ABSTRACT
In Southwestern Yukon, ice patches have shown substantial retreat since the Little Ice Age (1600–1900 AD) in response to warming trends. These ice patches support unique alpine wetlands that have formed habitats for diverse flora and fauna over millennia. With ice retreat, pristine bryophyte populations are exposed beneath accumulated ancient dung. Given that bryophytes have been shown to survive extreme conditions including ice entombment and can regenerate from viable cells, emergent ice margin bryophyte and dung samples from the Granger and Gladstone ice patches were assayed for regrowth potential under growth chamber conditions. Diaspore (spore/fragment) generation of species found in the original subfossil material was indicated in 68 percent of assays, emphasizing the cyclical establishment of ancient ice patch vegetation. One of the oldest samples, dating 4036 calibrated years BP from the Gladstone ice patch margin, showed remarkable bryophyte generation from diaspores in dung. These Yukon ice patches form reservoirs of cryopreserved biota and have a critical role in maintaining alpine diversity, which provides summer refuge for caribou and other alpine fauna. Ice margin fluctuations, which bury and release populations through time, are part of a complex revegetation sequence in alpine regions that has followed deglaciation.
Introduction
Within Arctic Canada, summer temperatures have been significantly higher over the past 100 years compared to historical records (G. H. Miller et al. Citation2013), with temperatures in northwest Canada (Yukon) exceeding the Holocene thermal maximum (Porter et al. Citation2019). Unprecedented warming has accelerated retreat and thinning of ice margins (Gardner et al. Citation2011; Koch, Clague, and Osborn Citation2014) and can suspend the accumulation of alpine ice (Farnell et al. Citation2004; Meulendyk et al. Citation2012). With ice retreat in northern environments, plant communities have been exhumed in pristine condition (Falconer Citation1966; Bergsma, Svoboda, and Freedman Citation1984; La Farge, Williams, and England Citation2013). Bryophytes often form the dominant taxa in deglaciated foreland communities of arctic and alpine glaciers that facilitate reestablishment (Bliss and Gold Citation1999; G. A. Jones and Henry Citation2003; Breen and Lèvesque Citation2006; Gornall et al. Citation2007) through nutrient and substrate modification, as well as emergent biota (La Farge, Williams, and England Citation2013). In addition to pioneer species or organic substrate, their role as resilient populations that maintain continuity in alpine ice patch ecosystems through millennia is explored here.
Bryophytes have adaptive traits that enable persistence in extreme climates (Longton Citation1997). Cold-adapted arctic and alpine bryophytes are often desiccation tolerant, which facilitates cryptobiosis until rehydration (Proctor Citation2000). During freezing, minimal intracellular ice is formed that would otherwise damage the cells (Lenne et al. Citation2010). Bryophytes also have molecular mechanisms that prevent cell damage from diurnal freeze–thaw cycles. Metabolically they produce dehydrins, late embryogenesis abundant proteins, that preserve membrane and enzyme integrity during freezing events (Bewley, Reynolds, and Oliver Citation1993; Graether and Boddington Citation2014) and accumulate sucrose for vitrification, preserving cell viability (Buitink, Hoekstra, and Leprince Citation2002). In addition, glutathione antioxidant levels are maintained to protect cells against reactive oxidative species produced by photosynthesis inhibition during desiccation (Proctor et al. Citation2007). Bryophytes are also totipotent, referring to cells that can dedifferentiate (stem cells) to regenerate an entirely new organism from injured or fragmented leaves or stems (Ishikawa et al. Citation2011; Kofuji and Hasebe Citation2014). In addition to field observations of in situ regeneration at the Teardrop Glacier margin, Sverdrup Pass (Ellesmere Island, Nunavut), growth chamber assays of Little Ice Age (LIA) exhumed bryophytes 404 to 460 calibrated years before present (cal BP) confirmed successful in vitro generation (La Farge, Williams, and England Citation2013). The in vitro assays of thawed Antarctic permafrost core samples have also demonstrated generation of bryophytes populations 1533 to 1697 cal BP (Roads, Longton, and Convey Citation2014).
The dominant mode of bryophyte reproduction in extreme environments is asexual, including specialized propagules (i.e., gemmae, bulbils, and tubers) and unspecialized fragmentation. Asexual structures and spores accumulate in the soil to form diaspore banks. These stress-tolerant structures remain dormant until ideal growth conditions arise (During Citation2001). Diaspores form genetic archives of successive generations, encapsulating phenotypic variation produced under variable environmental conditions (Hock et al. Citation2008). This maintains genetic diversity and persistence in habitats when faced with variable conditions through time (Thompson and Grime Citation1979; Frey and Kürschner Citation2011).
Within the southwest Yukon, a network of alpine ice patches (IPs), comprising small glacial remnants and snow deposits compacted into ice (100-m- to 1-km-wide margins; Farnell et al. Citation2004), have been selectively monitored since discovery in 1997 (Kuzyk et al. Citation1999). They are often stratified with accumulations of organic-rich layers from surface deposition of windblown sediment and dung from fauna (predominately caribou—Rangifer tarandus) that utilize the ice as critical habitats for refuge from summer heat and biting insects (Andrews and MacKay Citation2012; Chellman et al. Citation2021). IPs are significant archaeological sites in Yukon, exhuming preserved First Nations’ hunting artifacts (Hare et al. Citation2004, Citation2012; Greer and Strand Citation2012). Ice-entombed pollen has also indicated historical caribou foraging (Bowyer and Schweger Citation2001). IPs have been documented globally, particularly for archaeological productivity, in Alberta (Tirlea et al. Citation2022), Yukon (Kuzyk et al. Citation1999; Farnell et al. Citation2004; Hare et al. Citation2004, Citation2012; Dove, Hare, and Heacker Citation2005; Helwig, Monahan, and Poulin Citation2008; Greer and Strand Citation2012), Nunavut (Davesne, Fortier, and Domine Citation2022), Northwest Territories (Alix et al. Citation2012; Andrews, MacKay, and Andrew Citation2012; Galloway et al. Citation2012; Meulendyk et al. Citation2012), Alaska (Dixon, Manley, and Lee Citation2005; VanderHoek, Tedor, and McMahan Citation2007; VanderHoek et al. Citation2012), the Yellowstone region of Montana and Wyoming (Lee Citation2012; Lee and Pusemen Citation2017), the Teton mountains of Wyoming (Sgouros and Stirn Citation2015), Norway (Callanan Citation2012; Nesje et al. Citation2012; Ødegård et al. Citation2017; Pilø et al. Citation2021), Spain (Serrano et al. Citation2011; Serrano, González-Trueba, and González-García Citation2012; Ruiz-Fernández et al. Citation2016), Mongolia (Taylor et al. Citation2019), and the Julian Alps of Italy–Slovakia (Colucci Citation2016).
Retreating IP ice margins have exhumed bryophyte populations that have been utilized in radiocarbon dating for archaeological surveys, revealing ice preservation up to 7402 cal BP (CALIB 8.2; Farnell et al. Citation2004; Hare et al. Citation2004, Citation2012; Dove, Hare, and Heacker Citation2005). The ice retreat has created unique moisture-rich foreland habitats and downstream melt channels that support a diverse flora (Woo and Young Citation2003; Rosvold Citation2016) in an otherwise moisture limited tundra. The exhumed vegetation can be used for paleoreconstruction of the past environment (Birks and Birks Citation1980) and may contain viable diaspores and material that can contribute to the specialized IP foreland, sustaining the specialized plant populations and the fauna that relies on these habitats.
Two Yukon sites, Granger IP and Gladstone IP, were selected to document emergent vegetation with the following objectives: (1) determine the age and species composition of ice-exhumed bryophyte populations and (2) assay samples of the emergent bryophyte and dung in vitro for potential regrowth and diaspore generation. We predict that IPs have created a unique habitat within the alpine tundra environment that is enriched by ice-exhumed subfossil material as a growth substrate and as a significant diaspore source. The dung deposited from ungulates attracted to the IPs for a reprieve from summer heat is enriched with diaspores that are incorporated into the ice along with windblown material from the surrounding extant taxa. With an escalation of climate warming, habitats associated with IPs are vulnerable to desiccation (Hare et al. Citation2011), which would critically affect the local biodiversity. We investigate the paleovegetation record to understand the evolution of the alpine ice patch ecosystem and the role of ice patches as biological reservoirs that release entombed bryophytes with viable diaspores, maintaining the unique alpine habitats.
Methods
Study area
The Granger Ice Patch (GRIP) is located on the northern slope of Mount Granger, within the southern lakes ecoregion of the intermontane belt of the boreal cordillera ecozone, approximately 20 km southwest of Whitehorse, Yukon (; Smith, Meikle, and Roots Citation2004), traditional land of the Kwanlin Dün First Nation (60.54111° N, 135.25687° W; 1,781 m.a.s.l. at lowest basal ice margin). This region is semi-arid, with a mean annual temperature of −1°C to −2°C and annual precipitation from 20 to 23.5 cm. The soil is alkaline cryosols (permafrost soil) comprising glacial deposits and areas of discontinuous permafrost (Smith, Meikle, and Roots Citation2004).
Figure 1. The locality of IPs surveyed for exhumed subfossil material. Granger (GRIP 2015, 2016) at Mount Granger, Gladstone (GLIP 2016) at Mount Venus vicinity, including Little Gladstone (LGLIP 2016).
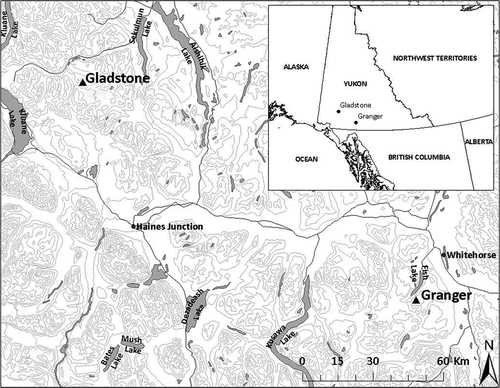
Approximately 170 km northwest of Mount Granger is the Gladstone Ice Patch (GLIP) below Venus Peak, situated in the Ruby Range ecoregion of the boreal cordillera ecozone, east of Kluane Lake, Yukon (Smith, Meikle, and Roots Citation2004), on the traditional land of the Champagne and Aishihik First Nations (61.27098° N, 137.97339° W; 2,010 m.a.s.l.). Annual temperature varies depending on elevation, ranging from −3°C to −7°C, and the precipitation average is 250 to 300 mm annually (Smith, Meikle, and Roots Citation2004). Soils are predominately cryosols, composed of glacial deposits and sporadic discontinuous permafrost. Little Gladstone (LGLIP) is approximately 2 km northwest of GLIP within the same ecoregion and environmental regime (61.28699° N, 137.99942° W; 1,965 m.a.s.l.).
GRIP, GLIP, and LGLIP are characterized by stratified layers of windblown sediment and dung accumulation. Accumulations of seasonal snow and ice fluctuate through time at each IP, with a basal core of Holocene ice. GRIP and GLIP are classified as “large” relative to the suite of surveyed IPs (Hare et al. Citation2012). The GRIP ice margin (IM) is 135 m wide and the GLIP IM is 1 km wide and have varied local responses to climate warming but are susceptible to rapid retreat (Farnell et al. Citation2004).
Ice patch retreat
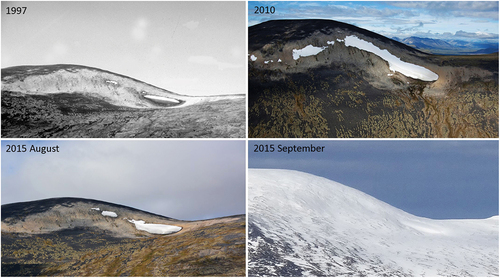
The perimeter of GRIP was measured at 10-m intervals by Global Positioning System on 4 August 2015, used to determine area and compared to previous area estimates by Farnell et al. (Citation2004). Comparisons of IM fluctuations from 1997 to 2015 were made with images taken by Farnell (Citation1997), Hare (Citation2010), La Farge (August 2015), and Svoboda (September 2015; ). To monitor maximum seasonal ice retreat, thirteen metal stakes were placed every 10 m at the lower margin on 4 August 2015. After five days, the distance from the IM to each stake was measured and recorded. At the end of the fieldwork, seven stakes were left in situ for reassessment the following year on 28 July 2016. The GLIP IM was measured using twenty-six stakes every 10 m along the central portion of the 1-km margin. Over a short duration (20–25 July 2016), an estimation of a single season of ice retreat rate was made.
Figure 2. Fluctuation of GRIP from 1997 to 2015. Each image was taken at the end of the melt season. LIA maximum is indicated by the light shaded terrain surrounding the ice patch in each image (lichen-free zone). The maximum retreat is indicated by the 1997 IP remnant (Farnell et al. Citation2004). By 2010 the GRIP shows expansion at the end of the seasonal melt (Greg Hare). In 2015 GRIP has retracted at the end of the melt season in August, with the season end indicated by snow cover in September (M. Svoboda, Canadian Wildlife Service, photo 9 September 2015, taken from ITEX site southeast of Mt. Granger). The global warming trend indicates that this ice patch will soon disappear, which has been documented in other Yukon IP (Hare et al. Citation2011). Global, seasonal, and local climate can have a varied impact within the overarching global trend of retreat.
An estimation of growing degree days (>0°C) at GRIP was recorded from the end of the growing season on 4 September 2015 to 28 July 2016. Eight Thermochron iButton data loggers were placed 5 cm into the soil every 10 m along the central portion of the GRIP IM on 12 August 2015. Data loggers were programmed to record temperatures every 240 minutes and overwrite the oldest data through “rollover.” Data loggers were analyzed using a 1-Wire/iButton viewer (Maxim Integrated).
Emergent bryophyte radiocarbon dates
Six IM samples from the GLIP and GRIP 2016 were submitted for radiocarbon dating (14C) at A.E. Lalonde AMS (Accelerator Mass Spectrometry) Laboratory, University of Ottawa (UOC), and four GRIP 2015 samples were submitted to W. M. Keck Laboratory, University of California, Irvine (UCIAMS) to determine the age range of emergent populations. Conventional 14C ages (yr BP) ranges were calculated as calibrated ages as years before present (cal BP) using CALIB 8.2 (Stuiver, Reimer, and Reimer Citation2022), with median values given in brackets, which will be used for discussion in the text (). The distal bryophyte stem apices of three GLIP and three GRIP populations () were subsampled (approximately ten to twenty apices per original sample). Stem sampling was restricted to the youngest material (~1 to 3 cm from the apex) to accurately represent the last growth period. Before submission, species composition was determined for each sample and washed in double-distilled water and then manually inspected to remove any contaminants (i.e., fungal hyphae, moss rhizoids, algae) using nitrile gloves and forceps sterilized in a 10 percent bleach (NaClO) solution.
Table 1. Radiocarbon dates from ten samples at the Granger (2015, 2016) and Gladstone (2016) ice patch margins.
Field samples
Emergent subfossil (ancient, nonfossilized) vegetation was collected from the GRIP (3–12 August 2015 and 28 July 2016), GLIP (17–24 July 2016), and LGLIP (25 July 2016) following maximum seasonal ice retreat. Bryophyte populations and dung were excavated from the IM or collected ≤1 m distance from the IM (DIM; ; Appendix II, Figure S1). Samples were placed in sterile polyethylene Whirl-Pak bags (Nasco) and kept in a cooler for transport to the University of Alberta where they were stored in a freezer at −20°C until subsampling. Bryophyte samples varied from ~100 to 200 g, depending on the original population size. Additional GRIP foreland bryophyte populations and modern dung samples >1 m DIM (up to 40 m) were collected for comparison to IM sample regrowth ().
Table 2. Growth chamber assays: The ice patch and survey year; number of samples, and percentage of jars with bryophyte(s) generated.
Diaspore traps (fourteen) were placed along the GRIP IM to capture any windblown spores, propagules, or fragments from the foreland and surrounding areas. Diaspore deposition could germinate and contribute to new growth in the assays on the exhumed subfossil substrate. Traps consisted of twice autoclaved soil samples (commercial brand Miracle-Gro potting soil) in open polystyrene Petri dishes (100 mm). Eight dishes were collected after ten days in 2015 (3–12 August). Six traps were left in situ alongside iButtons, both contained within octagonal open-top chambers to prevent them from being lost in winter snow or wind (Appendix II, Figure S2). The traps were collected on 28 July 2016, sealed with parafilm, and transported to the University of Alberta.
Bryophyte generation capacity
The viability of ice patch bryophyte populations (GRIP 2015, 2016, GLIP 2016, LGIP 2016) was assessed with in vitro growth chamber assays. Of the four ice patches surveyed, 91 collections were subsampled (20 to 30 g) for assays, including replicates for a total of 136 (; ). The assays consisted of fifty-eight IM samples (≤1 m DIM) and thirty-three foreland samples (>1–40 m DIM). Subsamples were seeded “as is” into sterile, autoclaved 100 mL glass Magenta jars and enclosed with B-cap (Sigma) after 6 hours of thawing at room temperate. Surfaces and nitrile gloves were washed and sterilized with 70 percent ethanol between samples.
The growth chamber was set at a mean irradiance of 74 ± 9.3 SD μmol−2s−1, simulating 16 hours of daylight and 8 hours of darkness with 215 W cool-white, fluorescent bulbs, with temperature maintained at 15°C (La Farge, Williams, and England Citation2013). Assay samples were hydrated using autoclaved (121°C for 60 minutes) distilled water every seven days or as needed. Observations of chronological vegetative emergence were made monthly. Generated species were determined nine months (2015 samples) and four months (2016 samples) after their seeding.
Diaspore trap samples were subjected to the same growing conditions and duration as the subfossil assays. Five controls of autoclaved commercial soil substrate were placed in the growth chamber to test and ensure that observed growth was due to field diaspores, not substrate contaminants.
A separate set of additional growth assays was used to further assess gametophytic regrowth from selected subfossil material. Ten specimens were subsampled (nine IM and one foreland; Appendix I) using ten to twelve stems from the original material, rinsed with distilled water, and inspected to remove any surface debris with a dissecting microscope. Stems were placed on growth media (Phytagel, Sigma-Aldrich) in polystyrene Petri dishes (100 mm) and placed in the growth chamber for approximately two months. Plates were hydrated with autoclaved distilled water as needed and documented for plant development.
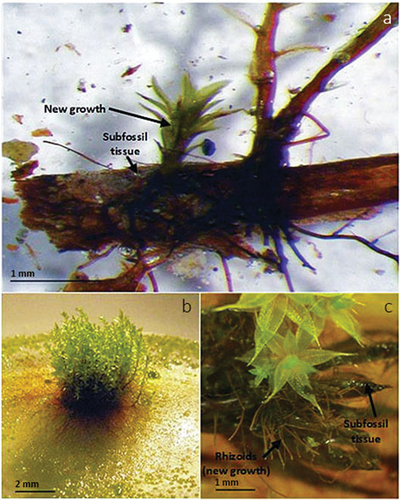
Subfossil and generated taxa from all assays were determined by floating each population in distilled water and removing portions for identification. Regrowth was concluded if new growth was directly from subfossil tissue or if the new growth represented the same taxa/taxon of the original subfossil material. Generated species not documented on the original subfossil sample provided evidence of diaspore germination from either extant or ice-entombed material or a mixed source. Growth was documented photographically, compiled in Zerene Stacker (Citation2016), and edited in Adobe Photoshop CC (Citation2015) ().
Figure 3. GLIP and LGLIP IPs 2016. (a) The 1-km-wide GLIP shows the multilayered glacier ice remnant and more recent seasonal snow deposits. The distinct layers of remnant glacier (darker area) ice in the central region of the IP with more recent seasonal snow (white carapace) above and below. Along the bottom ice margin, persistent seasonal snow that extends the functional margin was present at the end of the growing season. Central portions of the ice margin were defined by “old ice” (26 July 2016). (b) GLIP shows the upper and lower portions of the IP defined by season snow deposits. The central portion shows the “old ice” portion of the IP. The lower margin of the old ice shows the vertical lines (dark) that indicate sediment and dung melt out.

Figure 4. Ice patch emergent subfossil bryophyte material in situ, in vitro, and resulting growth chamber assay generation of bryophyte species from selected samples ≤1 m from ice margins. (a) In situ population of Ceratodon purpureus; (b) growth chamber assay “as is” subsample (20–30 g) from BLM GR-SBG-9, collected August 2015; (c) GLIP 2016 (BLM GL-Z1-PT 38–2) showing growth of Aulacomnium palustre, Aulacomnium turgidum, Ceratodon purpureus, Leptobryum pyriforme, Marchantia polymorpha, Pogonatum urnigerum, Pohlia nutans, Polytrichum juniperinum, Polytrichum piliferum, and Ptychostomum pallescens; (d) LGLIP 2016 (BLM GL(2)-3): Polytrichum hyperboreum, Polytrichum juniperinum, C. purpureus, P. nutans, M. polymorpha, L. pyriforme, and P. pallescens; (e) GRIP 2015 (BLM GR-SBG-11-1): Pohlia drummondii, Cephalozia bicuspidata subsp. ambigua, Imbribryum alpinum, P. juniperinum, P. nutans, P. urnigerum, L. pyriforme, and M. polymorpha; (f) GRIP 2016 (CLF GR 2): Dicranum fuscescens, Trichostomum cf. arcticum, P. pallescens, P. piliferum, P. urnigerum, P. drummondii, P. juniperinum, P. nutans; (g) GLIP 2016 dung (CLF GL-SBG-6): C. purpureus, P. nutans, M. polymorpha, L. pyriforme, and P. piliferum; and (h) GRIP 2015 dung (BLM GR-SBG-(8-9)): Cephalophoziella sp., C. purpureus, I. alpinum, L. pyriforme, P. urnigerum, P. drummondii, P. nutans, P. hyperboreum, P. juniperinum.

Species and nomenclature were determined using the following: mosses (Flora of North America Citation2007, Citation2014), liverworts (Damsholt Citation2009), vascular plants (Cody Citation1996), and fungi (Arora Citation1986). Some specimens were not determined to species, lacking diagnostic features. These included Saxifraga, Polytrichum, Peziza, Sphagnum, Lophozia, Cephaloziella, and one juvenile fern species. Vouchers of all taxa were deposited in the Cryptogamic Herbarium, Department of Biological Sciences, University of Alberta.
Ordinal analysis
Ordinal analyses were used to determine the similarity of subfossil and generated bryophyte assemblages between each ice patch survey (GRIP 2015, 2016; GLIP and LGLIP 2016). The variation in composition of subfossil assays was assessed using nonmetric multidimensional scaling (NMDS; Legendre and Legendre Citation1998; McCune and Grace Citation2002) in PC-ORD 6.19 (McCune and Mefford Citation2011). The NMDS was run with presence/absence species data of subfossil samples where material was discernible: forty-nine assays and sixteen taxa; GRIP 2015, twenty; GRIP 2016, four; GLIP, twenty; LGLIP, five; ; ). A Jaccard distance metric was used to minimize the influence of the several single occurrence taxa (Legendre and Legendre Citation1998). Joint plot overlays of the species variables with high correlations to ordinal axes (r > 0.2) were included to indicate the species contributing most to the spatial organization (). Significant differences between subfossil composition at each ice patch were tested with a multiresponse permutation procedure (MRPP; McCune and Grace Citation2002) with a Jaccard distance metric in PC-ORD 6.19 (McCune and Mefford Citation2011). The MRPP T statistic indicates pairwise dissimilarities between groups, and the A statistic indicates the variability between species and variables within groups.
Analysis of generation composition similarity between each ice patch was conducted with the same statistical procedure: fifty-seven assays, forty-two taxa; GRIP 2015, twenty-eight; GRIP 2016, four; GLIP, twenty; LGLIP, five; ; ). Significant differences between the subfossil and generation composition of each ice patch were also assessed with MRPP. The percentage frequency of species occurrences within ice patch assays was determined through indicator species analysis (ISA; Dufrene and Legendre Citation1997) in PC-ORD 6.19 (McCune and Mefford Citation2011) with 9,999 permutations for Monte Carlo analysis of indicator value significance ().
Table 3. Species diversity of assays ≤1 m from ice patch margins.
Table 4. Species diversity of foreland assays >1–40 m from the ice patch margins.
Results
Ice margin retreat
In 2015, GRIP’s area was estimated as 1.71 ha, representing 13 percent of the 13.26 ha Little Ice Age maximum defined by the lichen-free zone (). After five days in August 2015, the retreat distance of the IM from the thirteen stakes was an average of 0.74 m, ranging from 0 to 1.17 m. In 2016, the distance from the IM to seven stakes was remeasured, showing a retreat range of 1.5 to 4.73 m with the maximum at the center of the ice patch, for an average retreat rate of 2.1 m per year (Appendix II, Figure S2). Based on five days in July 2016, the GLIP IM retreat average was 0.88 m from twenty-six stakes, ranging from 0.24 to 1.97 m.
Data loggers recorded soil temperatures from 4 September 2015 to 28 July 2016, showing growing degree days above 0°C from 13 to 24 July 2016 (0.5°C–6.2°C), indicating the maximum thaw of the 2016 IM was twelve days. Mount Granger had complete snow cover by 9 September 2015 (), approximating a ten-month freeze at GRIP (9 September 2015 to 13 July 2016).
Emergent bryophytes age and composition
AMS 14C radiocarbon dates were based on ten exhumed or foreland bryophyte populations, including bryophytes from one dung sample. Along the GRIP IM, ages ranged from modern to 6388 cal BP and modern within the foreland (median values; ). Emergent organics from within 1.5 m of the GLIP IM ranged from 4036 to 4629 cal BP.
The exhumed bryophyte populations (≤1 m DIM) showed pristine preservation (i.e., leaf attachment, intact cellular structure). Sixteen species were found across the three ice patches (GRIP, GLIP, LGLIP), based on 133 subfossil taxonomic determinations with 0 to 5 per assay. The most frequent subfossil species were Ceratodon purpureus Hedw., Pogonatum urnigerum (Hedw.) P. Beauv., Polytrichum juniperinum Hedw., and Polytrichum piliferum Hedw. (). GLIP had the highest subfossil richness with eleven species, followed by GRIP 2015 with nine (). GRIP 2015 and 2016 assemblages shared four taxa and had five species restricted to the 2015 samples, Pohlia crudoides (Sull. & Lesq.) Broth, Polytrichum hyperboreum R. Br., Polytrichastrum alpinum (Hedw.) G.L. Sm., Pohlia drummondii (Müll. Hal.) A.L. Andrews, and Pohlia nutans (Hedw.) Lindb., and one species restricted to 2016, Ptychostomum pallescens (Schleich. ex Schwägr.) J.R. Spence (). The richness from subfossil assemblages of the GRIP (2015, 2016) IM represents 10 percent of the surveyed extant species richness at Mount Granger (B. L. Miller and La Farge, unpublished data).
Bryophyte generation capacity
From the emergent growth chamber assays (≤1 m DIM), 99 percent indicated moss gametophore development (; ). Forty-two species were generated from fifty-seven assays, based on 352 taxonomic determinations, 219 more than in the subfossil material (1–17 per assay; ; Appendix I). From the assays, 68 percent generated at least one of the original subfossil taxa and 35 percent contained growth of all species determined in the original subfossil assemblage (Appendix I). On average, assays yielded 30 percent more taxonomic determinations than the original subfossil material, with 57 percent of generated taxa observed as generation without parental subfossil material. Fifteen species were present within the IM assays as generated growth or subfossil material that were not present in the foreland assays (). Six generated species found in assays from all ice patches also had the highest frequencies of occurrence: C. purpureus, Pohlia drummondii, Leptobryum pyriforme (Hedw) Wilson, Pohlia nutans, Polytrichum juniperinum, and Polytrichum piliferum (). Twenty-six generated species were not determined in the subfossil assays, representing 62 percent of the total richness (generated and subfossil) from all IP sites. Two species were present as subfossil material but did not regrow: Niphotrichum canescens (Hedw.) Bedn., Ochyra & Ochyra and Dicranum acutifolium (Lindb. & Arnell) C.E.O. Jensen (forty-two total; ). Development from parental subfossil tissue was not detected in any IM assay.
GRIP IM assays (2015, 2016) generated thirty-four species, including twenty-four that were not determined in the original subfossil samples (). GRIP 2015 contained the highest richness, representing eleven to twenty more taxa than the other IPs surveyed (). Twenty-four species were generated from GLIP and LGLIP IM assays (). GLIP IM generated species represented 52 percent of the subfossil taxa, and LGLIP represented 33 percent.
Foreland samples (>1–40 m from IM) had 100 percent gametophore development (). Of these samples, 86 percent generated at least one species found in the original material and 30 percent contained growth of all parental species (six assays; Appendix I). Based on new growth, on average there were 27 percent more taxonomic determinations per jar than from the original subfossil material.
All IP foreland taxa were present in the IM assays, except for six species: Hypnum revolutum (Mitt.) Lindb., Jungermannia borealis Damsh. & Váňa, Pohlia filum (Schimp.) Mårtensson, Saxifraga L. and two entomophilous and coprophilous species, Tetraplodon mnioides (Hedw.) Bruch & Schimp. and Splachnum vasculosum Hedw. (). The entomophilous species developed from two of the modern dung samples not recorded from any other assay. The six dominant IM taxa (C. purpureus, L. pyriforme, P. drummondii, P. nutans, P. juniperinum, and P. piliferum) were frequent within the foreland assays.
The additional growth assays of nine emergent IM samples and one foreland sample showed prolific development of species other than the seeded taxa, with only three samples developing taxa that were originally assayed (Appendix I). The most frequent taxon observed was Pohlia nutans, which commonly germinated on Polytrichum sp. leaves (). Direct regrowth from stem or leaf tissue was not observed.
Figure 5. Diaspore generation on subfossil Polytrichum leaves in additional growth assays on Phytagel growth media. (a) Ceratodon purpureus growing on the base of a detached Polytrichum piliferum leaf (GRIP 2015 BLM GR-SBG-6-2, <1 m from ice margin), (b) dense population of Pohlia nutans with extensive rhizoidal mats developed on Polytrichum sp. leaves (GRIP 2015 CLF GR-SBG-17, <10 cm from ice margin), and (c) Ptychostomum pallescens with rhizoid development on Polytrichum sp. leaves (GRIP 2015 CLF GR-SBG-20, >1–10 m from ice margin).
Diaspore trap and sterile control samples lacked any growth after nine months in the growth chamber. On 18 August 2015, twenty-one days before snowfall (9 September), the majority of Polytrichaceae taxa had intact calyptrae on the capsules, indicating that spores had not been dispersed during that growing season. IM samples collected in 2015 would not have been subject to a 2015 spore rain of Polytrichaceae taxa.
Ordinal analysis
The NMDS output of subfossil composition from the GRIP (2015, 2016), GLIP, and LGLIP data sets indicates the similarity of ice patch assemblages (). However, GRIP 2015 differed from GLIP 2016 (T = −6.19, A = 0.04; p < .01) and GRIP 2016 differed from GLIP 2016 (T = −3.81, A = 0.03; p < .01). Sample size did not drastically influence the strength of the ordination; 8.914 stress from the two-dimensional solution with GRIP 2015 and GLIP 2016 samples compared to a final stress of 10.55 for all ice patches (p < .01). The species with significant effects on the IP spatial relationships included Dicranum acutifolium and Dicranum elongatum, due to their presence in the LGLIP and GLIP assemblages; Polytrichum juniperinum, which had a high abundance in LGLIP and GRIP 2015 and 2016; Pohlia nutans, due to its similar presence in all sites except for GRIP 2016 where it was absent; and Ceratodon purpureus, being most prevalent in GRIP 2016.
Figure 6. Ordination of ice patch subfossils and growth chamber generated species. NMDS ordination joint plots of (a) ice margin (≤1 m) subfossil species composition and (b) growth chamber assay generated species from Granger, Gladstone, and Little Gladstone ice patches, based on presence/absence data. Species vectors with significant contributions to the ordinal output (r2 > 0.2) are shown. Vector length and direction indicate the strength of correlation with each ordinal axis. Assemblages that have more similarity in species composition show a higher degree of overlap. Final stress for the two-dimensional solution = (a) 10.54862, (b) 17.77608; number of iterations = (a) 93, (b) 77; final instability = 0 for both ordinations.
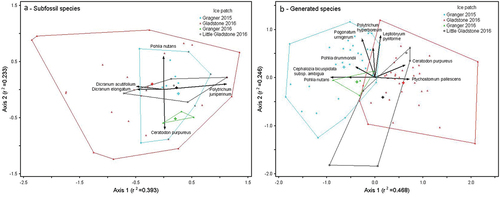
The GRIP 2015 and GLIP 2016 subfossil taxa and the generated composition were significantly different, with GLIP assemblages showing a great dissimilarity between assemblages (T = −19.25, A = 0.09; p < .01) compared to GRIP 2015 assemblages (T = −12.75, A = 0.12; p < .01). GRIP 2016 assemblages were weakly dissociated (T = −2.86, A = 0.19; p = .013), and LGLIP was not considered significantly different (T = −1.83, A = 0.048; p = .051), likely due to the small sample sizes (four and xi, respectively). An NMDS comparison of subfossil to generated assemblages for each ice patch was unsuccessful owing to a few heavily weighted taxa (more frequent) and many species occurring only once within the data set (zero-inflated).
NMDS comparative analysis of species generation composition from the four IP surveys showed variable similarity (). To ensure that differences in sample size did not affect the ordinal output, an additional NMDS was run with only GRIP 2015 and GLIP 2016. The resulting stress for the two-dimensional solution was similar when all sites were included (17.62 and 17.77, respectively; p < .01). Species significantly contributing to the IP relationships included Pohlia nutans, which was abundant in the GRIP 2015, 2016, and LGLIP assemblages Pohlia drummondii, which was abundant in the GRIP 2015, 2016; Cephalozia bicuspidata subsp. ambigua (C. Massal.) Husn., which was only present in the GRIP 2015 assemblages; Pogonatum urnigerum, Polytrichum hyperboreum, and Leptobryum pyriforme, which were most abundant in the GRIP 2015 assemblages; and Ceratodon purpureus and Ptychostomum pallescens, which were prevalent in the GLIP 2016 assemblages.
MRPP analysis showed that generated assemblages between each ice patch were overall dissimilar (T = −16.14, A = 0.84; p < .01). GRIP 2015 and GLIP 2016 were significantly distinct (T = −24.30, A = 0.083; p < .01), and differences between other sites were moderately strong (T between −5 to −1, A < 0; p < .01). GRIP 2016 was not distinct from GRIP 2015 and LGLIP (T = −1.2; p > .01).
Discussion
Ice patch dynamics
Radiocarbon dates of exhumed organic material from GRIP 2015 IM samples had a broad range in age from modern to 6388 cal BP (), indicating that ice margin expansion has entombed material of different ages. In 1997 the IP was reduced to 6 percent of the LIA maximum of 13.62 ha and re-expanded to 32 percent in 2001 (Farnell et al. Citation2004). In 2015, GRIP was reduced to 13 percent of the LIA maximum. This twenty-year record indicates that IM populations are exposed and reburied through time. Previously dated dung and vegetation, ranging from modern to 8330 yr BP at GRIP (Farnell et al. Citation2004; Hare et al. Citation2012), highlights the expected ages at the margin, in contrast to a simple linear increase in age. The monitoring of GRIP (1995–2015) shows an overall decrease in permanent ice. Seasonal snow can accumulate over several years but is more susceptible to overall global warming trends. The vulnerability of IPs to temporal fluctuations has also been observed on Ward Hunt Island, Nunavut, where warming over sixteen years (2008–2012) resulted in ice loss, followed by periods of summer cooling where ice accumulated (Davesne, Fortier, and Domine Citation2022).
The multiple-layered ice of GLIP () represents older remnant glacial events, as well as younger seasonal deposits of snow, windblown mineral and organic debris, and dung that previously dated to 3690 yr BP (Hare et al. Citation2012). The GLIP IM samples (2016) ranged from 4036 to 4629 cal BP, showing a more stable and progressive IM retreat, given where samples were collected than those from GRIP.
Summer temperature increase can accelerate IM retreat, resulting in a progressive shrinkage of the ice patches. Records of summer temperatures have shown an average increase of 2°C over the past fifty years in the Yukon (Streicker Citation2016). Ice patch susceptibility to loss with temperature rise is exemplified by the 87 percent reduction of GRIP from its LIA maximum and average retreat of 3.6 m between 2015 and 2016. The GLIP IM is comparatively wide (1 km), but both are highly sensitive to temporal temperature variance. Detected dung-rich, ice-free sites within southwest Yukon are presumed to be former ice patches that have disappeared with warming (Farnell et al. Citation2004). Without a consistent source of seasonal IP meltwater, alpine ice patch ecosystems would be threatened and changed by desiccation.
Bryophyte generation capacity
The generative capacity of exhumed bryophytes was remarkably high, with 68 percent of the in vitro IM assays (≤1 m) containing growth of the same species as found in the original subfossil sample. This generation is from three potential sources: (1) direct regrowth from parental subfossil material (i.e., stems, leaves, rhizoids), (2) growth of diaspores (i.e., spores, gemmae, bulbils etc.) buried with the original material of the same species, and/or (3) growth of diaspores deposited on original populations (same or different taxa) during periodic IM fluctuations. Direct generation was not observed from parental subfossil material, and differentiating ancient versus modern diaspores is not possible within a sample. One exhumed dung sample from the GLIP IM was dissected and dated (4036 cal BP; ) from within 4 cm of the northwest GLIP margin, within the average annual retreat distance of 0.88 m, where exposed material is presumed predominantly ancient (CLF GL-3; Appendix II, Figure S3). The subsample of cleaned subfossil bryophytes constrained the dung sample 14C age, and the remainder of the dung sample was placed in the growth chamber. These original subfossil dung species had passed through the caribou digestive system over 4,000 years ago. None of the original species were generated in the growth chamber, indicating viable ancient diaspores buried at the time of ice expansion. GRIP seasonal snow and ice accumulation have entombed populations up to 6388 cal BP and integrated modern material resulting in mixed ages (). However, given that the average retreat rate of 2.1 m per year at GRIP and the short growing season (~13 July to 9 September 2016), generation from samples collected ≤1 m DIM has the highest potential of ancient origin. Generation of mid-Holocene bryophyte diaspores, which were either buried with the bryophytes or deposited on the dung before ice entombment, indicates the resilience of these upland bryophyte communities.
Six bryophytes formed the dominant components of the generated and subfossil assemblages at each ice patch: Ceratodon purpureus, Leptobryum pyriforme, Pohlia drummondii, Pohlia nutans, Polytrichum juniperinum, and Polytrichum piliferum (; ). These species represent perennial stayers (i.e., Polytrichum spp.) or rapid colonizers (i.e., L. pyriforme, C. purpureus) of perturbed areas within these ice patch habitats (Miles and Longton Citation1990; During Citation2001). Ceratodon purpureus was found with sporophytes and produces specialized filamentous stem propagules or rhizome tubers. Polytrichaceae populations produce abundant sporophytes that can release up to 85 million spores per square meter of moss cover (Longton and Miles Citation1982) and form dense networks of rhizoids that generate clonal populations (Wigglesworth Citation1947). Polytrichaceae species had prolific sporophyte production within the IP forelands (i.e., P. juniperinum). In high-altitude or -latitude environments, asexual reproduction is commonly exhibited for habitat colonization and maintenance (Longton Citation1988; Frey and Kürschner Citation2011). Pohlia drummondii and L. pyriforme produce asexual propagules (i.e., axillary bulbils and axillary or rhizoidal brood bodies, respectively). Pohlia nutans typically lacks specialized asexual structures but has clonal development from dense rhizoidal mats, as seen in the additional growth assays. The totipotency of bryophyte tissue also includes potential regrowth from gametophytic leaf or stem fragments (La Farge, Williams, and England Citation2013). These adaptive features contribute to the long-term persistence and dominance of these bryophytes in marginal IP environments.
Ice margin growth chamber assays generated twenty-six species across all ice patches that were not detected from any of the subfossil samples, resulting in overall dissimilarity between subfossil and generated species. These species that lacked parental subfossil gametophores represent populations generated from diaspores within the subfossil samples. Many of these species do not produce sporophytes frequently and more likely were dispersed as propagules. Propagule density is highest close to the generating gametophyte (Miles and Longton Citation1992), favoring short-distance dispersal for an increased chance of survival, but propagules can be found at long-range distances from the original source (Pohjamo et al. Citation2006; Lonnell et al. Citation2012; Sunberg Citation2013), particularly where anemochory is not limited by canopy cover (e.g., high alpine tundra). Pollen analysis from ice-embedded debris layers at GRIP showed a dominance of Picea spp. and Pinus sp. (Bowyer and Schweger Citation2001). These taxa are known for widespread dispersal and are common within lower altitude forests. In vitro growth of a fern thallus (CLF GL-3; ), characteristic of montane forests of lower altitude, also suggests long-distance dispersal.
During the melt season, organic and mineral deposits within the ice strata melt out and accumulate at the ice margin (Appendix II, Figures S1–S3). Windblown propagules incorporated into the ice can intermix with older emergent material along the receding ice margin during the melt season. In the alpine, windblown fragments accumulated on late-lying snow drifts have shown successful germination of gametophytic propagules (N. G. Miller and Ambrose Citation1976) with rare success from spores (Robinson and Miller Citation2013). Bryophyte asexual propagules adapted to high-altitude environments may develop a significant component of IP associated flora. The diaspore traps placed along the GRIP IM did not show successful germination, likely due to insufficient time to accumulate airborne diaspores or the constriction of the open-top chambers. The generation richness results from the foreland and IM samples (48 and 57 percent, respectively) indicate the prevalence of diaspore dispersal.
Arctic and alpine herbivores have been shown to disperse bryophyte propagules including within caribou dung accumulated in the ice patch strata. Germination from emergent GRIP and GLIP dung showed the contribution of bryophyte propagules from ancient dung deposits, suggesting that caribou function as endozoochorous diaspore vectors for Yukon alpine flora. These data corroborate previous results that found bryophytes material in caribou dung dating up to 5070 cal BP from Selwyn Mountain ice patches in the Northwest Territories (Galloway et al. Citation2012). Fragment analysis of caribou dung from Thandlät, Yukon, showed that 11 percent of their diet consisted of moss (Kuzyk et al. Citation1999). Similarly, dung from Svalbard barnacle geese (Branta leucopsis) contained ten bryophyte species (Stech et al. Citation2010). Yukon herbivore utility of IPs and the resulting plant dispersal are critical components of the formation of IPs as a rich diaspore source, contributing to bryophyte persistence in alpine environments.
Taxa that were not detected as subfossils (i.e., as individual stems, leaves, or established colonies) but that were generated in vitro under ideal growth chamber conditions potentially hindered establishment in situ (IM: 62 percent ≤1 m; foreland: 48 percent >1–40 m). The saturated substrate at the IM could constrain microhabitat conditions for germination of taxa adapted to mesic to xeric environments (i.e., Ditrichum flexicaule (Schwägr.) Hampe, Dicranum fuscescens Turner). The propagules of these taxa remain dormant until adequate environmental conditions stimulate their growth (During Citation2001; Hock et al. Citation2008). GRIP generated IM species representing 65 percent of foreland species (B. L. Miller and La Farge, unpublished data) show that the soil conditions were more suitable for the establishment within the IP foreland. Conversely, subfossil gametophytes found ≤1 m at all IPs are species that typically exhibit habitat plasticity and were previously able to establish near the IM (i.e., Polytrichum juniperinum).
Despite the similar dominant species and ecosystem role of IPs, a significant difference in generated and subfossil diversity between GLIP and GRIP (2015) was observed. This variation is attributed to the different environmental conditions between Mount Granger and Venus Peak (i.e., precipitation, temperature, elevation; Smith, Meikle, and Roots Citation2004), which support distinct alpine assemblages that are dispersed into the IPs. Increased species richness may be detected with further surveys, as shown in subfossil species composition differences between GRIP 2015 and 2016, increasing the resolution on the specific assemblages supported by each ice patch.
Bryophytes can survive long-term cryopreservation owing to their inherent physiological traits of cold acclimation, desiccation tolerance, and totipotency (Longton Citation1988; Segreto et al. Citation2010). This has been demonstrated by direct generation from exhumed 400 cal BP LIA gametophytes in Ellesmere Island of the Canadian High Arctic (La Farge, Williams, and England Citation2013) and in >1500 yr BP Antarctic permafrost peat cores (Roads, Longton, and Convey Citation2014). Direct regrowth from subfossil gametophytes at the IM (≤1 m DIM) was not observed from the Yukon IPs; however, given that bryophyte cells can dedifferentiate and develop new gametophytes through the protonemal phase (Ishikawa et al. Citation2011), it cannot be excluded as a potential mechanism. Direct regrowth from foreland assays (>1–10 m DIM) and in situ populations (>10–20 m DIM) illustrate the growth capacity of selected taxa (P. juniperinum and A. turgidum; Appendix II, Figures S4 and S5).
On deglaciated forelands, plant establishment requires water and nutrients (IP meltwater) in addition to soil stability (Gold and Bliss Citation1995; Hodkinson, Coulson, and Webb Citation2003; Breen and Lèvesque Citation2008; C. Jones and Del Moral Citation2009). In the IP forelands, exhumed subfossil bryophyte populations provide an organic-rich substrate, including paleo-diaspores, which facilitate colonization of exposed terrain. Temporal deposits of spores, gemmae, and rhizoidal tubers from distinct growing seasons provide efficient means for species maintenance, despite changing environmental conditions at a given site (Hock et al. Citation2008). The additional growth assays of individual IM stems and leaves also showed that plant structures can form microhabitats (i.e., Polytrichum juniperinum in-rolled leaves; ) facilitating the growth of superficial diaspores of other species, contributing to the dense colonization of bryophytes in IP forelands.
Future environments
Diaspores, both ancient and modern, entombed within ice patches have the capacity to generate new growth and contribute to the colonization of deglaciated terrain. Diaspore banks accumulate genetic diversity with propagules and spores representing haplotypes absent in extant populations (Hock et al. Citation2008). Genetic variation from successive plant populations produces diaspores adapted to a range of growing conditions, providing a cyclical mechanism to maintain genetic diversity without population loss due to climate change (Jump, Marchant, and Penuelas Citation2009). Thus, cryopreserved diaspores can enhance the genetic diversity of the modern flora with new populations, maintaining the specialized alpine wetland community.
Conclusions
Bryophytes form persistent alpine communities that are ice entombed, released, and reestablished from IPs through time. The IP subfossil records had similar species composition and shared generated bryophyte taxa from growth chamber assays. The exhumed material indicated that these species have formed the dominant floristic components of Yukon IP ecosystems in alpine environments since the mid-Holocene. The comparison of subfossil and foreland plant composition shows that abiotic conditions of the ice patches have supported a unique suite of taxa adapted to thrive in moisture-rich alpine environments. These taxa show resilience and persistence, given the generation from ancient dung dating 4036 cal BP at GLIP. This assay substantially expands our understanding of bryophyte viability in glacial environments. The viability of this material indicates that IP cryopreservation of past populations has the potential to contribute biotic and genetic material to alpine ecosystems that diversify and sustain these Yukon environments as high-altitude oases.
Supplemental Material
Download Zip (1.9 MB)Acknowledgments
This project was conducted on the traditional land of the Kwalin Dün First Nations (KDFN) and Champagne and Aishihik First Nations (CAFN), with permission granted by the respective First Nations members. Senior lands and resource planner of the Kwalin Dün First Nation John Meikle and Champagne Aishihik First Nations Heritage Manager Shelia Greer provided support for this study, as well as perspectives on the significance of the ice patches. This project was inspired by the initial ice patch research done by Richard Farnell (Yukon Department of Environment), Greg Hare (Yukon Department of Culture and Tourism), Gerry Kuzyk, and other members of the Yukon Ice Patch Research and Site Inventory Project. Greg Hare provided invaluable direction for ice patch selection and, with the CBC Nature of Things film crew, provided helicopter support to LGLIP. Michael Svoboda from Canadian Wildlife Services (CWS) Northern Conservation Division, as well as the Kluane Lake Research Station (KLRS), provided essential logistical support and supplies for fieldwork. David Hik (Polar Knowledge Canada) provided insights and support for project design and field supplies. John England (University of Alberta) provided glaciological insights, field support, and radiocarbon dates (UOC). Dr. Alberto Reyes (University of Alberta) provided study design insights and radiocarbon dates (UCIAMS). Primary funding was provided by the Northern Scientific Training Program (NSTP) through Polar Knowledge Canada and the University of Alberta Northern Research Award (UANRA). Taxonomic analysis was conducted in the Cryptogamic Herbarium, Department of Biological Sciences, University of Alberta. We also thank the two anonymous reviewers and David Kofrankek for their feedback and edits.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15230430.2023.2222034.
Additional information
Funding
References
- Adobe Photoshop. 2015. Version CC 2015. San Jose, CA: Adobe Inc.
- Alix, C., G. P. Hare, T. D. Andrews, and G. MacKay. 2012. A thousand years of lost hunting arrows: Wood analysis of ice patch remains in Northwestern Canada. Arctic 65, no. 5: 95–19. doi:10.14430/arctic4187.
- Andrews, T. D., and G. MacKay. 2012. The archaeology and paleoecology of alpine ice patches: A global perspective. Arctic 65, no. 5: iii–vi. doi:10.14430/arctic4181.
- Andrews, T. D., G. MacKay, and L. Andrew. 2012. Archaeological investigations of alpine ice patches in the Selwyn Mountains, Northwest Territories, Canada. Arctic 65: 1–21.
- Arora, D. 1986. Mushrooms demystified. 2nd ed. Berkeley, California: Ten Speed Press.
- Bergsma, B. M., J. Svoboda, and B. Freedman. 1984. Entombed plant communities by a retreating glacier at central Ellesmere Island, Canada. Arctic 37, no. 1: 49–52. doi:10.14430/arctic2162.
- Bewley, J. D., T. L. Reynolds, M. J. Oliver. 1993. Evolving strategies in the adaptation to desiccation. In Current topics in plant physiology, ed. T. J. Close and E. A. Bray, Plant responses to cellular dehydration during environmental stress, vol. 10, 193–201. American Society Plant Physiology.
- Birks, H. J. B., and H. H. Birks. 1980. Quaternary palaeoecology. London: Edward Arnold.
- Bliss, L. C., and W. G. Gold. 1999. Vascular plant reproduction, establishment, and growth and the effects of cryptogamic crusts within a polar desert ecosystem, Devon Island, NWT, Canada. Canadian Journal of Botany 77, no. 5: 623–36. doi:10.1139/b99-031.
- Bowyer, V., and C. E. Schweger. 2001. Ice patch as context: Reconstructing Holocene alpine environments in the southern Yukon. Paper presented at the 34th Annual Conference, Canadian Archaeological Association, Banff, Alberta.
- Breen, K., and E. Lèvesque. 2006. Proglacial succession of biological soil crusts and vascular plants: Biotic interactions in the High Arctic. Canadian Journal of Botany 84, no. 11: 1714–31. doi:10.1139/b06-131.
- Breen, K., and E. Lèvesque. 2008. The influence of biological soil crusts on soil characteristics along a High Arctic glacier foreland, Nunavut, Canada. Arctic, Antarctic, and Alpine Research 40, no. 2: 287–97. doi:10.1657/1523-0430(06-098)[BREEN]2.0.CO;2.
- Buitink, J., F. A. Hoekstra, and O. Leprince. 2002. Biochemistry and biophysics of tolerance systems. In Desiccation and survival in plants: Drying without dying, ed. M. Black and H. W. Pritchard, 293–318. Wallingford, UK: CABI Publishing.
- Callanan, M. 2012. Central Norwegian snow patch archaeology patterns past and present. Arctic 65, no. 5: 1–202. doi:10.14430/arctic4192.
- Chellman, N. J., G. T. Pederson, C. M. Lee, D. B. McWethy, K. Puseman, J. R. Stone, S. R. Brown, and J. R. McConnell. 2021. High elevation ice patch documents Holocene climate variability in the Northern Rocky Mountains. Quaternary Science Advances 3: 100021. doi:10.1016/j.qsa.2020.100021.
- Cody, W. J. 1996. Flora of the Yukon Territory. Ottawa: National Research Council, Research Press.
- Colucci, R. R. 2016. Geomorphic influence on small glacier response to post-Little Ice Age climate warming: Julian Alps, Europe. Earth Surface Processes and Landforms 41, no. 9: 1227–40. doi:10.1002/esp.3908.
- Damsholt, K. 2009. Illustrated flora of Nordic liverworts and hornworts. 2nd ed. Lund: Nordic Bryological Society.
- Davesne, G., D. Fortier, and F. Domine. 2022. Properties and stratigraphy of polar ice patches in the Canadian High Arctic reveal their current resilience to warm summers. Arctic Science 8, no. 2: 414–49. doi:10.1139/as-2021-0011.
- Dixon, E. J., W. F. Manley, and C. M. Lee. 2005. The emerging archaeology of glaciers and ice patches: Examples from Alaska’s Wrangell-St. Elias National Park and Preserve. American Antiquity 70, no. 1: 129–43. doi:10.2307/40035272.
- Dove, C. J., G. Hare, and M. Heacker. 2005. Identification of ancient feather fragments found in melting alpine ice patches in southern Yukon. Arctic 58: 38–43.
- Dufrene, M., and P. Legendre. 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological monographs 67, no. 3: 345–66. doi:10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2.
- During, H. 2001. Diaspore banks. Bryologist 104, no. 1: 92–7. doi:10.1639/0007-2745(2001)104[0092:DB]2.0.CO;2.
- Falconer, G. 1966. Preservation of vegetation and patterned ground under thin ice body in northern Baffin Island, N.W.T. Geographical Bulletin 8: 194–200.
- Farnell, R., G. P. Hare, E. Blake, V. Bowyer, C. Schweger, S. Greer, and R. Gotthardt. 2004. Multidisciplinary investigations of alpine ice patches in southwest Yukon, Canada: Paleoenvironmental and paleobiological investigations. Arctic 57, no. 3: 247–59. doi:10.14430/arctic502.
- Flora of North America Editorial Committee, eds. 2007. Flora of North America North of Mexico. [Online]. Vol. 27. New York and Oxford. http://beta.floranorthamerica.org
- Flora of North America Editorial Committee, eds. 2014. Flora of North America North Vol. 28. New York and Oxford. http://beta.floranorthamerica.org
- Frey, W., and H. Kürschner. 2011. Asexual reproduction, habitat colonization and habitat maintenance in bryophytes. A review. Flora 206, no. 3: 173–84. doi:10.1016/j.flora.2010.04.020.
- Galloway, J. M., J. Adamczewski, D. M. Schock, T. D. Andrews, G. MacKay, V. E. Bowyer, T. Meulendyk, B. J. Moorman, and S. J. Kutz. 2012. Diet and habitat of mountain woodland caribou inferred from dung preserved in 5000-year-old alpine ice in Selwyn Mountains, Northwest Territories, Canada. Arctic 65, no. 5: 59–79. doi:10.14430/arctic4185.
- Gardner, A. S., G. Moholdt, B. Wouters, G. J. Wolken, D. O. Burgess, M. J. Sharp, J. G. Cogley, C. Braun, and C. Labine. 2011. Sharply increased mass loss from glaciers and ice caps in the Canadian Arctic Archipelago. Nature 473: 357–60.
- Gold, W., and L. Bliss. 1995. Water limitations and plant community development in a polar desert. Ecological Society of America 76: 1558–68.
- Gornall, J. L., I. S. Jondottir, S. J. Woodin, and R. Van der Wal. 2007. Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 153, no. 4: 931–41. doi:10.1007/s00442-007-0785-0.
- Graether, S. P., and K. F. Boddington. 2014. Disorder and function: A review of the dehydrin protein family. Frontiers in Plant Science 5: 576. doi:10.3389/fpls.2014.00576.
- Greer, S., and D. Strand. 2012. Cultural landscapes, past and present, and the south Yukon ice patches. Arctic 65, no. 5: 136–52. doi:10.14430/arctic4189.
- Hare, P. G., S. Greer, R. Gotthardt, R. Farnell, V. Bowyer, C. Schweger, and D. Strand. 2004. Ethnographic and archaeological investigations of alpine ice patches in southwest Yukon, Canada. Arctic 57, no. 3: 260–72. doi:10.14430/arctic503.
- Hare, P. G., S. Greer, H. Jones, R. Mombourquette, J. Fingland, M. Nelson, J. Shorty, and T. Evans. 2011. The frozen past: Yukon ice patches. Government of Yukon.
- Hare, P. G., C. D. Thomas, T. N. Topper, and R. M. Gotthardt. 2012. The archaeology of Yukon ice patches: New artifacts, observations, and insights. Arctic 65, no. 5: 118–35. doi:10.14430/arctic4188.
- Helwig, K., V. Monahan, and J. Poulin. 2008. The identification of hafting adhesive on a slotted antler point from a Southwest Yukon ice patch. American Antiquity 73, no. 2: 279–88. doi:10.1017/S000273160004227X.
- Hock, Z., P. Szövényi, J. Schneller, Z. Tóth, and E. Urmi. 2008. Bryophyte diaspore bank: A genetic memory? Genetic structure and genetic diversity of surface populations and diaspore bank in the liverwort Mannia fragrans (Aytoniaceae). American Journal of Botany 95, no. 5: 542–8. doi:10.3732/ajb.2007283.
- Hodkinson, I. D., S. J. Coulson, and N. R. Webb. 2003. Community assembly along proglacial chronosequences in the high Arctic: Vegetation and soil development in north-west Svalbard. Journal of Ecology 91, no. 4: 651–63. doi:10.1046/j.1365-2745.2003.00786.x.
- Ishikawa, M., T. Murata, Y. Sato, T. Nishiyama, Y. Hiwatashi, A. Imai, A. Kimura, et al. 2011. Physcomitrella cyclin-dependent kinase a links cell cycle reactivation to other cellular changes during reprogramming of leaf cells. The Plant Cell 23, no. 8: 2924–38. doi:10.1105/tpc.111.088005.
- Jones, C., and R. Del Moral. 2009. Dispersal and establishment both limit colonization during primary succession on a glacier foreland. Plant Ecology 204, no. 2: 217–30. doi:10.1007/s11258-009-9586-3.
- Jones, G. A., and G. H. R. Henry. 2003. Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. Journal of Biogeography 30, no. 2: 277–96. doi:10.1046/j.1365-2699.2003.00818.x.
- Jump, A. S., R. Marchant, and J. Penuelas. 2009. Environmental change and the option value of genetic diversity. Trends in Plant Science 14, no. 1: 51–8. doi:10.1016/j.tplants.2008.10.002.
- Koch, J., J. J. Clague, and G. Osborn. 2014. Alpine glaciers and permanent ice and snow patches in western Canada approach their smallest sizes since the mid-Holocene, consistent with global trends. The Holocene 24, no. 12: 1639–48. doi:10.1177/0959683614551214.
- Kofuji, R., and M. Hasebe. 2014. Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Current Opinion in Plant Biology 17: 13–21. doi:10.1016/j.pbi.2013.10.007.
- Kuzyk, G. W., D. E. Russell, R. S. Farnell, R. M. Gotthardt, P. G. Hare, and E. Blake. 1999. In pursuit of prehistoric caribou on Thandlät, Southern Yukon. Arctic 52, no. 2: 214–9. doi:10.14430/arctic924.
- La Farge, C., K. H. Williams, and J. H. England. 2013. Regeneration of Little Ice Age bryophytes emerging from a polar glacier with implications of totipotency in extreme environments. PNAS 110, no. 24: 9839–44. doi:10.1073/pnas.1304199110.
- Lee, C. M. 2012. Withering snow and ice in the mid-latitudes: A new archaeological and paleobiological record for the Rocky Mountain region. Arctic 65, no. 5: 165–77. doi:10.14430/arctic4191.
- Lee, C. M., and K. Pusemen. 2017. Ice patch hunting in the Greater Yellowstone Area, Rocky Mountains, USA: Wood shafts, chipped stone projectile points, and bighorn sheep (Ovis canadensis). American Antiquity 82, no. 2: 223–43. doi:10.1017/aaq.2016.32.
- Legendre, P., and L. Legendre. 1998. Numerical ecology. Second English ed. Amsterdam, Netherlands: Elsevier Science, Developments in environmental modeling 20.
- Lenne, T., G. Bryant, C. H. Hocart, C. X. Huang, and M. C. Ball. 2010. Freeze avoidance: A dehydrating moss gathers no ice. Plant, Cell & Environment 33, no. 10: 1731–41. doi:10.1111/j.1365-3040.2010.02178.x.
- Longton, R. E. 1988. Biology of polar bryophytes and lichens. Cambridge: Cambridge University Press.
- Longton, R. E., and C. J. Miles. 1982. Studies on the reproductive biology of mosses. Journal of the Hattori Botanical Laboratory 52: 219–40.
- Longton, R. E. 1997. The role of bryophytes and lichens in polar ecosystems. In Ecology of Arctic environments, ed. S. J. Woodin and M. Marquiss, 69–96. Oxford: Blackwell.
- Lonnell, N., K. Hylander, B. G. Jonsson, and S. Sunberg. 2012. The fate of the missing spores — Patterns of realized dispersal beyond the closest vicinity of a sporulating moss. PLoS ONE 7, no. 7: e41987. doi:10.1371/journal.pone.0041987.
- McCune, B., and J. B. Grace. 2002. Analysis of ecological communities. Oregon, USA: MjM Software, Gleneden Beach.
- McCune, B., and M. J. Mefford. 2011. PC-ORD. Multivariate analysis of ecological data. Version 6. MjM Software. Oregon, USA: Gleneden Beach.
- Meulendyk, T., B. J. Moorman, T. D. Andrews, and G. MacKay. 2012. Morphology and development of ice patches in Northwest Territories, Canada. Arctic 65, no. 5: 43–58. doi:10.14430/arctic4184.
- Miles, C. J., and R. E. Longton. 1990. The role of spores in reproduction in mosses. Botanical Journal of the Linnean Society 104, no. 1–3: 149–73. doi:10.1111/j.1095-8339.1990.tb02216.x.
- Miles, C. J., and R. E. Longton. 1992. Deposition of moss spores in relation to distance from parent gametophytes. Journal of Bryology 17, no. 2: 355–268. doi:10.1179/jbr.1992.17.2.355.
- Miller, N. G., and L. J. H. Ambrose. 1976. Growth in culture of wind-blown bryophyte gametophyte fragments from Arctic Canada. The Bryologist 79, no. 1: 55–63. doi:10.2307/3241866.
- Miller, G. H., S. J. Lehman, K. A. Refsnider, J. R. Southon, and Y. Zhong. 2013. Unprecedented recent summer warmth in Arctic Canada. Geophysical Research Letters 40, no. 21: 1–7. doi:10.1002/2013GL057188.
- Nesje, A., L. H. Pilø, E. Finstad, B. Solli, V. Wangen, R. S. Ødegård, K. Isaksen, E. N. Støren, D. I. Bakke, and L. M. Andreassen. 2012. The climatic significance of artefacts related to prehistoric reindeer hunting exposed at melting ice patches in southern Norway. The Holocene 22, no. 4: 485–96. doi:10.1177/0959683611425552.
- Ødegård, R. S., A. Nesje, K. Isaksen, L. M. Andreassen, T. Eiken, M. Schwikowski, and C. Uglietti. 2017. Climate change threatens archaeologically significant ice patches: Insights into their age, internal structure, mass balance and climate sensitivity. The Cryosphere 11, no. 1: 17–32. doi:10.5194/tc-11-17-2017.
- Pilø, L. H., J. H. Barrett, T. Eiken, E. Finstad, S. Grønning, J. R. Post-Melbye, A. Nesje, J. Rosvold, B. Solli, and R. S. Ødegård. 2021. Interpreting archeological site-formation process at a mountain ice patch: A case study from Langfonne, Norway. The Holocene 31, no. 3: 469–82. doi:10.1177/0959683620972775.
- Pohjamo, M., S. Laaka-Lindberg, O. Ovaskainen, and H. Korpelainen. 2006. Dispersal potential of spores and asexual propagules in the epixylic hepatic Anastrophyllum hellerianum. Evolutionary Ecology 20, no. 5: 415–30. doi:10.1007/s10682-006-0011-2.
- Porter, T. L., S. W. Schoenemann, L. J. Davies, E. J. Steig, S. Bandara, and D. G. Froese. 2019. Recent summer warming in northwestern Canada exceeds the Holocene thermal maximum. Nature Communications 10, no. 1: 1631. doi:10.1038/s41467-019-09622-y.
- Proctor, M. C. F., P. Alpert, N. Cleavitt, B. D. Mishler, M. J. Oliver, L. R. Stark, and A. J. Wood. 2007. Desiccation tolerance in bryophytes: A review. The Bryologist 110, no. 4: 595–621. doi:10.1639/0007-2745(2007)110[595:DIBAR]2.0.CO;2.
- Proctor, M. C. F. 2000. Physiological ecology. In Bryophyte biology, ed. A. J. Shaw and B. Goffinet, 237–43. Cambridge: Cambridge University Press.
- Roads, E., R. E. Longton, and P. Convey. 2014. Millennial timescale regeneration in a moss from Antarctica. Current Biology 24, no. 6: R222–R223. doi:10.1016/j.cub.2014.01.053.
- Robinson, S. C., and N. G. Miller. 2013. Bryophyte diversity on Adirondack alpine summits is maintained by dissemination and establishment of vegetative fragments and spores. The Bryologist 116, no. 4: 382–91. doi:10.1639/0007-2745-116.4.382.
- Rosvold, J. 2016. Perennial ice and snow-covered land as important ecosystems for birds and mammals. Journal of Biogeography 43, no. 1: 3–12. doi:10.1111/jbi.12609.
- Ruiz-Fernández, J., M. Oliva, F. Hrbáček, G. Vieira, and C. García-Hernández. 2016. Soil temperatures in an Atlantic high mountain environment: The Forcadona buried ice patch (Picos de Europa, NW Spain). CATENA 149: 637–47. doi:10.1016/j.catena.2016.06.037.
- Segreto, R., K. Hassel, R. Bardal, and H. K. Stenoien. 2010. Desiccation tolerance and natural cold acclimation allows cryopreservation of bryophytes without pretreatment or use of cryoprotectants. The Bryologist 113, no. 4: 760–9. doi:10.1639/0007-2745-113.4.760.
- Serrano, E., J. J. González‐Trueba, J. J. Sanjosé, and L. M. Del Río. 2011. Ice patch origin, evolution, and dynamics in a temperate high mountain environment: The Jou Negro, Picos de Europa (NW Spain). Geografiska Annaler: Series A, Physical Geography 93, no. 2: 57–70. doi:10.1111/j.1468-0459.2011.00006.x.
- Serrano, E., J. González-Trueba, and M. González-García. 2012. Mountain glaciation and paleoclimate reconstruction in the Picos de Europa (Iberian Peninsula, SW Europe). Quaternary Research 78, no. 2: 303–14. doi:10.1016/j.yqres.2012.05.016.
- Sgouros, R., and M. Stirn. 2015. An ice patch artifact and paleobiological specimen from the Teton Mountains, Wyoming, USA. Journal of Glacial Archaeology 2: 3–24. doi:10.1558/jga.v2i1.27649.
- Smith, C. A. S., J. C. Meikle, and C. F. Roots. 2004. Ecoregions of the Yukon Territory: Biophysical properties of Yukon landscapes. PARC Technical Bulletin No. 04–01, 313. Summerland, British Columbia: Agriculture and Agri-Food Canada.
- Stech, M., E. Kolvoort, L. Mjje, K. Vrieling, and J. D. Kruijer. 2010. Bryophyte DNA sequences from faeces of an artic herbivore, barnacle goose (Branta leucopsis). Molecular Ecology Resources 11, no. 2: 404–8. doi:10.1111/j.1755-0998.2010.02938.x.
- Streicker, J. 2016. Yukon Climate Change Indicators and Key Findings 2015. Northern Climate ExChange, 84. Yukon Research Centre, Yukon College.
- Stuiver, M., P. J. Reimer, and R. W. Reimer. 2022. CALIB 8.2. [WWW program]. http://calib.org (accessed June 10, 2022).
- Sunberg, S. 2013. Spore rain related to regional sources and beyond. Ecography 36, no. 3: 364–73. doi:10.1111/j.1600-0587.2012.07664.x.
- Taylor, W., J. K. Clark, B. Reichhardt, G. Hodgins, J. Bayarsaikhan, O. Batchuluun, J. Whitworth, M. Nansalmaa, C. M. Lee, and E. J. Dixon. 2019. Investigating reindeer pastoralism and exploitation of high mountain zones in northern Mongolia through ice patch archaeology. PloS one 14, no. 11: e0224741. doi:10.1371/journal.pone.0224741.
- Thompson, K., and J. P. Grime. 1979. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. Journal of Ecology 67, no. 3: 893–921. doi:10.2307/2259220.
- Tirlea, D., A. Osicki, T. Kristensen, R. Woywitka, B. Jensen, A. Criscitiello, K. Williams, et al. 2022. Ice patch archaeology and paleoenvironmental research in Jasper National Park (JNP), Alberta. Paper presented the 65th Annual Meeting of the Canadian Archaeological Association, April 27–30, Edmonton, Alberta.
- VanderHoek, R., J. E. Dixon, N. L. Jarman, and R. M. Tedor. 2012. Ice patch archaeology in Alaska: 2000-10. Arctic 65, no. 5: 153–64. doi:10.14430/arctic4190.
- VanderHoek, R., R. M. Tedor, and J. D. McMahan. 2007. Survey and monitoring of ice patches in the Denali Highway Region, Central Alaska. Alaska Journal of Anthropology 5: 76–86.
- Wigglesworth, G. 1947. Reproduction in Polytrichum commune L. and the significance of the rhizoid system. Transactions of the British Bryological Society 1, no. 1: 4–13. doi:10.1179/006813847804879520.
- Woo, M., and K. L. Young. 2003. Hydrogeomorphology of patchy wetlands in the High Arctic, polar desert environment. Wetlands 23, no. 2: 291–309. doi:10.1672/8-20.
- Zerene Stacker. 2016. Version 1.04. Richland, WA: Zerene Systems, LLC.
