Abstract
To provide a comprehensive account of the association of ACYP2 gene polymorphisms with susceptibility to cancer. A literature search for eligible candidate gene studies published before April 20, 2022 was conducted in the PubMed, Medline and Web of Science. The following combinations of main keywords were used: (ACYP2 OR acylphosphatase 2) AND (polymorphism OR mutation OR variation OR SNP OR genotype) AND (cancer OR tumor OR neoplasm OR malignancy OR carcinoma OR adenocarcinoma). Potential sources of heterogeneity were sought out via subgroup and sensitivity analysis. Publication bias were also estimated. Overall, a total of 10 articles with 5,230 cases and 5,086 controls for thirteen polymorphisms of ACYP2 gene were enrolled. We found that ACYP2 rs11125529, rs11896604, rs12615793, rs17045754, rs6713088, rs843645, rs843706, rs843711 and rs843752 were correlated with an increased risk of cancer. However, we found that ACYP2 rs12621038 might have less susceptibility to cancer. While for other polymorphisms, the results showed no significant association with cancer risk. ACYP2 rs11125529, rs11896604, rs12615793, rs17045754, rs6713088, rs843645, rs843706, rs843711 and rs843752 are associated with cancer risk. ACYP2 rs12621038 polymorphism is inversely associated with cancer risk.
Introduction
Telomeres are short repetitive hexamer (TTAGGGs) sequences located at the end of chromosomes.[Citation1] Chromosomes shorten with each cell division. However, cancer cells could prevent chromosome shortening by expressing telomerase.[Citation2] Many studies have proven that abnormal telomere length is associated with the development of many diseases, including cancers.[Citation3–5] The ACYP2 (Acylphosphatase 2) gene, located on chromosome 2p16.2, encodes cytosolic acylphosphatase enzyme which hydrolyzes a variety of membrane proteins, regulates the glycolysis pathway, pyruvate metabolism and cell apoptosis.[Citation6,Citation7] ACYP2 has been identified as an important telomere length related gene.[Citation8] Many studies have demonstrated that ACYP2 polymorphisms are associated with telomere length, which could be associated with various cancers.[Citation9–11]
Recently, many studies have demonstrated the association between ACYP2 polymorphisms and risks of various cancers. However, these results are inconsistent, which might be due to the heterogeneity within cancer types, ethnicities, source of control, Hardy-Weinberg equilibrium (HWE), small sample sizes, data source and so on. Therefore, it is necessary to perform a comprehensive meta-analysis to evaluate the effects of the genetic variation of ACYP2 gene on cancer risk.
Materials and methods
Literature search
We conducted a systematic literature search on PubMed, Medline and Web of Science to retrieve all eligible publications on the association between ACYP2 polymorphisms and the risk of cancer (up to April 20, 2022) with the following keywords: (ACYP2 OR acylphosphatase 2) AND (polymorphism OR mutation OR variation OR SNP OR genotype) AND (cancer OR tumor OR neoplasm OR malignancy OR carcinoma OR adenocarcinoma). The language of enrolled studies was restricted to English. After carefully screening, thirteen polymorphisms were left for further investigation.
Inclusion criteria and exclusion criteria
Articles enrolled in our meta-analysis satisfied the following inclusion criteria: (1) case-control studies that evaluated the association between ACYP2 polymorphisms and cancer risk; (2) publications focusing on population genetic polymorphisms; (3) articles with sufficient genotype data to assess ORs and the corresponding 95%CIs; (4) the control subjects satisfied Hardy-Weinberg equilibrium (HWE). The major exclusion criteria were: (1) case-only studies, case reports, or reviews; (2) studies without raw data for the ACYP2 genotype; (3) combined with other influencing factors.
Data extraction
Two investigators (XC and ZY) independently extracted the data. All the case-control studies satisfied the inclusion criteria and consensus for any controversy was achieved. The data from the eligible articles comprise the first author’s name, year of publication, ethnicity, source of control, cancer type and numbers of cases and controls in ACYP2 genotypes. Ethnicity was categorized as “Asian,” “Mix.”
Statistical analysis
The risk between the ACYP2 polymorphisms and cancer was evaluated using summary ORs and the corresponding 95% CIs in allelic (B versus A), dominant (BA + BB versus AA), and recessive (BB versus BA + AA) models (A: wild allele; B: mutated allele). The Cochrane’s Q-statistic test was used to assess the heterogeneity between studies, and the inconsistency was quantified with the I2 statistic. The substantial heterogeneity was considered significant when I2 > 50% or PQ ≤ 0.1, then, a random effects model was used; otherwise, the fixed effects model was applied. Subgroup meta-analysis were performed by ethnicity and the source of control. We also conducted sensitivity analysis to assess stability of the results by omitting one study each time to exclude studies. HWE was estimated by the asymptotic test, and deviation was considered when P < 0.05. The potential publication bias of the eligible studies was evaluated by Begg’s and Egger’s regression test quantitatively. Trial sequential analysis (TSA) was performed to minimize random errors and strengthen the robustness of our conclusions.[Citation12] The data was analyzed using the Stata 14.0 software (version 14.0; State Corporation, College Station, Texas, USA). A two-tailed P < 0.05 was considered statistically significant.
In-silico analysis using GTEx website
For the purpose of investigating the influence of polymorphisms on ACYP2, we obtained the association between polymorphism and ACYP2 expression level using the GTEx cohort.[Citation13]
Results
Main characteristics of the enrolled studies
The study selection processes were presented in . A total of 10 case-control studies with 5,230 cases and 5,086 controls for thirteen polymorphisms of ACYP2 gene met the inclusion criteria.[Citation14–23] Nine studies of them were performed in Asians, one study was performed in Mix ethnicity. Controls of 6 studies were population-based controls, and 4 studies were hospital-based controls. All studies were in compliance with HWE. showed the characteristics of all the eligible studies and genotype frequency distributions of the thirteen ACYP2 polymorphisms included in our meta-analysis. Newcastle-Ottawa scale (NOS) was used to evaluate the quality of the enrolled studies, as shown in .
Table 1. Characteristics of eligible case-control studies included in the meta-analysis.
Table 2. Methodological quality of the enrolled studies according to the Newcastle-Ottawa scale.
Quantitative synthesis
rs11125529
The pooled results based on 8 included studies (including 4,468 cases and 4,284 controls) indicated that rs11125529 was significantly related to an increased risk of cancer in dominant model (AB + BB versus AA: OR = 1.12, 95%CI = 1.02–1.23, P = 0.018) and recessive model (BB versus AA + AB: OR = 1.75, 95%CI = 1.34–2.27, P < 0.001).[Citation14,Citation16–21,Citation23] Then, in the stratification analysis by ethnicity, we observed that rs11125529 polymorphism was significantly related to an increased cancer risk for Asians in dominant model (BB + AB versus AA: OR = 1.17, 95%CI = 1.05–1.31, P = 0.005) and recessive model (BB versus AA + AB: OR = 2.18, 95%CI = 1.58–2.99, P < 0.001). (, and )
Figure 2. Forest plot of ACYP2 rs11125529 polymorphism and cancer risk in recessive model stratified by ethnicity.
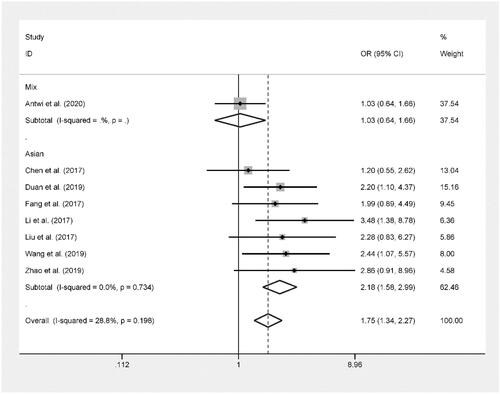
Table 3. Meta-analysis of ACYP2 polymorphisms.
Table 4. Meta-analysis of rs11125529.
rs11896604
The pooled results based on 8 included studies (including 3,570 cases and 3,431 controls) indicated that rs11896604 was significantly related to an increased risk of cancer in dominant model (AB + BB versus AA: OR = 1.20, 95%CI = 1.09–1.33, P < 0.001) and recessive model (BB versus AA + AB: OR = 1.44, 95%CI = 1.12–1.85, P = 0.004).[Citation15–21,Citation23] Then, in the stratification analysis by source of control, population-based control group was significantly related to an increased risk of cancer in dominant model (BB + AB versus AA: OR = 1.18, 95%CI = 1.02–1.36, P = 0.023). In addition, hospital-based control group was significantly related to an increased risk of cancer in allelic contrast (B versus A: OR = 1.25, 95%CI = 1.11–1.40, P < 0.001), dominant model (BB + AB versus AA: OR = 1.22, 95%CI = 1.06–1.40, P = 0.005) and recessive model (BB versus AA + AB: OR = 1.93, 95%CI = 1.35–2.77, P < 0.001). (, and )
Figure 3. Forest plot of ACYP2 rs11896604 polymorphism and cancer risk in dominant model stratified by source of control.
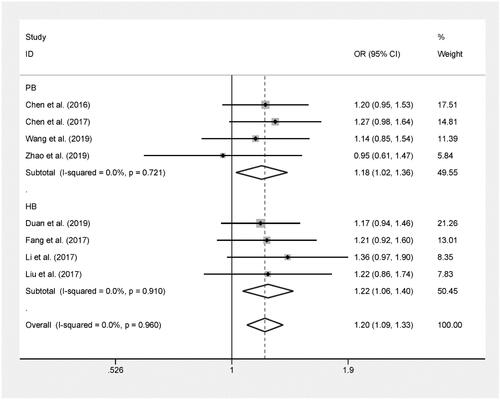
Table 5. Meta-analysis of rs11896604.
rs17045754
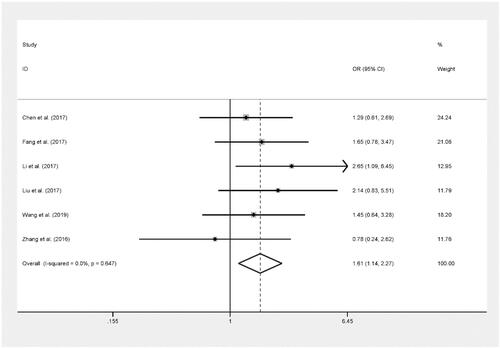
The pooled results based on 6 included studies (including 1,873 cases and 2,349 controls) indicated that rs17045754 was significantly related to an increased risk of cancer in allelic contrast (B versus A: OR = 1.23, 95%CI = 1.10–1.38, P < 0.001), dominant model (BB + AB versus AA: OR = 1.21, 95%CI = 1.07–1.38, P = 0.003) and recessive model (BB versus AA + AB: OR = 1.61, 95%CI = 1.14–2.27, P = 0.007).[Citation16,Citation18–22] (, )
rs843711
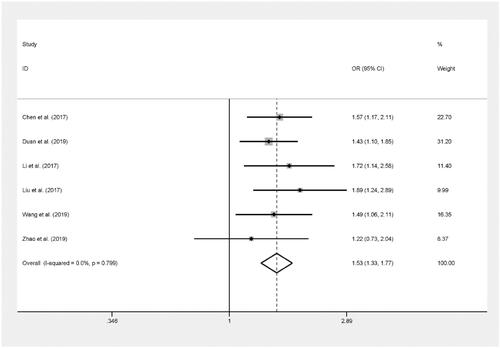
The pooled results based on 6 included studies (including 2,636 cases and 2,333 controls) indicated that rs843711 was significantly related to an increased risk of cancer in allelic contrast (B versus A: OR = 1.22, 95%CI = 1.12–1.32, P < 0.001), dominant model (BB + AB versus AA: OR = 1.25, 95%CI = 1.10–1.42, P = 0.001) and recessive model (BB versus AA + AB: OR = 1.53, 95%CI = 1.33–1.77, P < 0.001).[Citation16,Citation17,Citation19–21,Citation23] (, ).
rs6713088
The pooled results based on 5 included studies (including 1,495 cases and 1,851 controls) indicated that rs6713088 was significantly related to an increased risk of cancer in allelic contrast (B versus A: OR = 1.21, 95%CI = 1.10–1.33, P < 0.001), dominant model (BB + AB versus AA: OR = 1.25, 95%CI = 1.08–1.44, P = 0.003) and recessive model (BB versus AA + AB: OR = 1.35, 95%CI = 1.13–1.62, P = 0.001).[Citation16,Citation19–22] ().
rs12615793
The pooled results based on 4 included studies (including 1,190 cases and 1,344 controls) indicated that rs11896604 was significantly related to an increased risk of cancer in recessive model (BB versus AA + AB: OR = 2.01, 95%CI = 1.30–3.11, P = 0.002)[Citation16,Citation19,Citation20,Citation23] ().
rs843752
The pooled results based on 4 included studies (including 1,193 cases and 1,553 controls) indicated that rs843752 was significantly related to an increased risk of cancer in dominant model (BB + AB versus AA: OR = 1.21, 95%CI = 1.04–1.41, P = 0.014)[Citation16,Citation20–22] ().
rs843645
The pooled results based on 3 included studies (including 1,008 cases and 1,359 controls) indicated that rs843645 was significantly related to an increased risk of cancer in allelic contrast (B versus A: OR = 1.20, 95%CI = 1.05–1.36, P = 0.007), dominant model (BB + AB versus AA: OR = 1.29, 95%CI = 1.10–1.52, P = 0.002)[Citation16,Citation20,Citation21] ().
rs843706
The pooled results based on 3 included studies (including 1,008 cases and 1,158 controls) indicated that rs843706 was significantly related to an increased risk of cancer in allelic contrast (B versus A: OR = 1.33, 95%CI = 1.18–1.50, P < 0.001), dominant model (BB + AB versus AA: OR = 1.37, 95%CI = 1.13–1.65, P = 0.001) and recessive model (BB versus AA + AB: OR = 1.59, 95%CI = 1.29–1.96, P < 0.001)[Citation16,Citation19,Citation20] ().
rs12621038
The pooled results based on 4 included studies (including 1,191 cases and 1,550 controls) indicated that rs12621038 was significantly related to a reduced risk of cancer in allelic contrast (B versus A: OR = 0.90, 95%CI = 0.80–1.00, P = 0.047) and recessive model (AB + BB versus AA: OR = 0.80, 95%CI = 0.66–0.97, P = 0.023)[Citation16,Citation20–22] ().
rs1682111
The pooled results based on 6 included studies (including 1,914 cases and 2,337 controls) indicated that no significant association between rs1682111 polymorphism and cancer risk was found[Citation15,Citation16,Citation20–23] ().
rs10439478
The pooled results based on 5 included studies (including 1,361 cases and 1,728 controls) indicated that no significant association between rs10439478 polymorphism and cancer risk was found[Citation16,Citation20–23] ().
rs843720 rs10439478 rs1682111
The pooled results based on 4 included studies (including 1,455 cases and 1,662 controls) indicated that no significant association between rs843720 polymorphism and cancer risk was found[Citation15,Citation16,Citation20,Citation22] ().
Sensitivity analysis and publication bias
Sensitivity analysis were performed to evaluate the influence of each separate case-control study. The results showed that there was no material alteration in corresponding pooled ORs for thirteen polymorphisms of ACYP2 gene (Supplementary Figure S1–S13). In addition, Begg’s test and Egger’s regression test were performed to evaluate the publication bias. Publication bias was detected for rs11125529 in recessive model, rs12621038, and rs843706 in allelic contrast. (Supplementary Table S1 and Supplementary figure S14–S26 2)
Trial sequential analysis
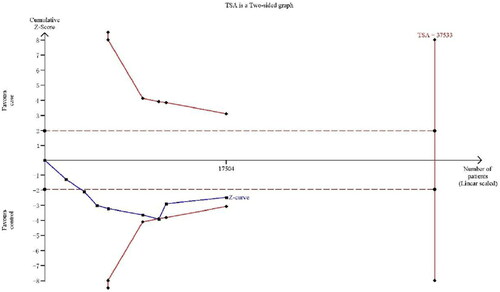
To evaluate random errors, we performed TSA (). This analysis showed that the cumulative z-curve didn’t cross the trial sequential monitoring boundary and the required information size, suggesting that more evidences are needed to verify the conclusions.
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist is reported in .
In-silico analysis using GTEx website
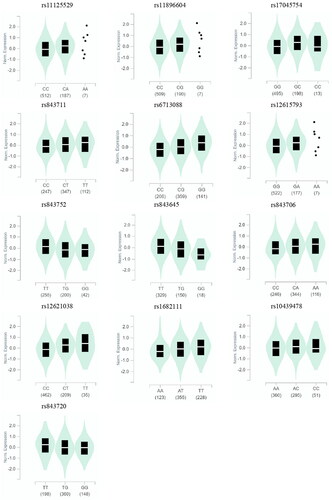
Based on the results of the GTEx database, we observed that the mutant allele increased the expression of ACYP2 mRNA in rs11125529(P = 1.1*10−6), rs11896604(P = 1.1*10−6), rs17045754 (P = 1.1*10−7), rs843711 (P = 4.8*10−5), rs6713088 (P = 8.6*10−13), rs12615793 (P = 3.8*10−7), rs843706 (P = 1.8*10−10), rs12621038 (P = 1.4*10−17), rs1682111 (P = 3*10−5) and rs10439478 (P = 2.7*10−6), while the mutant allele of rs843752 (P = 7.4*10−8) and rs843645 (P = 6*10−5) resulted in a reduction in the expression of ACYP2 ().
Discussion
ACYP2 encodes cytosolic acylphosphatase enzyme which can hydrolyze the phosphoenzyme intermediate of different membrane pumps, especially the Ca2+/Mg2+-ATPase from skeletal muscle sarcoplasmic reticulum.[Citation24] ACYP2 has been shown to play a key role in pyruvate metabolism, and its activation is an important factor in malignant transformation.[Citation25,Citation26] Furthermore, ACYP2 has been confirmed as an important telomere length related gene.[Citation8] Many studies have investigated the relationship between the SNP polymorphisms of ACYP2 gene and cancer risk. However, the results are inconsistent. Therefore, we conducted this systematic review and meta-analysis to investigate the association between genetic variation of ACYP2 gene and cancer susceptibility.
In this systematic review and meta-analysis, we analyze 10 case-control studies among 10,316 participants to verify the association between the thirteen SNPs of ACYP2 gene and cancer risk. Our findings suggest that rs11125529, rs11896604, rs12615793, rs17045754, rs6713088, rs843645, rs843706, rs843711 and rs843752 are able to increase cancer risk under different genetic models. Meanwhile, rs12621038 polymorphism is associated with reduced cancer risk in different genetic models. However, rs843720, rs10439478 and rs1682111 are not found to be significantly associated with cancer risk.
It has been reported that rs11125529 of ACYP2 was associated with reduced telomere length.[Citation27] However, publication bias was detected for rs11125529 in recessive model. The main source of significant heterogeneity might be ethnicity. In subgroup meta-analysis stratified by ethnicity, we found that rs11125529 was significantly associated with cancer risk in Asians in dominant model and recessive model. We only included one study of mix races. And our results indicated that rs11125529 was not associated with cancer susceptibility in various models in mix races group. So, our study indicated that rs11125529 might increase cancer susceptibility in Asian population. However, more studies are needed to confirm this result.
Many studies have identified that there is a strong linkage disequilibrium (LD) between each pair of eight SNPs (rs1682111, rs843752, rs10439478, rs843645, rs11125529, rs12615793, rs843711, and rs11896604) of ACYP2 gene.[Citation17,Citation20,Citation21,Citation23] However, the results of various studies are not consistent, so it is necessary to analyze each SNP of ACYP2 gene.
For ACYP2 rs11896604, our meta-analysis showed that rs11896604 was significantly increased cancer susceptibility in dominant model and recessive model. In subgroup meta-analysis stratified by source of control, we found that rs11896604 was significantly increased cancer susceptibility in HB group in allele contrast, dominant model and recessive model. We also found that rs11896604 was significantly increased cancer susceptibility in dominant model. But no publication bias was detected. This result indicated that rs11896604 might increase cancer susceptibility. Nevertheless, the result needs to be confirmed by more large sample size studies.
In this meta-analysis, we also found that ACYP2 rs12615793, rs17045754, rs6713088, rs843645, rs843706, rs843711 and rs843752 were significantly associated with cancer risk in various genetic models. In addition, no publication bias was detected except for rs843706 in allelic contrast. Although there are few studies on these SNPs, these results still indicated a positive correlation with cancer risk. More studies are needed to verify the results.
For ACYP2 rs12621038, our meta-analysis showed that rs12621038 was significantly reduced cancer susceptibility in allele contrast and recessive model. However, publication bias was detected in allele contrast dominant model and recessive model. Due to the small sample size, there is not enough data to analyze the potential sources of heterogeneity. This result need more large sample size studies for further evaluation.
Our results showed that ACYP2 rs843720, rs10439478 and rs1682111 were not associated with cancer susceptibility in various models. More studies with larger sample sizes are needed to reevaluate the results.
In this study, we conducted a systematic and comprehensive search to obtain accurate and reliable results. Then, NOS was used to evaluate the quality of the included studies. Sensitivity analysis, Egger’s and Begg’s tests were used to better control the quality of the included studies. However, there are some limitations in this meta-analysis. First, the sample size was relatively small, which limited the reliability of the results. Second, we included studies only reported in English, which may influence the effects of the polymorphisms. Third, most studies only reported Chinese Han population. Fourth, we cannot obtain enough data to assess the relationship between ACYP2 polymorphisms and cancer types. Fifth, publication bias was detected for rs11125529, rs12621038 and rs843706, which might cause large deviations. Sixth, the previous reported linkage disequilibrium of the ACYP2 SNPs reflected inconsistent function. More large sample size case-control studies of various ethnicities are needed to investigate the functions of ACYP2 polymorphisms.
Conclusion
Our meta-analysis suggests that ACYP2 rs11125529, rs11896604, rs12615793, rs17045754, rs6713088, rs843645, rs843706, rs843711 and rs843752 are associated with the risk of cancer in Asian population. While, ACYP2 rs12621038 polymorphism is inversely associated with cancer risk for Asians. Further well-designed case-control studies of various ethnicities with larger sample size are needed to confirm these findings.
Author’s contribution
Zhenwei Han had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Zhenwei Han and Xueliang Chang learned concept and design. Xueliang Chang and Zhan Yang conducted data analysis and interpretation. Xueliang Chang and Zhenwei Han Drafted of the manuscript. Yaxuan Wang, Xueliang Chang and Jingdong Li reviewed important academic content. Yaxuan Wang, Yanping Zhang, Zhihai Teng and Hu Wang conducted the statistical analysis.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplemental Material
Download MS Word (66.5 KB)Supplemental Material
Download MS Word (18.8 KB)Supplemental Material
Download JPEG Image (100.9 KB)Supplemental Material
Download JPEG Image (100.1 KB)Supplemental Material
Download JPEG Image (67.7 KB)Supplemental Material
Download JPEG Image (64.8 KB)Acknowledgments
We thank Dr. Chawnshang Chang at University of Rochester Medical Center for helping with the preparation of the manuscript.
Conflicts of interest
All authors declare that they have no conflict of interest.
Data availability
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Hewitt, G.; Jurk, D.; Marques, F. D. M.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J. F.; et al. Telomeres Are Favoured Targets of a Persistent DNA Damage Response in Ageing and Stress-Induced Senescence. Nat Commun. 2012, 3, 708. DOI: 10.1038/ncomms1708.
- Palm, W.; de Lange, T. How Shelterin Protects Mammalian Telomeres. Annu Rev Genet. 2008, 42, 301–334. DOI: 10.1146/annurev.genet.41.110306.130350.
- Cheng, Y.; Yu, C.; Huang, M.; Du, F.; Song, C.; Ma, Z.; Zhai, X.; Yang, Y.; Liu, J.; Bei, J.-X.; et al. Genetic Association of Telomere Length with Hepatocellular Carcinoma Risk: A Mendelian Randomization Analysis. Cancer Epidemiol. 2017, 50, 39–45. DOI: 10.1016/j.canep.2017.07.011.
- Ko, E.; Jung, G. Positive Association of Long Telomeres with the Invasive Capacity of Hepatocellular Carcinoma Cells. Biochem Biophys Res Commun. 2014, 447, 358–363. DOI: 10.1016/j.bbrc.2014.04.022.
- Maguire, D.; Neytchev, O.; Talwar, D.; McMillan, D.; Shiels, P. G. Telomere Homeostasis: Interplay with Magnesium. Int J Mol Sci. 2018, 19, 157. DOI: 10.3390/ijms19010157.
- Xu, H.; Robinson, G. W.; Huang, J.; Lim, J. Y.-S.; Zhang, H.; Bass, J. K.; Broniscer, A.; Chintagumpala, M.; Bartels, U.; Gururangan, S.; et al. Common Variants in Acyp2 Influence Susceptibility to Cisplatin-Induced Hearing Loss. Nat Genet. 2015, 47, 263–266. DOI: 10.1038/ng.3217.
- Wellmann, R.; Borden, B. A.; Danahey, K.; Nanda, R.; Polite, B. N.; Stadler, W. M.; Ratain, M. J.; O’Donnell, P. H. Analyzing the Clinical Actionability of Germline Pharmacogenomic Findings in Oncology. Cancer. 2018, 124, 3052–3065. DOI: 10.1002/cncr.31382.
- Codd, V.; Nelson, C. P.; Albrecht, E.; Mangino, M.; Deelen, J.; Buxton, J. L.; Hottenga, J. J.; Fischer, K.; Esko, T.; Surakka, I.; CARDIoGRAM Consortium.; et al. Identification of Seven Loci Affecting Mean Telomere Length and Their Association with Disease. Nat Genet. 2013, 45, 422–427. DOI: 10.1038/ng.2528.
- Thiesen, S.; Yin, P.; Jorgensen, A. L.; Zhang, J. E.; Manzo, V.; McEvoy, L.; Barton, C.; Picton, S.; Bailey, S.; Brock, P.; et al. Tpmt, Comt and Acyp2 Genetic Variants in Paediatric Cancer Patients with Cisplatin-Induced Ototoxicity. Pharmacogenet Genom. 2017, 27, 213–222. DOI: 10.1097/FPC.0000000000000281.
- Won, H.-H.; Lee, J.; Park, J. O.; Park, Y. S.; Lim, H. Y.; Kang, W. K.; Kim, J.-W.; Lee, S.-Y.; Park, S. H. Polymorphic Markers Associated with Severe Oxaliplatin-Induced, Chronic Peripheral Neuropathy in Colon Cancer Patients. Cancer. 2012, 118, 2828–2836. DOI: 10.1002/cncr.26614.
- Lin, S.; Wang, M.; Liu, X.; Zhu, W.; Guo, Y.; Dai, Z.; Yang, P.; Tian, T.; Dai, C.; Zheng, Y.; et al. Association of Genetic Polymorphisms in Mif with Breast Cancer Risk in Chinese Women. Clin Exp Med. 2017, 17, 395–401. DOI: 10.1007/s10238-016-0439-9.
- Xie, S.; Shan, X. F.; Shang, K.; Xu, H.; He, J.; Cai, Z. G. Relevance of Lig4 Gene Polymorphisms with Cancer Susceptibility: Evidence from a Meta-Analysis. Sci Rep. 2014, 4, 6630. DOI: 10.1038/srep06630.
- Gibson, G. Human Genetics. Gtex Detects Genetic Effects. Science. 2015, 348, 640–641. DOI: 10.1126/science.aab3002.
- Antwi, S. O.; Bamlet, W. R.; Rabe, K. G.; Cawthon, R. M.; Umudi, I.; Druliner, B. R.; Sicotte, H.; Oberg, A. L.; Jatoi, A.; Boardman, L. A.; et al. Leukocyte Telomere Length and Its Interaction with Germline Variation in Telomere-Related Genes in Relation to Pancreatic Adenocarcinoma Risk. Cancer Epidemiol Biomarkers Prev. 2020, 29, 1492–1500. DOI: 10.1158/1055-9965.EPI-19-1597.
- Chen, N.; Yang, X.; Guo, W.; You, J.; Wu, Q.; Zhang, G.; Li, H.; Geng, D.; Jin, T.; Fu, J.; et al. Association of Polymorphisms in the Telomere-Related Gene Acyp2 with Lung Cancer Risk in the Chinese Han Population. Oncotarget. 2016, 7, 87473–87478. DOI: 10.18632/oncotarget.13870.
- Chen, Z.; Sun, Y.; Xu, Z.; Xu, J.; Li, J.; Yan, M.; Li, J.; Jin, T.; Lin, H. Acyp2 Polymorphisms Are Associated with the Risk of Liver Cancer in a Han Chinese Population. Oncotarget. 2017, 8, 67723–67731. DOI: 10.18632/oncotarget.18574.
- Duan, X.; Hong, J.; Wang, F.; Wei, K.; Wang, P.; Hou, F.; Zhang, M.; Liu, D.; Yuan, D.; Liu, S.; et al. The Influence of Acyp2 Polymorphisms on Gastrointestinal Cancer Susceptibility in the Chinese Han Population. Mol Genet Genomic Med. 2019, 7, e00700. DOI: 10.1002/mgg3.700.
- Fang, Q.; Hui, L.; Min, Z.; Liu, L.; Shao, Y. Leukocyte Telomere Length-Related Genetic Variants in Acyp2 Contribute to the Risk of Esophageal Carcinoma in Chinese Han Population. Oncotarget. 2017, 8, 25564–25570. DOI: 10.18632/oncotarget.16071.
- Li, J.; Ma, G.; Zhu, X.; Jin, T.; Wang, J.; Li, C. Association Analysis of Telomere Length Related Gene Acyp2 with the Gastric Cancer Risk in the Northwest Chinese Han Population. Oncotarget. 2017, 8, 31144–31152. DOI: 10.18632/oncotarget.16097.
- Liu, F.; Zhang, Z.; Zhang, Y.; Chen, Y.; Yang, X.; Li, J.; Zhao, J. Genetic Polymorphisms in the Telomere Length-Related Gene Acyp2 Are Associated with the Risk of Colorectal Cancer in a Chinese Han Population. Oncotarget. 2017, 8, 9849–9857. DOI: 10.18632/oncotarget.14219.
- Wang, Y.; Zhang, Y.; Sun, Y.; Wu, J.; Chang, J.; Xiong, Z.; Niu, F.; Gu, S.; Jin, T. Association between Acyp2 Polymorphisms and the Risk of Renal Cell Cancer. Mol Genet Genomic Med. 2019, 7, e966. DOI: 10.1002/mgg3.966.
- Zhang, F.; Zhang, Y.; Deng, Z.; Xu, P.; Zhang, X.; Jin, T.; Liu, Q. Genetic Variants in the Acylphosphatase 2 Gene and the Risk of Breast Cancer in a Han Chinese Population. Oncotarget. 2016, 7, 86704–86712. DOI: 10.18632/oncotarget.13495.
- Zhao, W.; Niu, F.; Xie, Z.; et al. Assessment of the Association between Acyp2 and Laryngeal Squamous Cell Carcinoma Risk in Chinese Males. Mol Genet Genomic Med. 2019, 7, e00731. DOI: 10.1002/mgg3.731.
- Nassi, P.; Nediani, C.; Liguri, G.; Taddei, N.; Ramponi, G. Effects of Acylphosphatase on the Activity of Erythrocyte Membrane Ca2+ Pump. J Biol Chem. 1991, 266, 10867–10871. DOI. http://www.ncbi.nlm.nih.gov/pubmed/1645713.
- Conde, V. R.; Oliveira, P. F.; Nunes, A. R.; Rocha, C. S.; Ramalhosa, E.; Pereira, J. A.; Alves, M. G.; Silva, B. M. The Progression from a Lower to a Higher Invasive Stage of Bladder Cancer is Associated with Severe Alterations in Glucose and Pyruvate Metabolism. Exp Cell Res. 2015, 335, 91–98. DOI: 10.1016/j.yexcr.2015.04.007.
- Szlosarek, P. W.; Lee, S.; Pollard, P. J. Rewiring Mitochondrial Pyruvate Metabolism: Switching off the Light in Cancer Cells? Mol Cell. 2014, 56, 343–344. DOI: 10.1016/j.molcel.2014.10.018.
- Pooley, K. A.; Bojesen, S. E.; Weischer, M.; Nielsen, S. F.; Thompson, D.; Amin Al Olama, A.; Michailidou, K.; Tyrer, J. P.; Benlloch, S.; Brown, J.; et al. A Genome-Wide Association Scan (Gwas) for Mean Telomere Length within the Cogs Project: Identified Loci Show Little Association with Hormone-Related Cancer Risk. Hum Mol Genet. 2013, 22, 5056–5064. DOI: 10.1093/hmg/ddt355.