ABSTRACT
Climate change accelerates glacier retreat, leading to extensive exposure of sediment to light and ecological succession. Succession has traditionally been studied as a chronosequence, where vegetation development is directly correlated with time since glacier retreat or distance from the retreating glacier margin. More recent work has challenged this model, arguing that succession seems to be mainly influenced by heterogeneous conditions at the local scale. The aim of this study was to identify the factors influencing the local-scale establishment of plant communities following glacier recession. Vascular plants and their cover were inventoried in 100 plots (1 m2) for a thirty-year-old alluvial plain in front of the Otemma glacier (Swiss Alps). Depth to water table, distance to the glacial main river and to the nearest channel, sediment size, moss, lichen, and biological soil crust cover were measured. Results showed that proglacial margins develop hydrological heterogeneity over a small scale, reflected in the four observed plant communities. These range from the dry Sempervivum-dominated community, on gravel-rich sediments with a deep water table, to the Trifolium-dominated community, close to secondary channels, with the highest plant cover and species richness and incorporating grassland species. Heterogeneity in water availability exerted a critical control on vegetation development.
Introduction
Glacier retreat opens up extensive surfaces to phototrophic conditions and hence provides opportunities for potential colonization by pioneering organisms (Matthews Citation1992; Miller and Lane Citation2019). The associated ecosystems that develop comprise plants and animals but also bryophytes (like mosses and liverworts), fungi, lichens, algae, and microbial communities (Bradley, Singarayer, and Anesio Citation2014; Wietrzyk, Węgrzyn, and Lisowska Citation2016; Roncoroni et al. Citation2019, Citation2022). This colonization of newly exposed land is the first stage of ecological succession. In alpine environments, such colonization generally involves species highly adapted to extreme conditions (Körner Citation2003), as a result of intense solar radiation, a short growing season, high disturbance frequency, and low water retention within substrates (Miller and Lane Citation2019). Local variability in sediment caliber creates highly heterogeneous ecosystems with a multitude of microhabitats and high species diversity (Körner Citation2003).
There have many studies of ecological succession in glacier forefields (Coaz Citation1887; Schreckenthal-Schimitschek Citation1935; Matthews Citation1979a, Citation1979b, Citation1992; see most recent reviews in Burga et al. Citation2010; Erschbamer and Caccianiga Citation2017; Miller and Lane Citation2019). Originally, successional pathways were perceived as following a longitudinal chronosequence, with longitudinal gradients in ecological succession directly correlated with the age of the land surface since glacier retreat (Matthews Citation1979a, Citation1979b; Matthews and Whittaker Citation1987). A major assumption in the longitudinal chronosequence approach is that factors other than time are less important or can be held constant (Matthews and Whittaker Citation1987; Heckmann, McColl, and Morche Citation2016). More recent studies have highlighted the limitations of this approach (Matthews Citation1992; Raffl et al. Citation2006; Burga et al. Citation2010; Eichel et al. Citation2013; Rydgren et al. Citation2014; Miller and Lane Citation2019) whether due to the heterogeneous nature of abiotic processes, including availability of water and disturbance, or because of feedbacks between plant development and the environment (Eichel et al. Citation2013; Eichel Citation2019). The fact that heterogeneity and feedbacks were overlooked until relatively recently may result from sampling bias; the latter tended to focus upon sampling in stable environments, which ignores the role of disturbance and other limiting factors, notably water supply, in plant colonization (Matthews Citation1999; Rydgren et al. Citation2014; Temme and Lange Citation2014; Miller and Lane Citation2019). Sediment erosion and deposition and nutrient dynamics, via weathering and leaching, play a fundamental role in primary ecological succession by modifying the substrate; damaging organisms, especially plants; and influencing nutrient and water availability (Eichel Citation2019; Miller and Lane Citation2019). If there is sufficient valley width, glacier forefields may form alluvial plains with braided rivers that are highly dynamic. Intense daily variation in discharge due to glacier melt (e.g., Malard et al. Citation2006; Lane and Nienow Citation2019) leads to rapid expansion and contraction of inundation as well as high rates of channel erosion and deposition. If there are prolonged periods where sediment transport capacity by the river exceeds sediment supplied by the glacier to it, then systematic erosion and terraces may form. Alluvial plains are of particular interest because relatively high water availability should facilitate colonization, but high rates of morphodynamic activity or the negative impacts of intrusion by harsh glacial meltwater may make biogeomorphic succession slower than that in more stable zones (Corenblit et al. Citation2009; Miller and Lane Citation2019; Roncoroni et al. Citation2023).
Burga et al. (Citation2010) demonstrated that the factors controlling plant establishment depends on the scale considered. Large-scale patterns are driven by time since deglaciation, topography, and major disturbance, including floods, rockfalls, and avalanches. At smaller spatial scales, vegetation development depends on species pools present beforehand around or on the glacier foreland, seed dispersal processes (Ferrarini Citation2015), and local influences (Burga et al. Citation2010). At such small spatial scales, plant distribution appears to be more influenced by environmental heterogeneity than the spatial manifestation of time since deglaciation (Rydgren et al. Citation2014). Because glacially derived sediment commonly has a low water retention capacity (Müller, Lane, and Schaefli Citation2022), water supply is critical and is influenced by microclimate, substrate grain size, and microrelief (Burga et al. Citation2010; Wietrzyk, Węgrzyn, and Lisowska Citation2016; Roncoroni et al. Citation2023). Substrate grain size significantly influences the establishment of plants (Burga Citation1999) because it determines moisture retention and may create microclimatically sheltered zones that increase the probability of germination and seedling survival. Local relief is also important with alluvial terraces tending to be more stable and so less prone to morphodynamic reworking (Roncoroni et al. Citation2023) even if their height above the alluvial plain means they have more restricted access to shallow groundwater (Müller, Lane, and Schaefli Citation2022).
Access to nutrients and to water in these environments is strongly influenced by soil development, and thus ecosystem engineers that can encourage young soil development are important (Miller and Lane Citation2019). Candidate ecosystem engineers include plants, bryophytes, lichen, and microorganisms (algae, bacteria) (Roncoroni et al. Citation2019). The latter may have a significant effect, stabilizing sediments, retaining water, and providing a source of nutrients (Belnap Citation2003; Bradley, Singarayer, and Anesio Citation2014; Miller and Lane Citation2019; Roncoroni et al. Citation2019, Citation2023). These engineers are designated biofilms when they are communities that grow in aquatic environments (Roncoroni et al. Citation2019, Citation2023) and biological soil crust, partly constituted of bryophytes, lichens, and cyanobacteria, when they grow in terrestrial environments (Belnap, Prasse, and Harper Citation2001).
The interactions between environmental heterogeneity, ecosystem development, and ecosystem engineering effects are commonly integrated in the notion of biogeomorphic succession (Corenblit et al. Citation2009), which was applied to proglacial alluvial plains by Miller and Lane (Citation2019). The development of plant and microbial communities is initially controlled by abiotic processes, hydrological and geomorphological. Once established, such communities can initiate feedbacks in the system (Corenblit et al. Citation2009), such as weathering, changes in water availability, substrate stability, and the supply of nutrients and carbon (Gurnell et al. Citation2000; Matthews and Owen Citation2008; Moreau et al. Citation2008; Eichel et al. Citation2013; Schulz et al. Citation2013). These feedbacks may begin to support further ecosystem succession. Subsequent colonization by plants with deeper rooting systems can then aid the stabilization of sediment deposits (e.g., Gurnell et al. Citation2019), and there then develops a strong interaction between ecological and abiotic processes (Corenblit et al. Citation2009), which Miller and Lane (Citation2019) showed for proglacial alluvial plains is conditioned by alluvial disturbance frequency. Eventually, ecosystem engineering effects are so strong that abiotic processes become significantly less important and ecological processes dominate the succession.
Current climate change is accelerating glacier retreat. This increases the rate at which zones become ice free and colonizable (Cannone et al. Citation2008; Fickert, Grüninger, and Damm Citation2017). With global warming, plants will be able to colonize these new surfaces faster if temperature is a limiting growth factor. Cannone et al. (Citation2008) showed that this colonization could begin as soon as 1 year after the retreat of a glacier. Other studies found it to be closer to 4 to 8 years after deglaciation (Stöcklin and Bäumler Citation1996; Burga Citation1999). Ecological succession appears generally to be faster in a warmer climate, involving a larger number of species than 100 years ago (Fickert, Grüninger, and Damm Citation2017). With rapid climate change, the stages of succession seem to be altering. Early stages of ecological succession seem to be being more rapidly replaced by advanced stages (Erschbamer Citation2007; Cannone et al. Citation2008). However, there are few studies to date that have established the extent to which ecosystem succession following deglaciation is related to temperature limitation.
A key theme emerges from this review that is commonly overlooked in discussions of vegetation development in formerly glaciated terrain: development is highly constrained by access to water (Roncoroni et al. Citation2023), which matters because the spatially heterogeneous microrelief and substrates of glacier forefields (Müller, Lane, and Schaefli Citation2022) impart a complex heterogeneity on successional processes. Although Roncoroni et al. (Citation2023) have recently shown how water availability conditions biofilm development, an important ecosystem engineer in proglacial margins, they did not consider the broader plant communities that subsequently develop. Here, we test the hypotheses that the development of plant communities is not random but driven by underlying environmental heterogeneity. Abiotic and biotic environmental factors, such as water availability, substrate grain size, and the presence of bryophyte and biological soil crust, were measured in a small zone that largely became ice free at the same time. This allows us to understand how heterogeneous environmental conditions influence the establishment of plant communities.
Study site
The study area is located on an alluvial plain in the forefield of the Otemma glacier (approximately 2,450 m.a.s.l., Val de Bagnes, Canton Valais, Switzerland), near the Italian border (). The plain is approximately 200 m wide and has been ice free since the early 1980s. Steep valley walls comprising mixed glacial till and bedrock outcrops line the plain, and these have been dissected by small torrents that have formed small debris cones that infringe upon the alluvial plain (Mancini and Lane Citation2020). Revegetation of these hillslopes is happening at a greater rate than in the alluvial plain itself, and this appears to be due to high rates of reworking of the plain by the proglacial stream (Roncoroni et al. Citation2023). The latter has formed low relief terraces that the secondary channels (nonglacial small tributaries) flow across. The study area is located on top of the lowest terrace at the downstream end of the alluvial plain, just before the proglacial stream steepens and becomes semi-alluvial and confined. Geologically, metagranitoids of Lower Paleozoic age dominate this valley (Federal Office of Topography Citation2005) and the substrate is coarse and siliceous. The average annual temperature is −0.3°C, with a maximum monthly average of 11.5°C in July. The long-term monitoring station of Col du Grand St-Bernard, at an elevation of 2,469 and 20 km away, recorded annual precipitation of 2,368 mm for the reference period 1981 to 2010 (MeteoSwiss Citation2016). However, measurements at the site recorded for the period 2019 to 2021 (Müller Citation2022) suggest that rainfall at the study site is 50 to 35 percent less than this amount.
Figure 1. Location of the study area in the alluvial plain of Otemma glacier, with the delimitation of the position of the glacier from 1988 to 2017 superimposed on an orthoimage from 2017 (UAV imagery). The limits for 1988 and 1994 have been defined in accordance with historical aerial photographs available on https://map.geo.admin.ch/ (Federal Office of Topography swisstopo Citationn.d.). The red dot in the inset shows the location of the Otemma glacier in Switzerland.
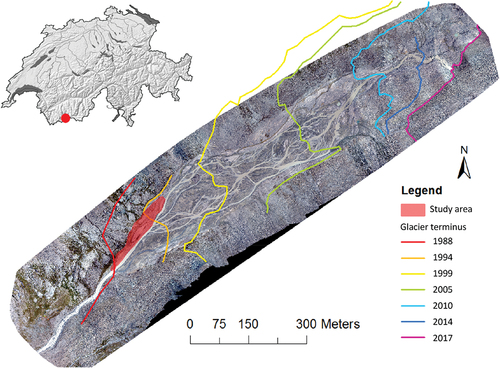
The Otemma glacier has been strongly impacted by climate change due to its southwest orientation and by the topography of the valley, which is flat and straight, resulting in a regular and homogeneous retreat of the glacier. The glacier lost 1 km in length between 1990 and 2016 (GALMOS Citation2018). It has negligible direct human impact (no grazing, no hydropower development) due to its isolation. Other research is being conducted at this site looking at biofilm development (Roncoroni et al. Citation2019), sediment flux and disturbance (Mancini and Lane Citation2020), water flow in the glacier (Egli et al. Citation2021), and the wider basin groundwater hydrology (Müller, Lane, and Schaefli Citation2022), which helps to place this study into a larger environmental context and allows for a better general understanding of this ecosystem.
Method
Sampling design
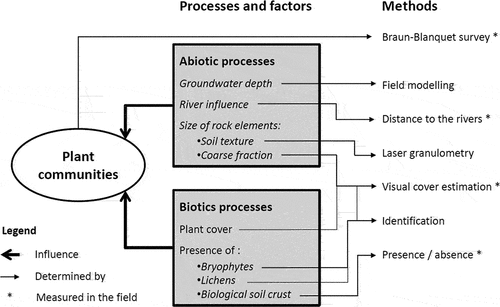
An area of about 5,500 m2 was the focus of this study, with fieldwork undertaken during the summer of 2018 (). This zone was chosen because it contained both early pioneer and more developed successional stages but actually became ice free in a relatively short window of time (between 1988 and 1994). Visual inspection of the study area suggested a wide range of abiotic conditions in relation to moisture availability (grain size of the substrate, distance to a small channel, elevation from the main river). Thus, it was ideal for identifying the drivers of heterogeneity in vegetation succession. In 2018, snowmelt in this area occurred between 19 and 29 June (on the basis of Sentinel satellite imagery, CitationSinergise Solution). Data collection was undertaken during the regional flowering period peak (24 July to 10 August). One hundred plots were selected by stratified random sampling according to the bed sediment size by qualitative visual estimation from 2017 UAV (unmanned aerial vehicle) imagery (Appendix I). Each plot shape was chosen to have a homogeneous topography, sediment size and plant cover distribution; these varied from a perfect square (1 × 1 m) to a long rectangle (0.30 × 3.33 m).
Composition of plant communities
In each plot, all vascular plants were identified and species cover was visually estimated in seven classes, following the standard method for describing plant communities in phytosociological studies (Blanquet 1964; Appendix V, Table V.1). The plant names follow the taxonomy of the Swiss floristic guide (Eggenberg, Bornand, and Juillerat Citation2018). For every plot, a sample of each bryophyte and lichen species was collected and identified using magnifying binoculars and a microscope. The taxonomy follows the specialized websites: Bryolich (Citation2017) and Swissbryophytes (Citation2019).
Site conditions
For each plot, the following site conditions were recorded in the field (): slope, aspect, distance to the main river and the closest secondary channel, and the presence of biological soil crust. The proportional coverage of vascular plants, bryophytes, lichens, litter, fine sediments (<2 mm), gravels (2 mm–2 cm), and coarse gravel and blocks (>2 cm) was visually estimated. The precise position of the corners of each plot was measured using a differential Global Positioning System (Appendix II), to allow return to the same sites in the future. To have better than ±1 cm precision, a fixed base station was used that had previously been precisely corrected into the Swiss coordinate system (CH 1903/LV03). To assess the elevation, a digital surface model (DSM) and an orthophoto (0.84 cm/pixel resolution; Appendix III) were created using 483 images in a grid mode taken by a drone DJI Phantom 3. The software Pix4D mapper (CitationArcMap v10.5) was used to georeference the UAV data using twenty-one ground control points that were measured with a differential Global Positioning System, following the methods reported in Roncoroni et al. (Citation2022). Finally, ten preferentially selected soil pits were dug down to the groundwater table. Soil profiles were described in the field.
Water availability
Water retention in soil is often well correlated with soil texture (Gee and Bauder Citation1986), so the latter was measured for samples from forty-six plots (top of the substrate). Samples were analyzed in the laboratory using a laser diffraction particle size analysis of the mineral part. To obtain a more accurate picture of water availability, a simplified groundwater model was developed. In a first step, the groundwater level was measured in eight pits at different terrace elevations, dug next to one vegetation plot, for two days (6 and 7 August 2018). The depth of the groundwater table was measured at different times of the day in the pits (Appendix IV). Measures were correlated with a record of the main river level taken at the outlet of the alluvial plain, about 100 m downstream of the studied area, derived from a continuously recording pressure transducer. To model the depth of the groundwater table, the elevations of the river and groundwater were assumed to be the same on a line perpendicular to the valley (Winter Citation1999). A grid of 5 m was drawn, where each line, perpendicular to the valley, corresponds to the elevation of the river according to the DSM (drone flight 7 August 2018). Groundwater levels measured in eight pits simultaneous to the drone flight were used to validate the model (control points). Finally, the map of the groundwater table depth was obtained by subtracting the DSM from the elevation given by the groundwater model. The limitation of this model is that it can only be realized in an environment where the substrate is sufficiently well drained so that there is a strong connection between the river level and the groundwater level. Müller et al. (Citation2023) confirmed that this is the case.
Statistical analyses
Statistical analyses followed Borcard, Gillet, and Legendre (Citation2011), who proposed an integrated R platform for the analysis of ecological data. Cover classes of plant species were transformed into average values of each class (abuntrans function in labdsv library, Appendix V). The Shannon index (Shannon and Weaver Citation1949) of diversity was calculated with the diversity function in the vegan library.
Based on plant composition, the surveys were separated into homogeneous groups (Borcard, Gillet, and Legendre Citation2011) to simplify the structure of our data. The Bray-Curtis dissimilarity was calculated between pairs of plots to allow comparison of their respective species composition. Ward’s minimum variance method was used to cluster sites into a smaller number of representative groups that could each be characterized by a diagnostic plant species and that then allowed statistical comparison of abiotic factors by group. The number of groups retained was a compromise between several criteria (based on silhouette, matrix correlation, and species fidelity analysis; Appendix V following Borcard, Gillet, and Legendre Citation2011). Diagnostic species were determined by calculating an indicator value for each species in each group, according to Dufrêne and Legendre (Citation1997), using the indval function in the labdsv library. The diagnostic species attributed to each group were the ones that had a p value < .05.
To identify causal linkages between the plant communities and abiotic variables, canonical redundancy analysis (the rda function in vegan library) was applied to the vascular plant surveys (response variables) and the environmental factors (explanatory variable). The most relevant variables were identified using a variance inflation factor, using the function vif.cca. Factors with a value greater than ten were deemed to be of insufficient importance for further consideration. Factors that had a correlation greater than 0.5 with another factor in the correlation matrix (Appendix VI) were also removed. The statistical analyses were realized with R v3.4.3 (R Core Team Citation2017).
Results
Distribution of vascular plants and development of plant communities
The visual cover of vascular plants shows a large difference between plots, with an average of 18 percent and a range between 0 and 88 percent. Plot 13 had no plants and was removed prior to statistical analyses. A total of 48 vascular plant species were identified, with an average of 10.4 species per plots (Appendix VII). The most frequently present species were Leucanthemopsis alpina (in eighty-seven plots), Cerastium uniflorum (eighty-one plots), and Agrostis rupestris (eighty plots). The plants with the largest cumulative cover were Trifolium pallescens, Trifolium badium, and Epilobium fleischeri. The plants identified are mostly grasses and forbs, with also some small shrubs of the genus Salix. We noted a young shoot of a pioneer tree, Betula pendula. Wind dispersal is likely the most common pathway used by plants in the Otemma alluvial plain, as with most alpine pioneer plants (Ferrarini Citation2015).
According to the analyses, four groups of plant inventories were retained (, , and Appendix V). These groups were labeled by the genus of one diagnostic species. One of the groups was not characterized by a plant species but rather by the presence of biological soil crust in the majority of the associated plots (). The plant cover of the Trifolium group exceeded that of the other groups (). This group had also the highest species richness (14.5 ± 2.2) and Shannon index (7.2 ± 1.5; ). The Sempervivum group showed the lowest species richness (3.2 ± 1.5) and Shannon index (2.1 ± 0.8) ().
Figure 3. (a) Distribution of plant community groups in the study area on an orthoimage (derived from UAV; see Appendix III). (b) Depth of groundwater level, modeled from a DMS (Digital Surface Model); the groundwater level measured in eight pits (red dots, number of the corresponding plant inventory); and the level of the river (see Appendix IV).
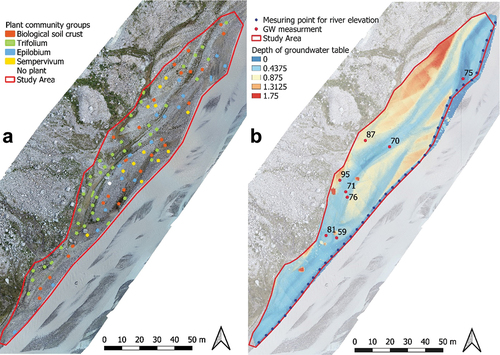
Figure 4. Boxplot of (a) vascular plant cover (%), (b) species richness, and (c) Shannon’s diversity index by group. The boxplot width is proportional to the number of surveys classified in the group. The black line is the median and the limits of the boxes are the first and third quantiles. The stars indicate the degree of significance of the p-value according to a global a Kruskal-Wallis test (*p ≤ .05, **p ≤ .01, ***p ≤ .001). Different letters indicate significant differences between groups with a post hoc comparison (Borcard, Gillet, and Legendre Citation2011).
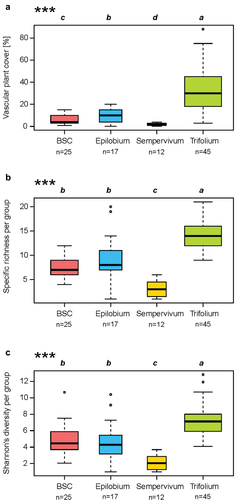
Table 1. Synoptic table of the four plant community groups in Otemma glacier forefield.
Rivers and groundwater
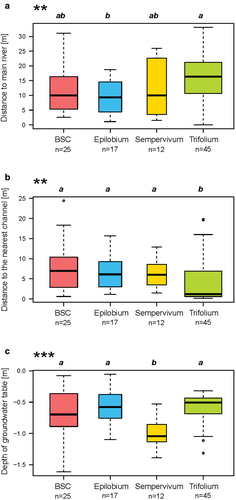
The difference in groundwater depths between pits was explained by proximity to the main river and the elevation of the alluvial terraces (Appendix III). It was possible to observe daily fluctuations of the groundwater table correlated with river flow (Appendix IV). This result confirmed the appropriateness of our modeling of groundwater depth relative to the main river level (). The modeled groundwater level tended to be 15 ± 10 cm lower (average and variance between model and field) than the measured values in the eight pits, with the largest differences (around 20–25 cm) explained by the proximity of tributaries, which raise the water table (Appendix IV).
The spatial distribution of groups in the study area showed that the Trifolium group was farther from the main glacial river than the Epilobium group but the Trifolium group was closer than any group to a secondary channel (). According to the model of the groundwater depth, the group Sempervivum was located higher on alluvial terraces with respect to the water table than the other groups ().
Soil description
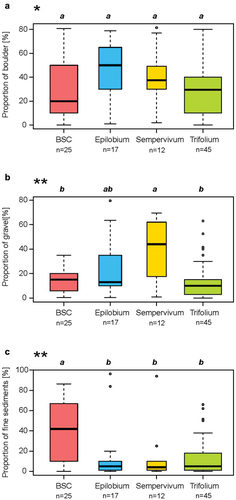
Across the full 5,500 m2 of the study site, block or gravel cover ranged between 0 and 80 percent and finer sediment between 0 and 96 percent. The Sempervivum group was associated with the highest proportion of gravel (2–20 mm) and the biological soil crust group with the highest proportion of sand and finer sediments (<2 mm; ). The texture of this fine material was distributed between sand, loamy sand, and light sandy loam but without any significant difference between plant groups (Appendix VIII). The soils of the alluvial plain of Otemma glacier were mostly poorly developed and came from river sediments or reworked moraines (Appendix IX). Among the ten described solums, six were Skeletic Fluvisols, three were Skeletic Leptosols, and one was a Skeletic Regosol, according to IUSS Working Group (Citation2015), all with para-humic systems (Zanella et al. Citation2018; Appendixes IX and X). Sorting of sediments by the river, with layers showing different textures, was observed in some solums. This indicates distinct deposition episodes associated with different river dynamics. These soils have been described as polylithic (Baize and Girard Citation2009). Two solums contained signs of oxidation–reduction (g horizon) in silty horizons, indicating temporary waterlogging, with alternance of reduction (mobilization) and oxidation (immobilization) of iron (Baize and Girard Citation2009). When the environment had been stable for a long enough time, the first signs of soil formation were recognized by the presence of a young horizon (Js; Baize and Girard Citation2009; Gobat and Guenat Citation2019), containing organic matter juxtaposed with fine mineral material. This was detected in two solums under the Trifolium group.
Distribution of bryophytes, lichens, and biological soil crust
Eleven different species of bryophytes were identified, with an average of 2.4 species per plot. Lichens were observed in twenty-six plots, and five different species were identified (Appendix XI). Three species of bryophytes were particularly frequent: (1) Polytrichum piliferum, present in dry, acidic, and very sunny areas (Urmi et al. Citation2016), present in 83 percent of the plots classified in the Sempervivum group; (2) Racomitrium canescens, with an approximately similar habitat (Swissbryophytes Citation2019), present in >75 percent of three groups (Epilobium, biological soil crust, and Sempervivum groups); and (3) Pohlia filum, present in 96 percent of the plots of the Trifolium group and growing in humid, acidic, and very sunny habitats (Köckinger and Hofmann Citation2017).
Among lichens, Stereocaulon alpinum was the most common species. It is considered to be the first lichen to establish after glacier retreat (Burga Citation1999), and it is preferably found in dry and poorly vegetated environments. It was present in all groups. The biological soil crust group had the highest proportions of lichen presence and diversity. Biological soil crusts were present in thirty-one plots, with their highest frequency (56 percent) in the biological soil crust group (). Conversely, they were rare to very rare in the Epilobium and Sempervivum groups.
Redundancy analysis
Under the redundancy analysis (RDA), seven driving variables were retained for analyses (): the proximity to the main river (Main_River) and to the nearest channel (Near_Channel), the depth of the groundwater (GW), the visual cover of bryophytes (Bryo), the presence of lichens (Lichen), the proportion of fine sediments (Fine_Sed), and the proportion of gravel (Gravel).
Figure 7. Pictures of each four groups (above) and RDA in scaling 1, based on species composition and constrained by six ecological variables (below): Visual cover of bryophytes (Bryo) and lichens (Lichen), proportion of fine sediments (Fine_Sed) and gravel (Gravel), distance to the main river (Main_River), distance to the nearest Channel (Near_Channel), and the depth of groundwater table (GW; negative values as in ). The first constrained axis explained 10.5 percent of the total variance and the second explained 4.0 percent.
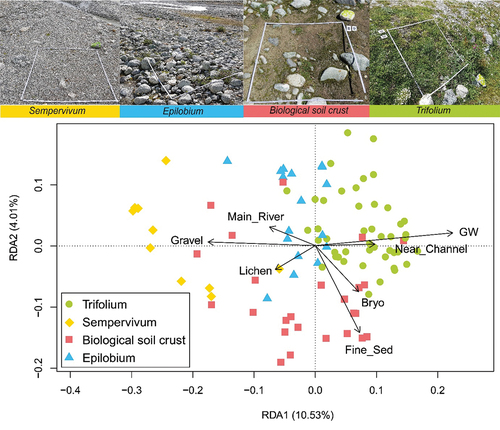
The plant community groups were relatively distinct on this RDA (). The first axis (10.5 percent of the variance) was mainly positively correlated to the depth of the groundwater and negatively to the proportion of gravel. The vertical axis (4.0 percent of the variance) was negatively correlated to the proportion of fine sediment. The Trifolium group occupied sites near a secondary channel and a high water table, whereas the Sempervivum group occupied areas with the deepest water table and the highest cover of gravel. The biological soil crust group was mostly located on substrates rich in fine sediments.
Discussion
The results of this study show that proglacial margins can develop hydrological heterogeneity over a small spatial scale that is then reflected in plant community development (). Five factors were identified as important: the depth of the water table, distance to the main river, distance to the nearest channel, sediment size, and presence of other organisms such as bryophytes, lichens, or biological soil crust. All of these factors directly or indirectly influence the availability of water for plants, although they can interact in complex ways.
Although the canonical RDA () only partially explained the total variance, it indicates the respective importance of these factors. According to these results, three plant community groups (Trifolium, Epilobium, and Sempervivum groups) were distributed along the main axis, which was explained mainly by the depth of the water table and the importance of gravels in sediments. The second axis separated the biological soil crust group, characterized by a high proportion of fine sediments. Sedimentation in these dynamic channels in alpine alluvial plains creates a high heterogeneity of conditions, here independent of time since glacier retreat.
Water supply and the stability of the substrate have been identified as key factors for the evolution of ecological succession in alpine environments (Jochimsen Citation1976; Matthews and Whittaker Citation1987). Soils with high water retention capacity allow more water-demanding plants to establish, with higher productivity, initiating soil development and the next stage of ecological succession according to the ecological optimum of the observed species (Raffl et al. Citation2006; Lauber, Wagner, and Gygax Citation2018). This was reflected in our results by the Trifolium group. The latter represents the least pioneering plant community with the highest species richness and cover and was generally found in locations with a good water availability, close to the water table or secondary channels. Conversely, communities characterized by coarse, draining substrates, high above the groundwater table, such as the Sempervivum group, had a low vegetation cover and species richness. The diagnostic species Sempervivum montanum is particularly well adapted to drought. In these sites, the ecological succession is slowed by this environmental constraint, with low productivity (Raffl et al. Citation2006) and hence slow soil development.
The discharge of glacier rivers, usually with a high sediment load, is subject to significant daily and annual variations (Marren Citation2005). Same-day variations can be important because of solar radiation that melts the surface of the glacier and can release large amounts of water in summer. The strong dynamics of the glacial river, through erosion and sediment deposition, slows the development of vegetation close to it (Roncoroni et al. Citation2019) and keeps the system in the geomorphic stage of biogeomorphic succession (Corenblit et al. Citation2009). Roncoroni et al. (Citation2023) showed that perennial biofilm cover could only develop at the Otemma glacier forefield on alluvial terrasses sufficiently above the active alluvial plain where disturbance frequency is reduced. In turn, in such environments, biofilm development was limited by water availability. We show here that this water availability also strongly influences wider plant community development. Availability is itself associated with relatively small differences in relief () in the presence of significant daily variation in water level in the main glacial stream. The most pioneering Sempervivum group was able to exploit the higher and drier zones, which are also the zones least prone to disturbance, whether by harsh glacier meltwater or erosion and deposition (Roncoroni et al. Citation2023). The Trifolium group, the least pioneering, needed access to water and so was found where the groundwater was closer to the surface, but this in turn exposed it to a greater risk of disturbance because of the small difference of elevation with the river. Thus, succession in this environment may be limited by the fact that as plant communities develop, their water demand increases. At least until ecosystem engineering and soil development cause glacial sediments to retain more water, such communities have to exploit zones with a high risk of disturbance. This prevents evolution to the biogeomorphic stage of succession (Corenblit et al. Citation2009) and keeps the system largely in the geomorphic stage (Miller and Lane Citation2019) with a strong association between abiotic factors and the communities that develop ().
Evolution to the biogeomorphic stage may be encouraged by the development of water-retaining conditions. Layers of fine sediments, often in temporary secondary channels of the river, promote the establishment of microorganisms (Raffl et al. Citation2006). Some microorganisms are also present as biofilms in minor tributaries that flow from the northern part of the study area to the main river. The roles of the biofilms as ecosystem engineers have already been described in Miller and Lane (Citation2019) and in Roncoroni et al. (Citation2019, Citation2023). They make the soil less permeable to infiltration, maintaining the water at the surface and creating a more favorable habitat for more water-demanding vascular plants (Miller and Lane Citation2019), such as Trifolium badium or Saxifraga stellaris (). An example of this process was identified in the Otemma glacier forefield (). A soil pit (soil 87, , Appendix IX, plant community classified in biological soil crust group) was dug on an alluvial terrace 30 m away from the main river but at 50 cm of a secondary channel. The groundwater table was found 72 cm below the soil surface, too deep to be reached by most of the plant roots. But the water from the secondary river was directly accessible to plants because maintained at the surface by a layer of biofilm (), and species of the Trifolium group have developed along the margins of this secondary channel. Thus, secondary channels on terraces, whether historically created by the alluvial plain or supplied by water from tributaries draining across the terraces, may be a primary means of countering dependence on deep groundwater supplied by the morphodynamically active main river. These then become hotspots for vegetation succession, something that is also clear in the development of biofilms (Roncoroni et al. Citation2023).
Figure 8. Roles of biofilms and biological soil crust in water availability. (a) Enlargement on a part of the study area with the distribution of the vegetation groups in relation to the depth of the groundwater table. (b) A soil pit located 50 cm away from a secondary channel with the groundwater table 72 cm below the soil surface. (c) Enlargement of the biofilm on the bed of the secondary channel, close to the pit in (b). (d) Flooded area strongly covered by bryophytes after a heavy rain. (e) Oxidation–reduction marks at the top of the soil of the plot in (d).
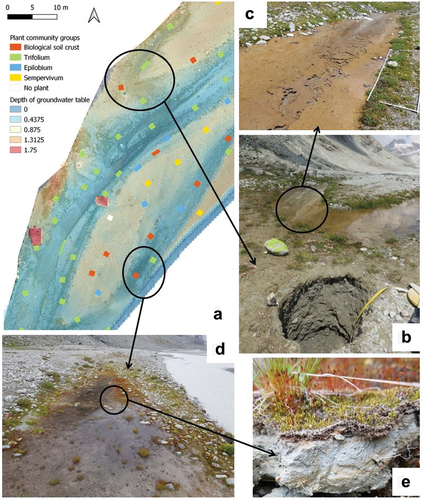
Bryophytes and biological soil crusts may also play a role as ecosystem engineers. Indeed, they slow water infiltration into the ground, can create temporary pools (), and, by their structure, slow down evaporation, maintaining a water at the surface for longer time. These wetter conditions were visible in signs of oxidation–reduction in the top of solums (), indicating water retention in the soil (Baize and Girard Citation2009). These improved conditions, with greater water availability, will be important in the establishment of vascular plants, especially for the survival of seedlings (Raffl et al. Citation2006). For example, Pohlia filum was a frequently found bryophyte, especially in Trifolium plots. It forms carpets that act as a seedbed and can offer a more stable and favorable site for young seedlings for some vascular plants (Stöcklin and Bäumler Citation1996). This can be one of the reasons for the high species diversity in the Trifolium group, including plants from the late successional stage, such as Phleum alpinum aggr., Euphrasia minima, or Festuca halleri. The role of early colonizers in stabilizing this environment and so further aiding the transition to a biogeomorphic condition has been shown for other glacial forelands (e.g., Eichel et al. Citation2013; Eichel Citation2019). That said, there are now also data that show that this stabilizing effect in alluvial plains (rather than on other landforms in such forelands) may be restricted (Roncoroni et al. Citation2023). This is because the increases in resistance to motion caused by the developing ecosystem remains small compared with the force of proglacial river channels (Roncoroni et al. Citation2023). High rates of reworking and then the geomorphic stage of biogeomorphological succession then remain dominant.
Another reason is the facilitation of nutrient supply. Indeed, the genus Trifolium is part of the Fabaceae family, which is known to establish symbiosis with nitrogen-fixing bacteria in root nodules.
However, many pioneer plant species do not require microorganism or bryophyte communities to colonize young and unstable substrates (Roncoroni et al. Citation2019). Some of these plant species are frequently found in proglacial alluvial plains, such as Saxifraga bryoides, Trifolium pallescens, or Poa cenisia (Burga Citation1999). For these species, fine sediments still have a direct influence on water and nutrient availability and so may accelerate their development. Burga et al. (Citation2010) linked vegetation establishment to sediment size in the forefield of the Morteratsch glacier and found that some plant communities were favored by certain substrates. The soils described in the Otemma glacier forefield are poorly developed, without any real soil structure and with a very low content of organic carbon (maximum 0.31 percent). Indeed, the retreat of the glacier is recent (less than thirty years), and the soils are young and with a low biomass input (Baize and Girard Citation2009). The evolution of fluvisols is conditioned by fluvial dynamics and the geologic origin of sediments (Bureau Citation1995). In the Otemma Valley, the slow weathering rate of granite slows down soil evolution and tends to produce sand (Gobat and Guenat Citation2019) and a very low clay content (Appendix VIII). Only silt and finer sediments retain water and nutrients, facilitating plant establishment and growth, explaining why hydrological heterogeneity is so important in this environment.
Wider climatic changes may be important. The tributaries that cross the zone studied here will be impacted by the magnitude of snow accumulation on the valley side walls as well as the timing of its melt. Reduced winter snow accumulation as well as early melt may reduce the extent of perennial tributary water supply. The melting of glaciers is also an important parameter in the evolution of alluvial plains. Glaciers produce large quantities of clastic material that are transported downstream. The combined effect of high flow, caused by increased ice melt, and high clastic availability explains the high sediment yields associated with deglaciation (Gurnell et al. Citation2000).
In a very simplified way, two scenarios are possible for the future evolution (before the extinction of the glacier) of the Otemma alluvial plain. These depend on the melting rate of the glacier—that is, the amount of water released (by the glacier melt, rainfall, residual snow)—and the sediment capacity of the river (Marren Citation2005) as well as the rate of sediment supply. In the first scenario, sediment transport capacity exceeds sediment supply, causing erosion and lowering the groundwater table. Water becomes less available to plants, but the terraces are less disturbed by the dynamics of the river (flooding, riverbed reworking) and therefore more stable. Water retained at the surface by biofilms and fine sediments will allow rapid vegetation development and succession toward alpine grasslands. In parallel, plants adapted to drought, like Sempervivum montanum, will be favored on coarse sediments, with a slow soil development.
In the second scenario, the sediment delivery exceeds sediment transport capacity, causing riverbed levels to rise and tending to lead to high rates of lateral river migration associated with braiding. Disturbances of the study area are frequent and important, maintaining the area mainly in pioneer conditions, with fine sediment banks, easily colonizable by biofilms (Roncoroni et al. Citation2019, Citation2023), and then promoting the establishment of species like Saxifraga stellaris, S. aizoides, or Trifolium badium and coarse sediments with low plant cover (Miller and Lane Citation2019).
The potential of plants as engineers in the ecosystem could become more important in both scenarios. The development of shrubs (present, but still small and in juvenile state), like Salix sp., will also have a function in stabilizing the sediments by their roots, which are deeper than most of the herbaceous plants in the study area. Which of these two options becomes dominant is likely to depend on the relative balance of sediment transport capacity and sediment supply, itself dependent on the glacier, its size, its rate of melt, and its erosion rate. This balance remains a very poorly understood element of glacial environments undergoing rapid climate change (Zhang et al. Citation2022), but it leads to a core hypothesis for further testing that it drives the nature of vegetation succession in alluvial plains through its combined impacts on water availability, microtopography, and disturbance regimes.
Conclusion
Among the four contemporary plant community groups recognized in the Otemma glacier forefield, the Trifolium group had the highest cover and species richness. This group was characterized by its proximity to the groundwater table and to a secondary channel, both ensuring good water availability during the whole summer but also exposing it to the risk of disturbance by the proglacial stream. The next two groups in decreasing order of plant cover and species richness were the biological soil crust and Epilobium groups. They shared a high water table with the Trifolium group but they were farther away from the secondary channel. On the other hand, the biological soil crust group was characterized by a high proportion of fine sediments, able to maintain soil moisture. This facilitates the growth of algae, lichens, or bryophytes, all improving superficial water retention. Finally, the Sempervivum group shared all of the poor conditions in terms of water availability: a high proportion of gravel, a low proportion of fine sediments, and a deep water table. Plant cover and species richness were very low in this group. These results are valid for the alluvial plain of the Otemma glacier, but other research in a wider range of proglacial alluvial plains must be realized to generalize these observations. Such research is likely to also need improved methods, notably in relation to determination of groundwater availability, given its observed importance in this study.
Despite the presence of a river fed by snow and ice melt, the glacier forefield is largely a dry surface for vascular plants. Indeed, glacial till, the predominant substrate in these landscapes, is a poor retainer of water. The sedimentological and geomorphological heterogeneity of the forefield then determines where within this aridity conditions are optimal for water access and succession can occur more rapidly. This is not a simple function of distance from streams or height above the stream. Zones closest to water are commonly the ones most likely to be impacted by erosion and/or deposition. Higher locations may be affected by tributary water supply. Thus, rapid vascular plant colonization is likely a trade-off between local stability and access to water, as observed with the Trifolium plant community. But the colonization by microorganisms (biofilms) and biological soil crusts slows down the infiltration of surface water and can locally improve access to water, creating an ecosystem engineering type of effect.
One of the striking differences between glacier recession at present and that which dominated the post–Little Ice Age period until the 1980s (in alpine environments at least) is the increase in the rate of retreat. This is likely to render the kinds of heterogeneous effects described here more dominant than patterns related to chronosequences. In effect, large longitudinal tracts of glacier forefield have become ice free at the same time. When taken with the effects of increased melt and sediment supply on the intensity of glacier stream morphodynamics, this may mean that in glacier forefields we no longer see traditional succession patterns. This may change when a glacier has shrunk sufficiently for the basin to become snow dominated. Future inventories of the same plots, along with studies of other proglacial alluvial plains, will allow a better understanding of the glacier forefield ecosystem in a context of global warming.
Supplemental Material
Download Zip (16.2 MB)Acknowledgments
We thank Luca Miserere and Mathias Vust for their help in identifying some lichen and bryophyte species. A big thank you to Hannah Miller for helping with the fieldwork at Otemma glacier.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15230430.2023.2259677
References
- Baize, D., and M. C. Girard. 2009. Référentiel pédologique 2008. Versailles, France: Editions Quae.
- Belnap, J. 2003. The world at your feet: Desert biological soil crusts. Frontiers in Ecology and the Environment 1, no. 4: 181–18. doi:10.1890/1540-9295(2003)001[0181:TWAYFD]2.0.CO;2.
- Belnap, J., R. Prasse, and K. T. Harper. 2001. Influence of biological soil crusts on soil environments and vascular plants. In Biological soil crusts: Structure, function, and management, 281–300. Berlin, Heidelberg: Springer.
- Borcard, D., F. Gillet, and P. Legendre. 2011. Numerical ecology with R. vol. 2, 688. New York: Springer.
- Bradley, J. A., J. S. Singarayer, and A. M. Anesio. 2014. Microbial community dynamics in the forefield of glaciers. Proceedings of the Royal Society B: Biological Sciences 281, no. 1795: 20140882. doi:10.1098/rspb.2014.0882.
- Bryolich. 2017. Bryolich; Association Suisse de Bryologie et de Lichénologie. http://www.bryolich.ch/bryologie/bryologie_de.html [WWW Document] (accessed June 11, 2019).
- Bureau, F. 1995. Evolution et fonctionnement des sols en milieu alluvial peu anthropisé. Doctoral diss., Verlag nicht ermittelbar.
- Burga, C. A. 1999. Vegetation development on the glacier forefield Morteratsch (Switzerland). Applied Vegetation Science 2, no. 1: 17–24. doi:10.2307/1478877.
- Burga, C. A., B. Krüsi, M. Egli, M. Wernli, S. Elsener, M. Ziefle, T. Fischer, and C. Mavris. 2010. Plant succession and soil development on the foreland of the Morteratsch glacier (Pontresina, Switzerland): Straight forward or chaotic? Flora-Morphology, Distribution, Functional Ecology of Plants 205, no. 9: 561–76. doi:10.1016/j.flora.2009.10.001.
- Cannone, N., G. Diolaiuti, M. Guglielmin, and C. Smiraglia. 2008. Accelerating climate change impacts on alpine glacier forefield ecosystems in the European Alps. Ecological Applications 18, no. 3: 637–48. doi:10.1890/07-1188.1.
- Coaz, J. 1887. Erste Ansiedelung phanerog. Pflanzen auf von Gletschern verlassenem Boden. Bern: Mitteilungen der Naturforschenden Gesellschaft.
- Corenblit, D., J. Steiger, A. M. Gurnell, E. Tabacchi, and L. Roques. 2009. Control of sediment dynamics by vegetation as a key function driving biogeomorphic succession within fluvial corridors. Earth Surface Processes and Landforms: The Journal of the British Geomorphological Research Group 34, no. 13: 1790–810. doi:10.1002/esp.1876.
- Dufrêne, M., and P. Legendre. 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs 67, no. 3: 345–66.
- Eggenberg, S., C. Bornand, and P. Juillerat. 2018. Flora Helvetica: Guide d’excursions. Bern: Haupt.
- Egli, P. E., B. Belotti, B. Ouvry, J. Irving, and S. N. Lane. 2021. Subglacial channels, climate warming, and increasing frequency of Alpine glacier snout collapse. Geophysical Research Letters 48, no. 21: e2021GL096031. doi:10.1029/2021GL096031.
- Eichel, J. 2019. Vegetation succession and biogeomorphic interactions in glacier forelands. In Geomorphology of proglacial systems, landform and sediment dynamics in recently deglaciated landscapes, geography of the physical environment, 327–49. Cham: Springer.
- Eichel, J., M. Krautblatter, S. Schmidtlein, and R. Dikau. 2013. Biogeomorphic interactions in the Turtmann glacier forefield, Switzerland. Geomorphology 201: 98–110. doi:10.1016/j.geomorph.2013.06.012.
- Erschbamer, B. 2007. Winners and losers of climate change in a central alpine glacier foreland. Arctic, Antarctic, and Alpine Research 39, no. 2: 237–44. doi:10.1657/1523-0430(2007)39[237:WALOCC]2.0.CO;2.
- Erschbamer, B., and M. S. Caccianiga. 2017. Glacier forelands: Lessons of plant population and community development. Progress in Botany 78: 259–84.
- Federal Office of Topography swisstopo. 2005. Geological Map of Switzerland 1:500 000. Geological Institute, University of Bern, and Federal Office for Water und Geology.
- Federal Office of Topography swisstopo. n.d. Aerial images swisstopo b/w. https://map.geo.admin.ch/ [www Document] (accessed May, 2018).
- Ferrarini, A. 2015. Where do diaspores come from? Reverse wind modelling unveils plant colonization trajectories. Proceedings of the International Academy of Ecology and Environmental Sciences 5, no. 4: 148.
- Fickert, T., F. Grüninger, and B. Damm. 2017. Klebelsberg revisited: Did primary succession of plants in glacier forelands a century ago differ from today? Alpine Botany 127, no. 1: 17–29. doi:10.1007/s00035-016-0179-1.
- GALMOS. 2018. The Swiss Glaciers 1880-2016/17, Glaciological Reports No 1-138, yearbooks of the Cryospheric commission of the Swiss Academy of Sciences (SCNAT). Zurich: VAW/ ETH.
- Gee, G. W., and J. W. Bauder. 1986. Methods of soil analysis: Part 1 physical and mineralogical methods, 5.1. 2nd ed., 383–411. Madison: John Wiley & Sons, Ltd.
- Gobat, J. M., and C. Guenat. 2019. Sols et paysages. Types de sols, fonctions et usages en Europe moyenne (No. BOOK). Lausanne: PPUR.
- Gurnell, A. M., W. Bertoldi, R. A. Francis, J. Gurnell, and U. Mardhiah. 2019. Understanding processes of island development on an island braided river over timescales from days to decades. Earth Surface Processes and Landforms 44: 624–40. doi:10.1002/esp.4494.
- Gurnell, A. M., P. J. Edwards, G. E. Petts, and J. V. Ward. 2000. A conceptual model for alpine proglacial river channel evolution under changing climatic conditions. Catena 38, no. 3: 223–42. doi:10.1016/S0341-8162(99)00069-7.
- Heckmann, T., S. McColl, and D. Morche. 2016. Retreating ice: Research in pro‐glacial areas matters. Earth Surface Processes and Landforms 41, no. 2: 271–6. doi:10.1002/esp.3858.
- IUSS Working Group. 2015. World reference base for soil resources 2014, update 2015: International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. Rome: FAO.
- Jochimsen, M. 1976. The development of pioneer-communities on raw soil above Alpine timberline (abridged version). In Verhandlungen der Gesellschaft für Ökologie Wien 1975, 61–3. Dordrecht: Springer.
- Köckinger, H., and H. Hofmann. 2017. Pohlia filum (Schimp.) Mårtensson. In Swissbryophytes Working Group (HRSG.), Moosflora der Schweiz. www.swissbryophytes.ch (accessed November 11, 2019).
- Körner, C. 2003. Alpine plant life: Functional plant ecology of high mountain ecosystems. Berlin: Springer Nature.
- Lane, S. N., and P. W. Nienow. 2019. Decadal‐scale climate forcing of alpine glacial hydrological systems. Water Resources Research 55, no. 3: 2478–92. doi:10.1029/2018WR024206.
- Lauber, K., G. Wagner, and A. Gygax. 2018. Flora helvetica, 1686. Bern, Switzerland: Haupt.
- Malard, F., U. Uehlinger, R. Zah, and K. Tockner. 2006. Flood‐pulse and riverscape dynamics in a braided glacial river. Ecology 87, no. 3: 704–16. doi:10.1890/04-0889.
- Mancini, D., and S. N. Lane. 2020. Changes in sediment connectivity following glacial debuttressing in an Alpine valley system. Geomorphology 352: 106987. doi:10.1016/j.geomorph.2019.106987.
- Marren, P. M. 2005. Magnitude and frequency in proglacial rivers: A geomorphological and sedimentological perspective. Earth-Science Reviews 70, no. 3–4: 203–51. doi:10.1016/j.earscirev.2004.12.002.
- Matthews, J. A. 1979a. The vegetation of the Storbreen gletschervorfeld, Jotunheimen, Norway. I. Introduction and approaches involving classification. Journal of Biogeography 6: 17–47. doi:10.2307/3038150.
- Matthews, J. A. 1979b. The vegetation of the Storbreen gletschervorfeld, Jotunheimen, Norway. II. Approaches involving ordination and general conclusions. Journal of Biogeography 6: 133–67. doi:10.2307/3038049.
- Matthews, J. A. 1992. The ecology of recently-deglaciated terrain: A geoecological approach to glacier forelands. Victoria: Cambridge University Press.
- Matthews, J. A. 1999. Disturbance regimes and ecosystem response on recently-deglaciated substrates. Ecosystems of the World 17–38.
- Matthews, J. A., and G. Owen. 2008. Endolithic lichens, rapid biological weathering and Schmidt hammer R‐values on recently exposed rock surfaces: Storbreen glacier foreland, Jotunheimen, Norway. Geografiska Annaler: Series A, Physical Geography 90, no. 4: 287–97. doi:10.1111/j.1468-0459.2008.00346.x.
- Matthews, J. A., and R. J. Whittaker. 1987. Vegetation succession on the Storbreen glacier foreland, Jotunheimen, Norway: A review. Arctic and Alpine Research 19, no. 4: 385–95. doi:10.2307/1551403.
- MeteoSwiss. 2016. Normes climatologiques Col du Grand St−Bernard - Période de référence 1981–2021. Zurich: Federal Office for Meteorology and Climatology.
- Miller, H. R., and S. N. Lane. 2019. Biogeomorphic feedbacks and the ecosystem engineering of recently deglaciated terrain. Progress in Physical Geography: Earth and Environment 43, no. 1: 24–45. doi:10.1177/0309133318816536.
- Moreau, M., D. Mercier, D. Laffly, and E. Roussel. 2008. Impacts of recent paraglacial dynamics on plant colonization: A case study on Midtre Lovénbreen foreland, Spitsbergen (79 N). Geomorphology 95, no. 1–2: 48–60. doi:10.1016/j.geomorph.2006.07.031.
- Müller, T. 2022. Weather dataset from Otemma glacier forefield, Switzerland (from 14 July 2019 to 18 November 2021) (v1.2021.02) Zenodo. doi:10.5281/zenodo.6106778.
- Müller, T., S. N. Lane, and B. Schaefli. 2022. Towards a hydrogeomorphological understanding of proglacial catchments: An assessment of groundwater storage and release in an Alpine catchment. Hydrology and Earth System Sciences 26, no. 23: 6029–54. doi:10.5194/hess-26-6029-2022.
- Müller, T., M. Roncoroni, D. Mancini, S. N. Lane, and B. Schaefli. 2023. Current and future role of meltwater-groundwater dynamics in a proglacial Alpine outwash plain. EGUsphere 1503: 1–34.
- Pix4D S.A. Pix4D mapper. ArcMap v10.5. https://www.pix4d.com/.
- Raffl, C., M. Mallaun, R. Mayer, and B. Erschbamer. 2006. Vegetation succession pattern and diversity changes in a glacier valley, Central Alps, Austria. Arctic, Antarctic, and Alpine Research 38, no. 3: 421–8. doi:10.1657/1523-0430(2006)38[421:VSPADC]2.0.CO;2.
- R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- Roncoroni, M., J. Brandani, T. I. Battin, and S. N. Lane. 2019. Ecosystem engineers: Biofilms and the ontogeny of glacier floodplain ecosystems. Wiley Interdisciplinary Reviews: Water 6, no. 6: e1390. doi:10.1002/wat2.1390.
- Roncoroni, M., D. Mancini, T. J. Kohler, F. Miesen, M. Gianini, T. J. Battin, and S. N. Lane. 2022. Centimeter-scale mapping of phototrophic biofilms in glacial forefields using visible band ratios and UAV imagery. International Journal of Remote Sensing 43: 4723–57. doi:10.1080/01431161.2022.2079963.
- Roncoroni, M., D. Mancini, F. Miesen, T. Müller, M. Gianini, B. Ouvry, M. Clémençon, F. Lardet, T. J. Battin, and S. N. Lane. 2023. Decrypting the stream periphyton physical habitat of recently deglaciated floodplains. Science of the Total Environment 867: Article number 161374. doi:10.1016/j.scitotenv.2022.161374.
- Rydgren, K., R. Halvorsen, J. P. Töpper, and J. M. Njøs. 2014. Glacier foreland succession and the fading effect of terrain age. Journal of Vegetation Science 25, no. 6: 1367–80. doi:10.1111/jvs.12184.
- Schreckenthal-Schimitschek, G. 1935. Der Einfluß des Bodens auf die Vegetation im Moränengelände des Mittelbergferners (Pitztal, Tirol). Zeitschrift für Gletscherkunde und Glazialgeologie 23, no. 1/3: 57–66.
- Schulz, S., R. Brankatschk, A. Dümig, I. Kögel-Knabner, M. Schloter, and J. Zeyer. 2013. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 10, no. 6: 3983–96. doi:10.5194/bg-10-3983-2013.
- Shannon, C. E., and W. Weaver. 1949. The mathematical theory of communication. Urbana, IL: University of Illinois Press.
- Sinergise Solution. Sentinelhub playground. www.sentinel-hub.com [www Document] (accessed June 2019).
- Stöcklin, J., and E. Bäumler. 1996. Seed rain, seedling establishment and clonal growth strategies on a glacier foreland. Journal of Vegetation Science 7, no. 1: 45–56. doi:10.2307/3236415.
- Swissbryophytes. 2019. Racomitrium canescens (Hedw.) Brid. 4. In Moosflora der Schweiz, ed. Swissbryophytes Working Group. www.swissbryophytes.ch (accessed November 11, 2019).
- Temme, A. J., and K. Lange. 2014. Pro‐glacial soil variability and geomorphic activity–the case of three Swiss valleys. Earth Surface Processes and Landforms 39, no. 11: 1492–9.
- Urmi, E., M. Baudraz, H. Berger, and H. Hofmann. 2016. Polytrichum piliferum Hedw. In Swissbryophytes Working Group (Hrsg.), Moosflora der Schweiz. www.swissbryophytes.ch (accessed November 11, 2019).
- Wietrzyk, P., M. Węgrzyn, and M. Lisowska. 2016. Vegetation diversity and selected abiotic factors influencing the primary succession process on the foreland of Gåsbreen, Svalbard. Polish Polar Research 37: 493–509.
- Winter, T. C. 1999. Ground water and surface water: A single resource. U.S. Geological Survey. North Dakota Water Science Center, Toxic Substances Hydrology Program, Dakota Water Science Center. doi:10.3133/cir1139.
- Zanella, A., J.-F. Ponge, I. Fritz, N. Pietrasiak, M. Matteodo, M. Nadporozhskaya, J. Juilleret, et al. 2018. Humusica 2, article 13: Para humus systems and forms. Applied Soil Ecology 122: 181–99. doi:10.1016/j.apsoil.2017.09.043.
- Zhang, T., D. Li, A. East, D. Walling, S. N. Lane, I. Overeem, A. Beylich, M. Koppes, and X. Lu. 2022. Warming-driven erosion and sediment transport in cold regions. Nature Reviews: Earth and Environment 3. doi:10.1038/s43017-022-00362-0.