ABSTRACT
Lichen heaths are decreasing in abundance in alpine and Arctic areas because of an increased competition with shrubs. This shift in vegetation might have important consequences for the soil temperature. The aim of this study is to find the drivers of the variation in soil temperature below lichen heaths and shrubs. Moreover, we want to gain more insight in the variability of the soil temperature below lichen heaths. We measured the soil temperature in thirty lichen plots and fifteen shrub plots in an alpine area in southern Norway during July and August 2019. We applied several treatments to study the drivers behind the variation in soil temperature between lichen heaths and shrub vegetation. We found that the average soil temperature was 1.45°C higher below lichen heaths than below shrub vegetation. Moreover, we measured a difference in soil temperature of 1.66°C between north- and south-facing lichen heaths, which contributes to the small-scale spatial variability in soil temperature below lichen heaths. Based on our experiments, we conclude that the buffering capacity of the litter layer below shrubs and shading of the soil by the shrub canopy lead to a lower soil temperature below shrubs compared to lichen heaths during the summer.
Introduction
Warming of the alpine and Arctic areas changes the local vegetation composition (Wilson and Nilsson Citation2009; Vanneste et al. Citation2017; Maliniemi et al. Citation2018). In these areas, lichens are one of the most vulnerable growing forms being subject to changes (Bjerke Citation2011; Macander et al. Citation2022). Lichen heaths are decreasing in abundance because of an increased competition with vascular plants as a result of climate change (Cornelissen et al. Citation2001; Joly, Jandt, and Klein Citation2009). For example, Fraser et al. (Citation2014) estimated that the average lichen cover decreased by 24 percent in the western Canadian Arctic since the 1980s. Shrubs are reported to increase in abundance at the expense of lichens (Pajunen, Oksanen, and Virtanen Citation2011; Fraser et al. Citation2014; Chagnon and Boudreau Citation2019). This shift in vegetation can have significant implications for the microclimate and soil temperature in alpine and Arctic areas.
The soil temperature in alpine and Arctic areas is an important variable that determines many ecosystem functions. For example, soil temperature is a driving force for the microbial activity, litter decomposition, and the carbon cycle (e.g., Hobbie Citation1996; Schimel, Bilbrough, and Welker Citation2004; Saito, Kato, and Tang Citation2009; Gavazov Citation2010; Hursh et al. Citation2017). Moreover, the soil temperature is an important factor determining the vegetation composition (Odland et al. Citation2017; Sundstøl and Odland Citation2017), which in turn can alter the functioning of the ecosystem and the soil temperature directly (Aalto, le Roux, and Luoto Citation2013; Myers‐Smith and Hik Citation2013; Olefeldt et al. Citation2013; Lafleur and Humphreys Citation2018; Heijmans et al. Citation2022). For example, the increasing soil temperature due to the recent climate change is one of the drivers of the greening of the Arctic (Berner et al. Citation2020; Mekonnen et al. Citation2021), and greening of the Arctic (e.g., shrubification) leads to lower soil temperatures during the summer (Blok et al. Citation2010; Myers-Smith et al. Citation2011). However, the soil temperature in alpine and Arctic areas varies over small spatial and temporal scales (Wundram, Pape, and Löffler Citation2010; Graham et al. Citation2012; Ford et al. Citation2013; Aalto et al. Citation2018). Moreover, numerous aspects of soil temperature conditions (e.g., mean temperatures, temperature extremes, thermal sums) can be of interest regarding the temperature regime that is optimal or destructive for plant species (Körner and Hiltbrunner Citation2017). Therefore, fine-scale temporal and spatial measurements are necessary to study the soil temperature in alpine and Arctic areas (Pape and Löffler Citation2017; Lembrechts et al. Citation2020).
Multiple studies have considered the effect of shrub expansion on the soil temperature. Shrubification leads to an increase in soil temperature during winter owing to the development of an insulating snowpack initiated by trapping of snow by the shrub canopy (Myers‐Smith and Hik Citation2013; Frost et al. Citation2018). However, the expansion of shrubs tends to lower the soil temperature during summer. Multiple studies found that shading of the soil by shrub canopies is an important driver of these lower soil temperatures (Blok et al. Citation2010; Myers‐Smith and Hik Citation2013; Aguirre, Benhumea, and McLaren Citation2021). For example, Frost et al. (Citation2018) reported that mature shrubs (Alnus viridis) cool the soil up to 9°C compared to open tundra in Siberia during the summer months.
The soil temperature below natural lichen heaths is less studied. Multiple studies have suggested or assumed that lichens keep the underlying soil cool compared to other vegetation types owing to the high albedo of lichens (e.g., Klein and Shulski Citation2011; Odland et al. Citation2017). Therefore, a shift from lichen heaths to shrub vegetation might be expected to lead to higher soil temperatures, despite the cooling effect of shading by the shrub canopy on the soil. However, studies that have aimed to measure the difference in soil temperature below lichen heaths and shrub vegetation are rare. The few, recent studies that have simultaneously measured the soil temperature below lichens and other vegetation types measured higher or equal summer soil temperatures below lichens compared to shrubs (Mikola et al. Citation2018; Grünberg et al. Citation2020; Aartsma et al. Citation2021). For instance, Grünberg et al. (Citation2020) measured the difference in soil temperature below different vegetation types including lichens and dwarf shrubs in Canada. They found that the soil temperature below lichen heaths was lower than that below dwarf shrubs during winter but could not find a difference in soil temperature between these two vegetation types during summer.
In an earlier paper (Aartsma et al. Citation2021), we measured the difference in microclimatic conditions between lichen heaths and shrub vegetation during summer using a paired sampling design. We found higher soil temperatures below lichens than below shrubs, despite the fact that the lichen heaths had a higher albedo and a lower net radiation. We proposed shading of the soil by the shrub canopy, the buffering capacity of the litter layer below the shrubs, and a higher evapotranspiration rate for shrub vegetation (e.g., more evaporative cooling) as potential reasons for the difference in soil temperature between lichens and shrubs. A higher evapotranspiration rate at shrub vegetation compared to lichen heaths can lead to lower temperatures below shrubs, because more of the available energy is used for evapotranspiration, the latent heat flux, than for heating the soil and the lowest air layer through sensible heat fluxes. A major factor in controlling the latent heat flux is the moisture availability. The cooling effect on the soil by shading of the shrub canopy has been studied before (Blok et al. Citation2010; Myers‐Smith and Hik Citation2013; Frost et al. Citation2018; Aguirre, Benhumea, and McLaren Citation2021), whereas the effect of a thicker litter layer and a higher evapotranspiration rate in shrubs relative to lichens on the soil temperature is less studied (Loranty et al. Citation2018).
With this study, we intend to advance the knowledge of the variability in soil temperature below alpine lichen heaths and shrub vegetation during the summer. Therefore, the first aim of this study is to assess whether and how the litter layer and moisture availability affect the soil temperature below lichens and shrubs. To address this aim, soil temperatures below plots with and without litter layers were compared, and moisture availability was varied by adding water to selected plots. The second aim is to quantify the difference in soil temperature between lichen heaths and shrub vegetation during the summer season. In addition, we want to gain more insight into the variation in soil temperature within lichen heaths. In the alpine environment of our study location, topographical variations are likely very important in this respect, and therefore the third aim is to quantify the variation in soil temperature initiated by topographical exposition. Small-scale variability in topography can play an important role in the plant species distribution and survival in mountainous areas (Scherrer and Körner Citation2011; Opedal, Armbruster, and Graae Citation2015) and can therefore be important for the distribution of vulnerable species like lichens. To reach these aims, we selected thirty lichen-dominated and fifteen shrub-dominated plots in an alpine area in southern Norway and monitored the soil temperature with a fine-scale temporal resolution during the summer of 2019. We applied specific treatments on some of these lichen and shrub plots to study the effect of the litter layer and evaporative cooling on the soil temperature. We hypothesize that (1) lichen plots have a higher soil temperature than shrub plots, (2) north-facing lichen plots have lower soil temperatures than south-facing lichen plots, (3) the litter layer decreases the average soil temperature and daily soil temperature variation below shrubs due to its buffering capacity, and (4) a higher soil moisture availability will generate lower soil temperatures.
Methods
Study area
The study area is Imingfjell, an alpine area in southern Norway (elevation range 1,100–1,350 m.a.s.l., 60.1901° N, 8.5724° E). The landscape in the study area has an undulating character with distinctive ridgetops and snowbeds, which have a height difference usually not more than approximately 5 m (). The ridgetops are mainly dominated by lichen heaths, with Cladonia, Flavocetraria, Cetraria, and Alectoria being the most dominant genera (Aartsma et al. Citation2020). Most common shrub species are Betula nana and Empetrum nigrum, which are located on the midslopes and on the ridgetops. The soils in the area consist of coarse-grained material with a silt and clay fraction of 5–20 percent in the matrix and are classified as podzols or show clear signs of podsolization (Aartsma et al. Citation2021). A nearby, but slightly lower elevation, climate station measured an average yearly air temperature of 0.5°C with a yearly precipitation of 550 mm (MET Norway Citation2019).
Figure 1. Location of Imingfjell in southern Norway and a picture of the study site. Lichen heaths were mainly located on the ridgetops, with Cladonia, Flavocetraria, Cetraria, and Alectoria being the most dominant genera. Shrubs (mainly B. nana and E. nigrum) were located on the midslopes and on the ridgetops. The lichen north-facing and lichen south-facing plots were located on the slopes of the ridgetops. Figure from Aartsma et al. (Citation2020).

Data collection
Within the study area, we selected a study site of approximately 0.5 km2. Within this study site, we subjectively selected thirty lichen-dominated and fifteen shrub-dominated plots distributed over the entire site. All selected plots were located on the ridgetops and had a size of 1 m2. Five of the lichen-dominated plots had a clear (>10°) north-facing slope and five of the lichen-dominated plots had a clear (>10°) south-facing slope. These slopes were located near the ridgetops and therefore represent small-scale topography (). All remaining plots had a slope lower than 6°, which we consider flat. In each of the plots, we buried two LogTag TRIX-8 soil temperature sensors at 5-cm depth below the mineral soil surface. These two sensors were buried 50 cm apart and 25 cm from the edge of the plot. The sensors measured the soil temperature every 15 minutes during the field season (4 July–8 August). After two weeks (4–18 July), we applied three treatments on fifteen lichen-dominated plots and two treatments on ten shrub-dominated plots. An overview of the treatments is given in . The litter treatments (lichen add litter and shrub remove litter) were applied to study the insulating capacity of the litter layer that is usually present below shrub vegetation. The watering treatments (lichen wet and shrub wet) were applied to increase the available moisture in the plots, which can enhance the evapotranspiration and can in turn affect the soil temperature pattern below the lichen- and shrub-dominated plots. By removing the lichens from the lichen plot (lichen bare soil), we wanted to study the insulating properties of lichens compared to the insulating properties of the litter below the shrub plots. The treatments were randomly assigned to the plots. Due to the weather conditions and other activities during the field campaign, the treatment implementation took six days. After the application of the treatments, we measured the soil temperature for sixteen days (24 July–8 August). However, it was not possible to water the plots for one day during this period, so this day and the following day were excluded from the analysis, leaving fourteen days of measurements for the period after the treatment.
Table 1. The different plot types and treatment methods that we used during this study.
In addition to the soil temperature, several other plot-specific variables were estimated. We determined the vegetation composition by visually estimating the percentage cover for all vascular plants and lichen species. We also measured the vegetation height along a north–south and east–west transect with 10 cm intervals, which resulted in twenty-two measurements per plot. Moreover, we determined the thickness of the litter layer above every soil temperature sensor. In addition, we measured the soil moisture once during the treatment period with a Delta-T SM150T soil moisture sensor on a dry day (3 August) after two days without rain. Four measurements were performed in each corner of every plot. We assessed the slope angle and the aspect of the plots with a compass and a clinometer. The background weather conditions were measured with a HOBO RX3000 remote weather station on one location in the study area. This weather station measured, among others, the air temperature at 2 m height, which was used for the data analysis.
Data analysis
For each treatment, we collected ten time series of soil temperature. We visually checked these time series for large deviations from the expected pattern. Time series from two sensors (one in a lichen north-facing plot and one in a lichen control plot) were omitted for further analysis, because one sensor did not conduct any measurements and the other sensor showed unrealistic measurements (i.e., a continuous soil temperature higher than 20°C).
We calculated the mean soil temperature of the entire field season (4 July–8 August) per soil temperature sensor for the plots on which no treatment was applied (lichen control, lichen north-facing, lichen south-facing, and shrub control). To test whether there was a difference in soil temperature between these plot types, we built a mixed effects model with plot type as a fixed effect and plot number as a random effect. Subsequently, Tukey’s test was used to test which plot types differed from each other.
To study the effect of the treatments on the soil temperature regime, we calculated various soil temperature variables (). These calculations led to one value per sensor for the period before the treatment (4–18 July) and one value per sensor for the period after the treatment (24 July–8 August) for each soil temperature variable. We recalculated the 5-minute air temperature measurements to 15-minute averages to match the sampling interval of the soil temperature measurements and to achieve a better estimation of the timing of the maximum and minimum of the daily air temperature wave, because this wave is smoothed from noise. Because our sample design was equivalent to a before–after–control–impact approach (Stewart-Oaten, Murdoch, and Parker Citation1986), we analyzed our data in a similar way as other studies that have followed this approach (e.g., Gaffney et al. Citation2020). Therefore, we built mixed effects models for each of the soil temperature variables and for each of the treatments. Each model contained a fixed effect of treatment (control vs. treatment), a fixed effect of time (before vs. after treatment). and their interaction (Treatment × Time). Plot number was included as a random effect. We considered a significant effect of the treatment on the soil temperature variable when the interaction term Treatment × Time had a p value < .05. All analyses were performed in R v4.0.2 (R Core Team Citation2020) with the packages lme4 (Bates et al. Citation2015) and multcomp (Hothorn, Bretz, and Westfall Citation2008).
Table 2. A list of the soil temperature variables that were used for analysis and their calculation method.
Results
General plot characteristics and weather conditions
All lichen plots contained a minimum of 90 percent pale-colored lichen species. Most of these lichen plots (twenty-five) were dominated by Flavocetraria nivalis, F. cucullata, and Alectoria ochroleuca. The other five lichen plots were dominated by Cladonia arbuscula and C. stellaris. The mean (± SE) vegetation height of the lichen plots was 6.7 (± 0.2) cm. All shrub plots consisted of at least 80 percent B. nana. The mean (± SE) vegetation height of the shrub plots was 27.8 (± 1.1) cm, and the mean (± SE) thickness of the litter layer below the shrub plots was 5.7 (± 0.5) cm.
The mean air temperature during the entire field season was 11.3°C. During the period before the treatments, the mean air temperature at 2 m height was 10.1°C with a total precipitation of only 4.8 mm. The period after the treatments was slightly warmer (12.8°C) but much wetter (27 mm). Time series of the air temperature, precipitation, relative humidity, wind speed, and incoming solar radiation during the field season are provided in Supplementary Information 1.
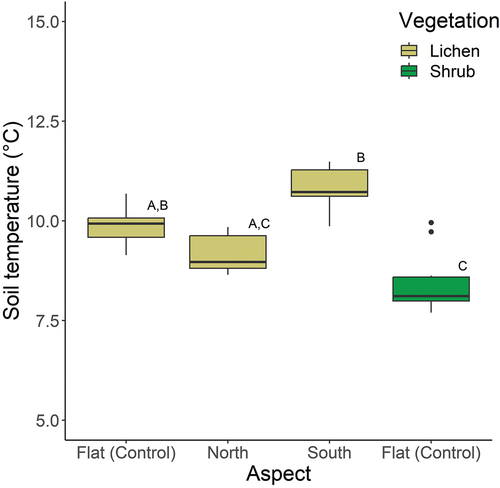
Difference in soil temperature between vegetation type and aspect
The mean (± SE) soil temperature below the lichen control plots was 9.89°C (± 0.16), and the mean (± SE) soil temperature below the shrub control plots was 8.44°C (± 0.25; ). The soils under the lichen control plots were thus on average 1.45°C warmer than the soils under the shrub control plots. The mean (± SE) soil temperature below the lichen north-facing plots was 9.16°C (± 0.16), which is 1.66°C lower than the mean (± SE) soil temperature below the lichen south-facing plots (10.82°C ± 0.16°C).
Treatment experiment
Removing the lichens (lichen bare soil) generated a higher mean soil temperature and affected other soil temperature characteristics (, ). Before the treatment, the mean soil temperature below the lichen bare soil plots was 0.15°C lower than the mean soil temperature below the lichen control plots, whereas after the treatment, the mean soil temperature below the lichen bare soil plots was 0.90°C higher than the lichen control plots (Supplementary Information 3). This indicates that lichens insulated the soil with 1.05°C (). Similarly, removing the lichens initiated an increase in daily amplitude of 4.0°C (), a decrease in delay in maximum soil temperature of 1.4 hours (), and a decrease in delay in minimum soil temperature of 1.1 hours () compared to the lichen control plots. In addition, the cumulative maximum soil temperature was 47.54 degree days higher (), the cumulative minimum soil temperature was 8.44 degree days lower () and the heat sum was 19.55 degree days higher () compared to the lichen control plots (Supplementary Information 3).
Figure 3. Effect of the different treatments and vegetation type on the soil temperature variables. Averages per treatment per period (before/after treatment) are given, with error bars indicating the standard error. ΔTimeMax is the mean of the difference in time between maximum air temperature and maximum soil temperature, ΔTimeMin is the mean of the difference in time between minimum air temperature and minimum soil temperature, CumSTMax is the sum of the maximum daily soil temperatures, and CumSTMin is the sum of the minimum daily soil temperatures.
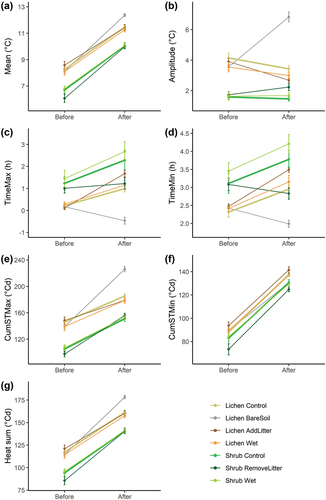
Table 3. Overview of the significance of the interaction Treatment × Time in the mixed effects models for the different treatments and different soil temperature variables.
Adding a 5-cm-thick layer of B. nana litter below the lichen mat (lichen add litter) cooled the soil and had an effect on some of the other soil temperature variables. The mean soil temperature decreased by 0.38°C below the lichen add litter plots compared to the lichen control plots (, Supplementary Information 3). Moreover, the delay in maximum and minimum soil temperature increased by 0.78 and 0.38 hours, respectively (). Furthermore, adding a litter layer below the lichen mat decreased the cumulative maximum soil temperature by 8.75 degree days () and the heat sum by 5.24 degree days () compared to the lichen control plots (Supplementary Information 3).
Removing the litter layer below the shrub plots (shrub remove litter) resulted, among others, in a warming of the soil. The difference in mean soil temperature between shrub remove litter and shrub control was 0.64°C before the treatment, whereas this difference was only 0.07°C after the treatment (Supplementary Information 3). Therefore, removing the litter layer caused an increase in mean soil temperature of 0.57°C relative to the shrub control plots (). Moreover, this treatment increased the amplitude by 0.63°C (), decreased the delay in minimum soil temperature by 0.92 hours (), increased the cumulative maximum soil temperature by 12.57 degree days (), and increased the heat sum by 8.15 degree days () compared to the shrub control plots (Supplementary Information 3). The mean (±SE) thickness of the litter layer that was removed in the shrub remove litter plots was 5.5 cm (±0.7).
Creating wet plots (lichen wet and shrub wet) did not have an effect on any of the soil temperature variables for both the lichen and shrub plots (), despite the wet plots having a higher soil moisture than the control plots (Supplementary Information 4).
Temporal dynamics
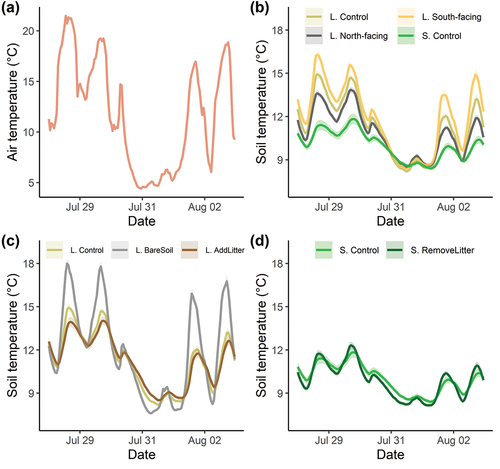
The effect of different plot types and treatments depends clearly on the background weather conditions, as is evident from an analysis of the response during six consecutive days that included a relatively cold period (). During these six days, the soil temperature response to a rapid decrease in air temperature emphasizes the buffering capacity of lichens and litter. The lichen bare soil plots and the shrub remove litter plots cooled down faster and more rigorously compared to the control plots (). The lichen add litter plots cooled down slower and less rigorously compared to the lichen control plots (). The soil temperature below the lichen north-facing plots did not differ from the soil temperature below lichen south-facing plots during cold and foggy days, whereas they had a lower soil temperature during warm and sunny days (). Moreover, the lichen control plots did not have a higher soil temperature than the shrub control plots during the cold and foggy period ().
Figure 4. Time series of six consecutive days of (a) the air temperature, (b) the soil temperature in the plots without treatment, (c) the soil temperature in the lichen plots with treatment, and (d) the soil temperature in the shrub plots with treatments. Each time series is the mean of all sensors per treatment with the standard error (shaded areas). Only the treatments with a significant effect on the soil temperature variables are displayed. See for details on the treatments.
Discussion
Vegetation type and aspect
The higher mean soil temperature below the lichen control plots compared to the shrub control plots during the summer is in line with our first hypothesis and consistent with earlier studies that have measured higher soil temperatures below lichens compared to shrubs during summer (Mikola et al. Citation2018; Aartsma et al. Citation2021). The average difference in soil temperature between our lichen and shrub plots (1.45°C) is close to the difference in soil temperature between shrubs and open tundra plots (2°C) found by Myers‐Smith and Hik (Citation2013) in Canada in July. The lower average soil temperature of the lichen north-facing plots compared to the lichen south-facing plots is in line with our second hypothesis and shows that lichen heaths are not an exception from the usual pattern that north-facing slopes have lower soil temperatures than south-facing slopes in alpine areas (Barry Citation2008; Wundram, Pape, and Löffler Citation2010; Winkler et al. Citation2016). These results reveal that even within the small-scale topography of our study area, the soil temperatures below lichen heaths on north- and south-facing slopes differ. This contributes to the large variation in soil temperature over short distances and might subsequently increase the plant species diversity in alpine areas (Scherrer and Körner Citation2011; Opedal, Armbruster, and Graae Citation2015). Furthermore, this variation is strongly dependent on the background weather conditions, because the difference in soil temperature between north- and south-facing slopes is present during sunny and warm days but is absent during cold and cloudy conditions ().
Treatment experiment
Our results of the treatment experiment are only partly in line with our third and fourth hypotheses. We found that the shrub litter indeed has the capacity to buffer the soil temperature below shrubs. The thermal conductivity of organic matter is low (0.25 W m−1 K−1 for organic matter vs, 1.70 W m−1 K−1 for a sandy alpine/Arctic soil (Beringer et al. Citation2001; Barrere et al. Citation2017) and, therefore, the soil with a litter layer will gain and lose heat less easily than a soil without litter layer (Oke Citation2002). This leads to lower soil temperatures and lower soil temperature fluctuations during the summer for soils with a litter layer (e.g., soils below shrubs) than soils without a litter layer (e.g., soils below lichens). Interestingly, shows that the CumSTMax (sum of the maximum daily soil temperatures; ) and the heat sum () were lower in the shrub removelitter plots than in the lichen control plots after the treatment was applied. This implies that, in addition to the litter layer, other processes or vegetation characteristics induce a lower soil temperature below shrubs relative to the soil temperature below lichens.
A factor that could lead to a lower soil temperature below shrubs compared to lichens is a higher evapotranspiration rate for the shrub plots compared to the lichen plots. However, we did not find an effect of an increased availability of moisture on any of the soil temperature variables for both the lichen wet plots and the shrub wet plots. Because we expected that the effect of an increase in available moisture and thereby a potential increase in evaporative cooling on the soil temperature would be the largest on warm days, we selected the five warmest days of the period after the treatment and repeated the analysis in the same way with the five warmest days representing the period after the treatment. However, during these five warm days, we did not find an effect of adding moisture on any of the soil temperature variables for both the lichen wet and shrub wet plots. Therefore, we found no evidence that a higher moisture availability resulted in lower soil temperatures, and our fourth hypothesis cannot be confirmed by our results. Conceivably, the evaporative cooling generated by the moist conditions was counterbalanced by an increased thermal conductivity of the soil and the lichens because of the higher moisture content (Beringer et al. Citation2001; O’Donnell et al. Citation2009; Beer et al. Citation2018). For instance, Oke (Citation2002) reported that the thermal conductivity of a saturated sandy soil (2.20 W m−1 K−1) is more than seven times larger than the thermal conductivity of a dry sandy soil (0.30 W m−1 K−1).
In an earlier study (Aartsma et al. Citation2021), we hypothesized that shading of the soil by the shrub canopy could also lead to a lower soil temperature below shrubs than below lichens. Although we did not test the effect of shading by the shrub canopy on the soil temperature during this study, multiple studies have reported this effect (Blok et al. Citation2010; Myers‐Smith and Hik Citation2013; Frost et al. Citation2018; Loranty et al. Citation2018; Aguirre, Benhumea, and McLaren Citation2021). Moreover, the findings in our study suggest that shading by shrub canopy causes a lower soil temperature below shrubs compared to lichens. The difference in CumSTMin (sum of the minimum daily soil temperatures) between the lichen- and shrub-dominated plots is marginal (), whereas the difference in CumSTMax between the lichen- and shrub-dominated plots is clear (). This could be an indication of the shading effect, because the shading by shrubs is absent during the time that the minimum soil temperatures occur (usually between 0400 and 0800), when the incoming solar radiation is absent or minor. The shading effect of the shrubs is present during the time that the maximum soil temperatures occur (usually between 1500 and 1900), whereas the incoming solar radiation is high at that time. Furthermore, the difference in delay in minimum soil temperature between lichen- and shrub-dominated plots is still present () because the litter layer buffers the soil temperature from a decreasing air temperature during the morning. Therefore, we hypothesize that the lower soil temperature below shrubs compared to lichens is governed by a combined effect of the buffering capacity of the litter layer below shrubs and shading of the soil by the shrub canopy.
The lichen bare soil treatment had the largest effect on the soil temperature variables. This confirms the importance of the buffering capacity of the lichens relative to the other treatments. Moreover, it supports other studies that have reported the strong insulating capacity of lichens (Beringer et al. Citation2001; Gold, Glew, and Dickson Citation2001; Macias-Fauria et al. Citation2008; Porada, Ekici, and Beer Citation2016; Nystuen et al. Citation2019; Van Zuijlen et al. Citation2020). In line with our results, Nystuen et al. (Citation2019) reported, in addition to a lower mean soil temperature, a lower maximum and higher minimum soil temperature below lichens compared to bare soil. In addition to a lower soil temperature below lichens compared to bare soil, both Nystuen et al. (Citation2019) and Van Zuijlen et al. (Citation2020) also measured different soil temperatures below monocultures of several lichen species driven by the distinct characteristics of the lichen species. Our lichen plots were dominated by mixtures of the genera Cladonia, Flavocetraria, Cetraria, and Alectoria. This might therefore have led to additional variation in the soil temperature between our lichen plots.
Implications for alpine and Arctic areas
Based on our results, several potential implications for alpine and Arctic areas can be foreseen. However, care needs to be taken, because our measurements were only conducted for one month in the summer. We thus did not measure temperatures for the whole growing season or directly after snowmelt. Moreover, we note that temperatures during winter and spring could potentially have influenced soil temperatures during the entire growing season.
According to our results, a shift from lichen heaths to shrub vegetation may induce lower variation in soil temperature on a daily scale during the summer, especially on warm, sunny days. However, this shift might also lead to less variation in soil temperature on a yearly scale. Our measurements indicate that this shift will generate lower soil temperatures in the summer, though other studies have reported that shrub expansion will increase the soil temperature during winter due to trapping of snow by the shrub canopy. Myers‐Smith and Hik (Citation2013) reported that the soil temperature is 4°C to 5°C higher below shrubs compared to open tundra patches during January. We expect that the difference in winter soil temperatures between lichen heaths and shrub vegetation will be larger than 4°C to 5°C, because lichen heaths are mainly located on windswept ridges, which are characterized by low soil temperatures during winter due to the absence of an insulating snow layer (Dahl Citation1956; Sundstøl and Odland Citation2017). Therefore, the potential implications of a change in soil temperature regime—for example, an increased nutrient availability for plant growth as a consequence of more microbial activity and higher litter decomposition rates (Schimel, Bilbrough, and Welker Citation2004; Gavazov Citation2010)—might be relatively large when a lichen heath shifts to shrub vegetation. An increased nutrient availability would further improve the growing conditions for shrubs and would therefore improve the competitiveness of shrubs over lichen heaths (Zamin and Grogan Citation2012).
Although we reveal that the soil temperature during summer may decrease after lichen heaths develop into a shrub-rich environment, this might be only a short-term effect. The ongoing climate change, amplified by the shrubification of alpine and Arctic areas (Chapin et al. Citation2005; Pearson et al. Citation2013), will lead to higher air temperatures. For example, the air temperature in Norway is projected to increase by 3°C during the current century (2000–2100) according to the intermediate scenario (RCP4.5) of the Intergovernmental Panel on Climate Change (Hanssen-Bauer et al. Citation2017). The increase in air temperature will ultimately lead to higher soil temperatures. This warming effect of an increase in air temperature on the soil temperature might overwhelm the cooling effect of the litter layer and the shading by the shrub canopy (Lawrence and Swenson Citation2011; Bonfils et al. Citation2012). Although the cooling effect of the litter and the shading of the shrub canopy will still be present under warmer conditions, both effects will probably not be able to keep future soil temperatures below or around current soil temperatures. Therefore, the net effect of the shift from lichen heaths to shrub vegetation might be an increase in soil temperature when longer timescales (e.g., multiple decades) are considered.
Conclusion
We hypothesized that (1) lichen plots have a higher soil temperature than shrub plots, (2) north-facing lichen plots have lower soil temperatures than south-facing lichen plots, (3) the litter layer decreases the soil temperature and daily soil temperature variation below shrubs due to its buffering capacity, and (4) evaporative cooling will generate lower soil temperatures. We found that the soil temperature below lichen heaths was 1.45°C higher than the soil temperature below shrub vegetation during summer. Moreover, we show that small-scale topography leads to more variation in soil temperature below lichen heaths because we measured a difference in soil temperature of 1.66°C between north- and south-facing lichen heaths. However, this difference in soil temperature between lichen heaths and shrub vegetation and the variability in soil temperature within lichen heaths is dependent on the weather conditions, because we found minor variation in soil temperature during cold and foggy days. Based on our treatment experiment, we conclude that the buffering capacity of the litter layer leads to the lower soil temperature below shrubs compared to lichens during the summer. Moreover, our results show indications that shading of the soil by the shrub canopy is an additional driver of the variation in soil temperature between lichen heaths and shrub vegetation. We were not able to find evidence that an increased moisture availability and therefore a potential increase in evaporative cooling induces the lower soil temperature below shrubs than below lichens. With this study, we advanced the knowledge of soil temperature dynamics in an alpine environment and gained insights into the consequences of the foreseen shift from lichen heaths to shrub vegetation.
Supplemental Material
Download Zip (890.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data are available from the authors on request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15230430.2023.2209397.
Additional information
Funding
References
- Aalto, J., P. C. le Roux, and M. Luoto. 2013. Vegetation mediates soil temperature and moisture in Arctic-alpine environments. Arctic, Antarctic, and Alpine Research 45, no. 4: 429–13. doi:10.1657/1938-4246-45.4.429.
- Aalto, J., D. Scherrer, J. Lenoir, A. Guisan, and M. Luoto. 2018. Biogeophysical controls on soil-atmosphere thermal differences: Implications on warming Arctic ecosystems. Environmental Research Letters 13, no. 7: 074003. doi:10.1088/1748-9326/aac83e.
- Aartsma, P., J. Asplund, A. Odland, S. Reinhardt, and H. Renssen. 2020. Surface albedo of alpine lichen heaths and shrub vegetation. Arctic, Antarctic, and Alpine Research 52, no. 1: 312–22. doi:10.1080/15230430.2020.1778890.
- Aartsma, P., J. Asplund, A. Odland, S. Reinhardt, and H. Renssen. 2021. Microclimatic comparison of lichen heaths and shrubs: Shrubification generates atmospheric heating but subsurface cooling during the growing season. Biogeosciences 18, no. 5: 1577–99. doi:10.5194/bg-18-1577-2021.
- Aguirre, D., A. E. Benhumea, and J. R. McLaren. 2021. Shrub encroachment affects tundra ecosystem properties through their living canopy rather than increased litter inputs. Soil Biology & Biochemistry 153: 108121. doi:10.1016/j.soilbio.2020.108121.
- Barrere, M., F. Domine, B. Decharme, S. Morin, V. Vionnet, and M. Lafaysse. 2017. Evaluating the performance of coupled snow–soil models in SURFEXv8 to simulate the permafrost thermal regime at a high Arctic site. Geoscientific Model Development 10, no. 9: 3461–79. doi:10.5194/gmd-10-3461-2017.
- Barry, R. G. 2008. Mountain weather and climate. 3rd ed. Cambridge: Cambridge University Press.
- Bates, D., M. Mächler, B. Bolker, and S. Walker. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, no. 1: 48. doi:10.18637/jss.v067.i01.
- Beer, C., P. Porada, A. Ekici, and M. Brakebusch. 2018. Effects of short-term variability of meteorological variables on soil temperature in permafrost regions. Cryosphere 12, no. 2: 741–57. doi:10.5194/tc-12-741-2018.
- Beringer, J., A. H. Lynch, F. S. Chapin, M. Mack, and G. B. Bonan. 2001. The representation of Arctic soils in the land surface model: The importance of mosses. Journal of Climate 14, no. 15: 3324–35. doi:10.1175/1520-0442(2001)014</>TROASI>2.0.CO;2.
- Berner, L. T., R. Massey, P. Jantz, B. C. Forbes, M. Macias-Fauria, I. Myers-Smith, T. Kumpula, et al. 2020. Summer warming explains widespread but not uniform greening in the Arctic tundra biome. Nature Communications 11, no. 1: 1–12. doi:10.1038/s41467-020-18479-5.
- Bjerke, J. W. 2011. Winter climate change: Ice encapsulation at mild subfreezing temperatures kills freeze-tolerant lichens. Environmental and Experimental Botany 72, no. 3: 404–8. doi:10.1016/j.envexpbot.2010.05.014.
- Blok, D., M. M. P. D. Heijmans, G. Schaepman-Strub, A. V. Kononov, T. C. Maximov, and F. Berendse. 2010. Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Global Change Biology 16, no. 4: 1296–305. doi:10.1111/j.1365-2486.2009.02110.x.
- Bonfils, C. J. W., T. J. Phillips, D. M. Lawrence, P. Cameron-Smith, W. J. Riley, and Z. M. Subin. 2012. On the influence of shrub height and expansion on northern high latitude climate. Environmental Research Letters 7, no. 1: 015503. doi:10.1088/1748-9326/7/1/015503.
- Chagnon, C., and S. Boudreau. 2019. Shrub canopy induces a decline in lichen abundance and diversity in Nunavik (Québec, Canada). Arctic, Antarctic, and Alpine Research 51, no. 1: 521–32. doi:10.1080/15230430.2019.1688751.
- Chapin, F. S., M. Sturm, M. C. Serreze, J. P. McFadden, J. R. Key, A. H. Lloyd, A. D. McGuire, et al. 2005. Role of land-surface changes in Arctic summer warming. Science 310, no. 5748: 657–60. doi:10.1126/science.1117368.
- Cornelissen, J. H. C., T. V. Callaghan, J. M. Alatalo, A. Michelsen, E. Graglia, A. E. Hartley, D. S. Hik, et al. 2001. Global change and Arctic ecosystems: Is lichen decline a function of increases in vascular plant biomass? Journal of Ecology 89, no. 6: 984–94. doi:10.1111/j.1365-2745.2001.00625.x.
- Dahl, E. 1956. Rondane. Mountain vegetation in South Norway and its relation to the environment. Skrifter utgittav det Norske Videnskaps-Akademi i Oslo, Mathematisk-Naturvidenskapelig Klasse. 3: 1–374.
- Ford, K. R., A. K. Ettinger, J. D. Lundquist, M. S. Raleigh, and J. H. R. Lambers. 2013. Spatial heterogeneity in ecologically important climate variables at coarse and fine scales in a high-snow mountain landscape. PLoS ONE 8, no. 6: e65008. doi:10.1371/journal.pone.0065008.
- Fraser, R. H., T. C. Lantz, I. Olthof, S. V. Kokelj, and R. A. Sims. 2014. Warming-induced shrub expansion and lichen decline in the Western Canadian Arctic. Ecosystems 17, no. 7: 1151–68. doi:10.1007/s10021-014-9783-3.
- Frost, G. V., H. E. Epstein, D. A. Walker, G. Matyshak, and K. Ermokhina. 2018. Seasonal and long-term changes to active-layer temperatures after tall shrubland expansion and succession in Arctic tundra. Ecosystems 21, no. 3: 507–20. doi:10.1007/s10021-017-0165-5.
- Gaffney, P. P. J., M. H. Hancock, M. A. Taggart, and R. Andersen. 2020. Restoration of afforested peatland: Immediate effects on aquatic carbon loss. Science of the Total Environment 742: 140594. doi:10.1016/j.scitotenv.2020.140594.
- Gavazov, K. S. 2010. Dynamics of alpine plant litter decomposition in a changing climate. Plant and Soil 337, no. 1–2: 19–32. doi:10.1007/s11104-010-0477-0.
- Gold, W. G., K. A. Glew, and L. G. Dickson. 2001. Functional influences of cryptobiotic surface crusts in an alpine tundra basin of the Olympic Mountains, Washington, USA. Northwest Science 75, no. 3: 315–26.
- Graham, E. A., P. W. Rundel, W. Kaiser, Y. Lam, M. Stealey, and E. M. Yuen. 2012. Fine-scale patterns of soil and plant surface temperatures in an alpine fellfield habitat, White Mountains, California. Arctic, Antarctic, and Alpine Research 44, no. 3: 288–95. doi:10.1657/1938-4246-44.3.288.
- Grünberg, I., E. J. Wilcox, S. Zwieback, P. Marsh, and J. Boike. 2020. Linking tundra vegetation, snow, soil temperature, and permafrost. Biogeosciences 17, no. 16: 4261–79. doi:10.5194/bg-17-4261-2020.
- Hanssen-Bauer, I., H. Drange, E. J. Førland, L. A. Roald, K. Y. Børsheim, H. Hisdal, D. Lawrence, et al. 2017. Climate in Norway 2100 - a knowledge base for climate adaptation. Oslo: NCCS.
- Heijmans, M. M., R. Í. Magnússon, M. J. Lara, G. V. Frost, I. H. Myers-Smith, J. van Huissteden, M. T. Jorgenson, et al. 2022. Tundra vegetation change and impacts on permafrost. Nature Reviews Earth & Environment 3, no. 1: 68–84. doi:10.1038/s43017-021-00233-0.
- Hobbie, S. E. 1996. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecological Monographs 66, no. 4: 503–22. doi:10.2307/2963492.
- Hothorn, T., F. Bretz, and P. Westfall. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50, no. 3: 346–63. doi:10.1002/bimj.200810425.
- Hursh, A., A. Ballantyne, L. Cooper, M. Maneta, J. Kimball, and J. Watts. 2017. The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Global Change Biology 23, no. 5: 2090–103. doi:10.1111/gcb.13489.
- Joly, K., R. R. Jandt, and D. R. Klein. 2009. Decrease of lichens in Arctic ecosystems: The role of wildfire, caribou, reindeer, competition and climate in north‐western Alaska. Polar Research 28, no. 3: 433–42. doi:10.1111/j.1751-8369.2009.00113.x.
- Klein, D. R., and M. Shulski. 2011. The role of lichens, reindeer, and climate in ecosystem change on a Bering Sea island. Arctic 64, no. 3: 353–61. doi:10.14430/arctic4124.
- Körner, C., and E. Hiltbrunner. 2017. The 90 ways to describe plant temperature. Perspectives in Plant Ecology, Evolution and Systematics 30: 16–21. doi:10.1016/j.ppees.2017.04.004.
- Lafleur, P. M., and E. R. Humphreys. 2018. Tundra shrub effects on growing season energy and carbon dioxide exchange. Environmental Research Letters 13, no. 5: 055001. doi:10.1088/1748-9326/aab863.
- Lawrence, D. M., and S. C. Swenson. 2011. Permafrost response to increasing Arctic shrub abundance depends on the relative influence of shrubs on local soil cooling versus large-scale climate warming. Environmental Research Letters 6, no. 4: 045504. doi:10.1088/1748-9326/6/4/045504.
- Lembrechts, J. J., J. Aalto, M. B. Ashcroft, P. De Frenne, M. Kopecký, J. Lenoir, M. Luoto, et al. 2020. SoilTemp: A global database of near-surface temperature. Global Change Biology 26, no. 11: 6616–29. doi:10.1111/gcb.15123.
- Loranty, M. M., B. W. Abbott, D. Blok, T. A. Douglas, H. E. Epstein, B. C. Forbes, B. M. Jones, et al. 2018. Reviews and syntheses: Changing ecosystem influences on soil thermal regimes in northern high-latitude permafrost regions. Biogeosciences 15, no. 17: 5287–313. doi:10.5194/bg-15-5287-2018.
- Macander, M. J., P. R. Nelson, T. W. Nawrocki, G. V. Frost, K. M. Orndahl, E. C. Palm, A. F. Wells, et al. 2022. Time-series maps reveal widespread change in plant functional type cover across Arctic and boreal Alaska and Yukon. Environmental Research Letters 17, no. 5: 054042. doi:10.1088/1748-9326/ac6965.
- Macias-Fauria, M., T. Helle, A. Niva, H. Posio, and M. Timonen. 2008. Removal of the lichen mat by reindeer enhances tree growth in a northern Scots pine forest. Canadian Journal of Forest Research 38, no. 12: 2981–93. doi:10.1139/X08-135.
- Maliniemi, T., J. Kapfer, P. Saccone, A. Skog, and R. Virtanen. 2018. Long‐term vegetation changes of treeless heath communities in northern Fennoscandia: Links to climate change trends and reindeer grazing. Journal of Vegetation Science 29, no. 3: 469–79. doi:10.1111/jvs.12630.
- Mekonnen, Z. A., W. J. Riley, L. T. Berner, N. J. Bouskill, M. S. Torn, G. Iwahana, A. L. Breen, et al. 2021. Arctic tundra shrubification: A review of mechanisms and impacts on ecosystem carbon balance. Environmental Research Letters 16, no. 5: 053001. doi:10.1088/1748-9326/abf28b.
- MET Norway. 2019. Meteorological data. www.met.no
- Mikola, J., T. Virtanen, M. Linkosalmi, E. Vähä, J. Nyman, O. Postagonova, A. Räsänen, et al. 2018. Spatial variation and linkages of soil and vegetation in the Siberian Arctic tundra–coupling field observations with remote sensing data. Biogeosciences 15, no. 9: 2781–801. doi:10.5194/bg-15-2781-2018.
- Myers‐Smith, I. H., and D. S. Hik. 2013. Shrub canopies influence soil temperatures but not nutrient dynamics: An experimental test of tundra snow–shrub interactions. Ecology and Evolution 3, no. 11: 3683–700. doi:10.1002/ece3.710.
- Myers-Smith, I. H., B. C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K. D. Tape, et al. 2011. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters 6, no. 4: 045509. doi:10.1088/1748-9326/6/4/045509.
- Nystuen, K. O., K. Sundsdal, Ø. H. Opedal, H. Holien, G. R. Strimbeck, and B. J. Graae. 2019. Lichens facilitate seedling recruitment in alpine heath. Journal of Vegetation Science 30, no. 5: 868–80. doi:10.1111/jvs.12773.
- Odland, A., G. Bandekar, I. Hanssen-Bauer, and S. M. Sandvik. 2017. Relationships between vegetation, air and soil temperatures on Norwegian mountain summits. Geografiska Annaler: Series A, Physical Geography 99, no. 4: 1–14. doi:10.1080/04353676.2017.1333324.
- O’Donnell, J. A., V. E. Romanovsky, J. W. Harden, and A. D. McGuire. 2009. The effect of moisture content on the thermal conductivity of moss and organic soil horizons from black spruce ecosystems in interior Alaska. Soil Science 174, no. 12: 646–51. doi:10.1097/SS.0b013e3181c4a7f8.
- Oke, T. R. 2002. Boundary layer climates. 2nd ed. London: Routledge.
- Olefeldt, D., M. R. Turetsky, P. M. Crill, and A. D. McGuire. 2013. Environmental and physical controls on northern terrestrial methane emissions across permafrost zones. Global Change Biology 19, no. 2: 589–603. doi:10.1111/gcb.12071.
- Opedal, Ø. H., W. S. Armbruster, and B. J. Graae. 2015. Linking small-scale topography with microclimate, plant species diversity and intra-specific trait variation in an alpine landscape. Plant Ecology & Diversity 8, no. 3: 305–15. doi:10.1080/17550874.2014.987330.
- Pajunen, A. M., J. Oksanen, and R. Virtanen. 2011. Impact of shrub canopies on understorey vegetation in western Eurasian tundra. Journal of Vegetation Science 22, no. 5: 837–46. doi:10.1111/j.1654-1103.2011.01285.x.
- Pape, R., and J. Löffler. 2017. Determinants of Arctic-alpine pasture resources: The need for a spatially and functionally fine-scaled perspective. Geografiska Annaler: Series A 99, no. 4: 1–18. doi:10.1080/04353676.2017.1368833.
- Pearson, R. G., S. J. Phillips, M. M. Loranty, P. S. A. Beck, T. Damoulas, S. J. Knight, and S. J. Goetz. 2013. Shifts in Arctic vegetation and associated feedbacks under climate change. National Climate Change 3, no. 7: 673–7. doi:10.1029/2005JG000013.
- Porada, P., A. Ekici, and C. Beer. 2016. Effects of bryophyte and lichen cover on permafrost soil temperature at large scale. Cryosphere 10, no. 5: 2291. doi:10.5194/tc-10-2291-2016.
- R Core Team. 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
- Saito, M., T. Kato, and Y. Tang. 2009. Temperature controls ecosystem CO2 exchange of an alpine meadow on the northeastern Tibetan Plateau. Global Change Biology 15, no. 1: 221–8. doi:10.1111/j.1365-2486.2008.01713.x.
- Scherrer, D., and C. Körner. 2011. Topographically controlled thermal‐habitat differentiation buffers alpine plant diversity against climate warming. Journal of Biogeography 38, no. 2: 406–16. doi:10.1111/j.1365-2699.2010.02407.x.
- Schimel, J. P., C. Bilbrough, and J. M. Welker. 2004. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biology & Biochemistry 36, no. 2: 217–27. doi:10.1016/j.soilbio.2003.09.008.
- Stewart-Oaten, A., W. W. Murdoch, and K. R. Parker. 1986. Environmental impact assessment: “Pseudoreplication” in time? Ecology 67, no. 4: 929–40. doi:10.2307/1939815.
- Sundstøl, S. A., and A. Odland. 2017. Responses of alpine vascular plants and lichens to soil temperatures. Ann. Annales Botanici Fennici 54, no. 1–3: 17–28. doi:10.5735/085.054.0304.
- Vanneste, T., O. Michelsen, B. J. Graae, M. O. Kyrkjeeide, H. Holien, K. Hassel, S. Lindmo, et al. 2017. Impact of climate change on alpine vegetation of mountain summits in Norway. Ecological Research 32, no. 4: 1–15. doi:10.1007/s11284-017-1472-1.
- van Zuijlen, K., R. E. Roos, K. Klanderud, S. I. Lang, and J. Asplund. 2020. Mat-forming lichens affect microclimate and litter decomposition by different mechanisms. Fungal Ecology 44: 100905. doi:10.1016/j.funeco.2019.100905.
- Wilson, S. D., and C. Nilsson. 2009. Arctic alpine vegetation change over 20 years. Global Change Biology 15, no. 7: 1676–84. doi:10.1111/j.1365-2486.2009.01896.x.
- Winkler, M., A. Lamprecht, K. Steinbauer, K. Hülber, J. P. Theurillat, F. Breiner, P. Choler, et al. 2016. The rich sides of mountain summits–a pan‐European view on aspect preferences of alpine plants. Journal of Biogeography 43, no. 11: 2261–73. doi:10.1111/jbi.12835.
- Wundram, D., R. Pape, and J. Löffler. 2010. Alpine soil temperature variability at multiple scales. Arctic, Antarctic, and Alpine Research 42, no. 1: 117–28. doi:10.1657/1938-4246-42.1.117.
- Zamin, T. J., and P. Grogan. 2012. Birch shrub growth in the low Arctic: The relative importance of experimental warming, enhanced nutrient availability, snow depth and caribou exclusion. Environmental Research Letters 7, no. 3: 034027. doi:10.1088/1748-9326/7/3/034027.