoesterase activity in the lichen Cladonia portentosa to nitrogen and phosphorus enrichment in a field manipulation experiment. New Phytologist 186:926–33. doi:https://doi.org/10.1111/j.1469-8137.2010.03221.x.
- Holmgren, M., C.-Y. Lin, J. E. Murillo, A. Nieuwenhuis, J. Penninkhof, N. Sanders, T. van Bart, H. van Veen, H. Vasander, M. E. Vollebregt, et al. 2015. Positive shrub–tree interactions facilitate woody encroachment in boreal peatlands. Journal of Ecology 103:58–66. doi:https://doi.org/10.1111/1365-2745.12331.
- Howe, H. F., and J. S. Brown. 1999. Effects of birds and rodents on synthetic tallgrass communities. Ecology 80:1776–81. doi:https://doi.org/10.1890/0012-9658(1999)080[1776:EOBARO]2.0.CO;2.
- Hurri, R. S. K., S. Makkonen, and M. Hyvärinen. 2007. Differential responses of lichen symbionts to enhanced nitrogen and phosphorus availability: An experiment with Cladina stellaris. Annals of Botany 99:877–84. doi:https://doi.org/10.1093/aob/mcm042.
- Ims, R. A., J.-A. Henden, and S. T. Killengreen. 2008. Collapsing population cycles. Trends in Ecology & Evolution 23:79–86. doi:https://doi.org/10.1016/j.tree.2007.10.010.
- Ims, R. A., N. G. Yoccoz, and S. T. Killengreen (2011) Determinants of lemming outbreaks. Proceedings of the National Academy of Sciences of the United States of America 108:1970–74. doi:https://doi.org/10.1073/pnas.1012714108.
- Jefferies, R. L., D. R. Klein, and G. R. Shaver. 1994. Vertebrate herbivores and northern plant communities – Reciprocal influences and responses. Oikos 71:193–206. doi:https://doi.org/10.2307/3546267.
- Johnson, D. R., M. J. Lara, G. R. Shaver, G. O. Batzli, J. D. Shaw, and C. E. Tweedie. 2011. Exclusion of brown lemmings reduces vascular plant cover and biomass in Arctic coastal tundra: Resampling of a 50 + year herbivore exlosure experiment near Barrow, Alaska. Environmental Research Letters 6:045507. doi:https://doi.org/10.1088/1748-9326/6/4/045507.
- Joly, K., R. R. Jandt, and D. R. Klein. 2009. Decrease of lichens in Arctic ecosystems: The role of wildfire, caribou, reindeer, competition and climate in north-western Alaska. Polar Research 28:433–42. doi:https://doi.org/10.1111/j.1751-8369.2009.00113.x.
- Lajtha, K., C. T. Driscoll, W. M. Jarrell, and E. T. Elliot. 1999. Soil phosphorus: Characterization and total element analysis. In Standard soil methods for long-term ecological research, ed. G. P. Robertson, D. C. Coleman, C. S. Bledsoe, and P. Sollins, 115–42. New York: Oxford University Press.
- Lara, M. J., D. R. Johnson, C. Andresen, R. D. Hollister, and C. E. Tweedie. 2016. Peak season carbon exchange shifts from a sink to a source following 50+ years of herbivore exclusion in an Arctic tundra ecosystem. Journal of Ecology 105:122–31. doi:https://doi.org/10.1111/1365-2745.12654.
- Li, X., Q. Huang, X. Mi, Y. Bai, M. Zhang, and X. Li. 2018. Grazing every month minimizes size but boosts photosynthesis in Stipa grandis in the steppe of Inner Mongolia, China. Journal of Arid Land 10:601–11. doi:https://doi.org/10.1007/s40333-018-0011-4.
- Mack, M. C., E. A. G. Schuur, M. S. Bret-Hart, G. R. Shaver, and F. S. Chapin. 2004. Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature 49:243–257. doi:https://doi.org/10.1038/nature02887.
- Maguire, A. J., and R. J. Rowe. 2017. Home range and habitat affinity of the singing vole on the North Slope of Alaska. Arctic, Antarctic, and Alpine Research 49:243–57. doi:https://doi.org/10.1657/AAAR0016-035.
- Mallen-Cooper, M., S. Nakagawa, and D. J. Eldridge. 2019. Global meta-analysis of soil-disturbing vertebrates reveals strong effects on ecosystem patterns and processes. Global Ecology and Biogeography 28:661–79. doi:https://doi.org/10.1111/geb.12877.
- McKendrick, J. D., G. O. Batzli, K. R. Everett, and J. C. Swanson. 1980. Some effects of mammalian herbivores and fertilization on tundra soils and vegetation. Arctic and Alpine Research 12:565–78. doi:https://doi.org/10.2307/1550501.
- McLaren, J. R. 2019. Soil biogeochemical variables collected on the Arctic Long Term Ecological Research (ARC LTER) experimental plots in moist acidic and dry heath tundra, Arctic LTER Toolik Field Station, Alaska 2017 ver 2. Environmental Data Initiative. doi:https://doi.org/10.6073/pasta/5a5cbb785bde48522bde7b87c65d3c13.
- McLaren, J. R., A. Darrouzet-Nardi, M. N. Weintraub, and L. Gough. 2018. Seasonal patterns of nitrogen availability in moist acidic tundra. Arctic Science 4:98–109.
- McLaren, J. R., and K. M. Buckeridge. 2019. The importance of phosphorus versus nitrogen in Alaskan tundra: Above- and belowground response to multi-decadal nutrient amendments in two ecosystems. Ecosphere 10 (7):e02735. doi:https://doi.org/10.1002/ecs2.2735.
- McLaren, J. R., K. M. Buckeridge, M. J. Van de Weg, G. R. Shaver, J. P. Schimel, and L. Gough. 2017. Shrub encroachment in Arctic tundra: Betula nana effects on above- and belowground litter decomposition. Ecology 98:1361–76. doi:https://doi.org/10.1002/ecy.1790.
- Moen, J., and L. Oksanen. 1998. Long-term exclusion of folivorous mammals in two arctic-alpine plant communities: A test of the hypothesis of exploitation ecosystems. Oikos 82:333–46. doi:https://doi.org/10.2307/3546974.
- Mosbacher, J. B., A. Michelsen, M. Stelvig, H. Hjermstad-Sollerud, and N. M. Schmidt. 2018. Muskoxen modify plant abundance, phenology, and nitrogen dynamics in a high arctic fen. Ecosystems. doi:https://doi.org/10.1007/s10021-018-0323-4.
- Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’hara, G. L. Simpson, P. Solymos, et al. 2019. Vegan: Community ecology package. R package version 2.5-5. https://CRAN.R-project.org/package=vegan
- Olofsson, J., H. Tommervik, and T. V. Callaghan. 2012. Vole and lemming activity observed from space. Nature Climate Change 2:880–83. doi:https://doi.org/10.1038/nclimate1537.
- Olofsson, J., J. Moen, and L. Oksanen. 2002. Effects of herbivory on competition intensity in two arctic-alpine tundra communities with different productivity. Oikos 96:265–72. doi:https://doi.org/10.1034/j.1600-0706.2002.960208.x.
- Olofsson, J., L. Oksanen, T. Callaghan, P. E. Hulme, T. Oksanen, and O. Suominen. 2009. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology 15:2681–93. doi:https://doi.org/10.1111/()1365-2486.
- Olofsson, J., S. Stark, and L. Oksanen. 2004. Reindeer influence on ecosystem processes in thetundra. Oikos 105:386–96. doi:https://doi.org/10.1111/oik.2004.105.issue-2.
- Pajunen, A., R. Virtanen, and H. Roininen. 2008. The effects of reindeer grazing on the composition and species richness of vegetation in forest–tundra ecotone. Polar Biology 31:1233–44. doi:https://doi.org/10.1007/s00300-008-0462-8.
- Pastor, J., and R. J. Naiman. 1992. Selective foraging and ecosystem processes in boreal forests. American Naturalist 139:690–705. doi:https://doi.org/10.1086/285353.
- Post, E., and C. Pedersen (2008) Opposing plant community responses to warming with and without herbivores. Proceedings of the National Academy of Sciences of the United States of America 105:12353–58. doi:https://doi.org/10.1073/pnas.0802421105.
- R Core Team. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org.
- Rhine, E. D., R. L. Mulvaney, E. J. Pratt, and G. K. Sims. 1998. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Science Society of America Journal 62:473–80. doi:https://doi.org/10.2136/sssaj1998.03615995006200020026x.
- Rowe, R. J., and J. Steketee. 2019. Unpublished data.
- Rydgren, K., R. H. Økland, F. X. Picó, and H. Kroon. 2007. Moss species benefits from breakdown of cyclic rodent dynamics in boreal forests. Ecology 88:2320–29. doi:https://doi.org/10.1890/06-1634.1.
- Saiya-Cork, K. R., R. L. Sinsabaugh, and D. R. Zak. 2002. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biology and Biochemistry 34:1309–15. doi:https://doi.org/10.1016/S0038-0717(02)00074-3.
- Shaver, G. R., J. A. Laundre, M. S. Bret-Harte, F. S. Chapin, J. A. Mercado Diaz, A. E. Giblin, L. Gough, W. A. Gould, S. E. Hobbie, G. W. Kling, et al. 2014. Terrestrial ecosystems at Toolik Lake, Alaska. In Alaska’s changing Arctic, ed. J. E. Hobbie and G. W. Kling, 90–142. New York: Oxford University Press.
- Sitters, J., M. Cherif, D. Egelkraut, R. Giesler, and J. Olofsson. 2019. Long-term heavy reindeer grazing promotes plant phosphorus limitation in arctic tundra. Functional Ecology 33:1233–42. doi:https://doi.org/10.1111/1365-2435.13342.
- Sitters, J., M. Te Beest, M. Cherif, R. Giesler, and J. Olofsson. 2017. Interactive effects between reindeer and habitat fertility drive soil nutrient availabilities in arctic tundra. Ecosystems 20:1266–77. doi:https://doi.org/10.1007/s10021-017-0108-1.
- Stark, S., D. Egelkraut, K.-Å. Aronsson, and J. Olofsson. 2019. Contrasting vegetation states do not diverge in soil organic matter storage: Evidence from historical sites in tundra. Ecology 100:e02731. doi:https://doi.org/10.1002/ecy.2019.100.issue-7.
- Stark, S., and D. Grellmann. 2002. Soil microbial responses to herbivory in an arctic tundra heath at two levels of nutrient availability. Ecology 83:2736–44. doi:https://doi.org/10.1890/0012-9658(2002)083[2736:SMRTHI]2.0.CO;2.
- Tikhomirov, B. A. 1959. Relationship of the animal world and the plant cover of the tundra. Botanical Institute, Academy of Sciences of the U.S.S.R. Trans. E. Issakoff and T. W. Barry TW. Edmonton: Barry Institute, University of Alberta.
- Tilman, D. 1988. Plant strategies and the dynamics and structure of plant communities. Princeton, NJ: Princeton University Press.
- Tuomi, M., S. Stark, K. S. Hoset, M. Vaisanen, L. Oksanen, F. J. Murguzur, H. Tuomisto, J. Dahlgren, and K. A. Brathen. 2019. Herbivore effects on ecosystem process rates in a low-productive system. Ecosystems 22:827–43. doi:https://doi.org/10.1007/s10021-018-0307-4.
- Vaisanen, M., H. Ylanne, E. Kaarlejarvi, S. Sjogersten, J. Olofsson, N. Crout, and S. Stark. 2014. Consequences of warming on tundra carbon balance determined by reindeer grazing history. Nature Climate Change 4:384–88. doi:https://doi.org/10.1038/nclimate2147.
- van der Wal, R., S. M. J. van Lieshout, and M. J. J. E. Loonen. 2001. Herbivore impact on moss depth, soil temperature and arctic plant growth. Polar Biology 24:29–32. doi:https://doi.org/10.1007/s003000000170.
- van Klink, R., M. Schrama, S. Nolte, J. P. Bakker, M. F. WallisDeVries, and M. P. Berg. 2015. Defoliation and soil compaction jointly drive large-herbivore grazing effects on plants and soil arthropods on clay soil. Ecosystems 18:671–85. doi:https://doi.org/10.1007/s10021-015-9855-z.
- Voroney, R. P., P. C. Brooks, and R. P. Beyaert. 2006. Soil microbial biomass C, N, P, and S. In Soil sampling and methods of analysis, ed. M. R. Carter and E. G. Gregorich, 637–652. Boca Raton: Lewis.
- Walker, D. A., J. G. Bockheim, F. S. Chapin III, W. Eugster, F. E. Nelson, and C. L. Ping. 2001. Calcium-rich tundra, wildlife, and the “Mammoth Steppe”. Quaternary Science Reviews 20:149–63. doi:https://doi.org/10.1016/S0277-3791(00)00126-8.
- Walsh, N. E., T. R. McCabe, J. M. Welker, and A. N. Parsons. 1997. Experimental manipulations of snow-depth: Effects on nutrient content of caribou forage. Global Change Biology 3:158–64. doi:https://doi.org/10.1111/j.1365-2486.1997.gcb142.x.
- Wardle, D. A., K. I. Bonner, and G. M. Barker. 2002. Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Functional Ecology 16:585–95. doi:https://doi.org/10.1046/j.1365-2435.2002.00659.x.
- Ylanne, H., and S. Stark. 2019. Distinguishing rapid and slow C cyling feedbacks to grazing in sub-arctic tundra. Ecosystems 22:1145–59. doi:https://doi.org/10.1007/s10021-018-0329-y.
- Zamin, T. J., and P. Grogan. 2013. Caribou exclusion during a population low increases deciduous and evergreen shrub species biomass and nitrogen pools in low Arctic tundra. Journal of Ecology 101:671–83. doi:https://doi.org/10.1111/1365-2745.12082.