Abstract
The nonpurine selective xanthine oxidase (XO) inhibitor febuxostat attenuates development of left ventricular (LV) hypertrophy and dysfunction in mice when treatment is initiated within 1 hour of transverse aortic constriction (TAC). This study investigated whether a 7-day delay of treatment with the XO inhibitors febuxostat or allopurinol would reverse TAC-induced changes after onset of heart failure (HF). Neither treatment significantly affected TAC-induced LV hypertrophy; only febuxostat caused a modest improvement in LV function (∼10% increase in LV ejection fraction). However, the purine analog allopurinol tended to increase mortality compared with vehicle or febuxostat in HF mice.
INTRODUCTION
Inhibition of xanthine oxidase (XO) with the purine analogs allopurinol or oxypurinol has been reported to improve left ventricular (LV) function in the failing human heart or in animal models with failing hearts[ Citation 1 – Citation 3 ] and to protect the heart against LV remodeling in animals after myocardial infarction.[ Citation 4 – Citation 6 ] We have reported that XO inhibition with the nonpurine selective XO inhibitor febuxostat (5 mg/kg p.o. for 8 days), given ∼60 minutes after transverse aortic constriction (TAC) in mice, can significantly attenuate systolic overload-induced LV hypertrophy, dysfunction, and fibrosis.[ Citation 7 ]The cardiac protective effect was associated with reduced oxidative stress as measured by myocardial nitrotyrosine levels. Moreover, febuxostat also attenuated the TAC-induced phosphorylation of the mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (Erk), events that are thought to mediate myocardial hypertrophy.
The purpose of this study was to investigate whether delayed XO inhibition with febuxostat (0.05 mg/ml in drinking water, ∼5 mg/kg/day) or allopurinol (0.15 mg/ml in drinking water, ∼15 mg/kg/day) could reverse TAC-induced changes at a time when heart failure (HF) was already established (7 days after TAC) using the mouse model described previously.[ Citation 7 ] Mortality rate was recorded during the 3-week treatment period. Echocardiography was performed to measure LV function and dimensions. Myocardial fibrosis and cardiac myocyte sizes were measured by histological staining.
MATERIALS AND METHODS
Minimally Invasive TAC Procedure
Male C57/BL6 mice 8–9 weeks of age (body weight 20–25 g) were used in the study. TAC was performed using the minimally invasive suprasternal approach as previously described,[ Citation 7 ] except that a 26-G needle was used with ligation of the aorta.
Experimental Protocol
Mice subjected to TAC or sham surgery were divided into 6 experimental groups. Beginning 7 days after surgery, TAC- and sham-operated animals were given access to drinking water treated with vehicle (0.01 mg/ml NaCl, salt content equivalent to that in the febuxostat drinking water), febuxostat (0.05 mg/ml, ∼5 mg/kg/day), or allopurinol (0.15 mg/ml, ∼15 mg/kg/day). Following 3 weeks of treatment, ventricular function was assessed by echocardiography. Blood samples and cardiac tissues were collected 2–3 hours after echocardiographic evaluation for determination of plasma uric acid (UA) levels and histological staining. The wet weights of the ventricle and lung of each animal were also measured with an electric balance and normalized as the ratio of organ weight to body weight.
Echocardiography
Echocardiography was performed on mice anesthetized with 1.5% isoflurane by inhalation as we previously described.[ Citation 7 – Citation 9 ]
Histological Staining and Measurement of Cardiac Myocyte Hypertrophy
Tissue sections of the central portion of the LV were stained with Masson's trichrome for detection of myocardial fibrosis as we previously described.[ Citation 9 ] Fluorescein isothiocyanate (FITC)-conjugated wheat germ agglutinin was used to stain the cell membrane for determination of cardiac myocyte size.[ Citation 8 , Citation 9 ]
Plasma UA Analysis
The concentration of UA in plasma was determined using UA reagent (ThermoDMA, Louisville, CO, USA) according to the manufacturer's protocol.
Data and Statistical Analyses
All values are expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to test each variable for differences among the treatment groups. Statistical significance was defined as P < 0.05. If the ANOVA indicated a significant effect, post hoc pairwise comparisons were made using the Fisher least significant difference test. The Fisher exact test was used to compare mortality data among the treatment groups.
RESULTS
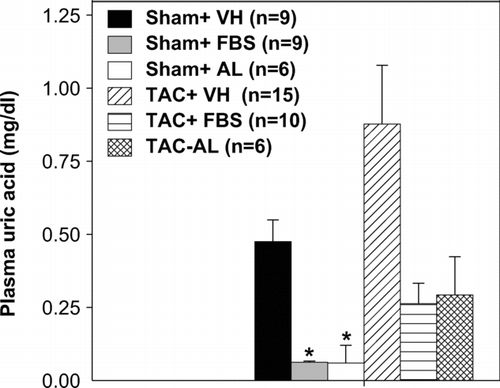
Effects of XO Inhibition on Plasma UA Levels
As expected, both febuxostat and allopurinol significantly decreased plasma UA in the sham-operated groups (). TAC tended to increase plasma UA levels relative to the sham-operated controls, and both XO inhibitors decreased plasma UA in TAC animals to a similar extent, although these changes did not reach statistical significance due to large variability between animals. Nevertheless, these data suggest that febuxostat and allo- purinol were given at similar XO inhibitory doses.
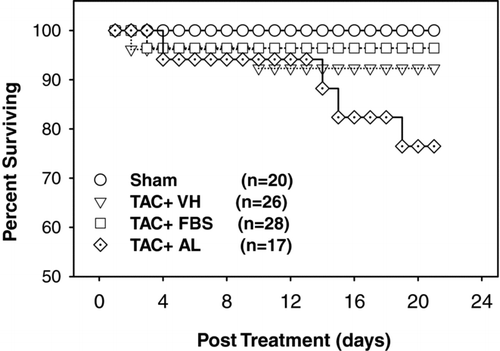
Effects of XO Inhibition on TAC-Induced Mortality Rate
Mortality was low over the 3-week treatment period in TAC mice treated with vehicle (2 of 26 mice died, 8% mortality) or febuxostat (1 of 28 mice died, 4% mortality). However, the mortality in TAC animals treated with allopurinol was 24% (4 of 17 died, P = 0.19 vs. vehicle control and P = 0.06 vs. febuxostat group; ).
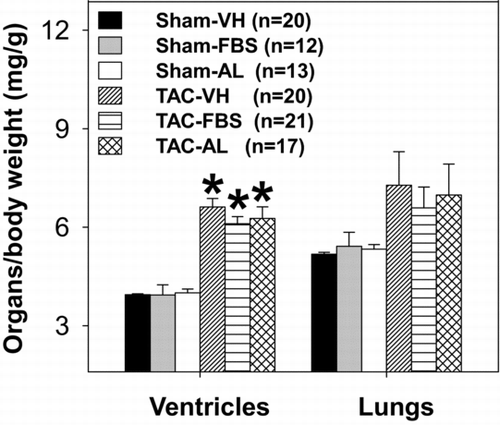
Effects of XO Inhibition on TAC-Induced LV Hypertrophy and Dysfunction
Febuxostat and allopurinol had no significant effects on ratios of ventricular and lung weights normalized to body weights in the sham groups. Chronic TAC resulted in a significant increase in body weight-normalized ventricular weight and tended to increase normalized lung weight; neither agent had a significant effect on these changes compared with vehicle (). These results suggest that, unlike what occurs with early treatment, a delay in XO inhibition until after the onset of cardiac hypertrophy and HF has no effect on TAC-induced ventricular hypertrophy.
FIGURE 3 Effects of 3-week febuxostat (FBS) or allopurinol (AL) treatment on ratios of ventricle/body and lung/body weights. Treatment was started 7 days following sham or TAC procedures and continued for 3 weeks. *p < 0.05 as compared with the corresponding sham control. VH = vehicle. TAC = transverse aortic constriction.
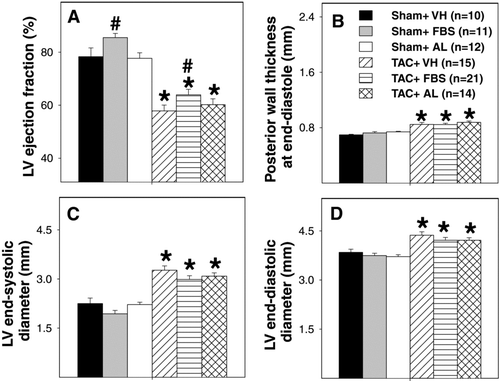
The effects of febuxostat and allopurinol on LV function and dimensions measured by echocardiography are presented in . In sham-operated animals, febuxostat resulted in a small increase in LV ejection fraction (∼9%, ) and fractional shortening (∼15%, data not shown). Although febuxostat had no effect on TAC-induced ventricular hypertrophy, it did induce a small, but statistically significant, improvement in the LV ejection fraction (∼10% increase) and LV fractional shortening (∼16%, data not shown) compared with vehicle-treated TAC animals (). Febuxostat also tended to attenuate the TAC-induced increase in LV end-systolic diameter, which correlates with the finding of improved fractional shortening (). In contrast, allopurinol had no effect on LV function or dimensions () in either sham or TAC mice.
FIGURE 4 Effects of 3-week febuxostat (FBS) or allopurinol (AL) treatment on LV function and dimensions. Data are for LV ejection fraction (A), LV end-systolic wall thickness (B), LV end-systolic diameter (C), and LV end-diastolic diameter (D). Treatment was started 7 days following sham or TAC procedures and continued for 3 weeks. *p < 0.05 as compared with the corresponding sham group. # p < 0.05 as compare with the corresponding vehicle group. LV = left ventricular. VH = vehicle. TAC = transverse aortic constriction.
Histological staining indicated that TAC resulted in significant ventricular fibrosis and increases in myocyte diameter (indicating cardiac hypertrophy). These changes were not affected by either febuxostat or allopurinol (data not shown), which is consistent with the results on ventricular dimensions as measured by echocardiography.
DISCUSSION
In our previous study, an 8 day-treatment of febuxostat beginning approximately 60 minutes after TAC significantly attenuated the TAC-induced ventricular hypertrophy, dilation, fibrosis, and dysfunction.[ Citation 7 ] Febuxostat also significantly attenuated the TAC-induced phosphorylation of mTOR and Erk, and the increase of myocardial atrial natriuretic peptide. In the present study, the effects of febuxostat treatment were diminished when treatment was initiated 7 days after TAC. Delayed treatment with febuxostat had no effect on LV hypertrophy and caused only modest improvement in LV function. Keeping in mind the limitations of experimental data from mice, a species in which uric acid is not the final product of purine catabolism, these results suggest that XO inhibition by febuxostat could be an effective therapeutic approach for the prevention of cardiac hypertrophy and dysfunction induced by systolic overload,[ Citation 7 ] but it may not be an effective treatment once HF has been established. These results are in agreement with findings from a study[ Citation 10 ] using a rabbit myocardial infarction model, in which 7 weeks of daily oral administration of febuxostat, starting a day after coronary artery ligation, significantly lessened the reduction in LV function. As in the current study, however, these effects were not seen if the initiation of treatment with febuxostat or allopurinol was delayed until after the onset of HF.[ Citation 10 ]
In the present study using the TAC mouse model, treatment with allo- purinol had no beneficial, and perhaps even a deleterious, effect as a treatment for established HF. Although administration of allopurinol resulted in a decrease in plasma UA levels that was similar to that of febuxostat, allopurinol treatment produced no improvement in either LV hypertrophy or function. Moreover, allopurinol treatment tended to increase mortality (24%) compared with vehicle (8%) or febuxostat (4%) treatment of mice with TAC.
Whether there is a true difference in the mortality rates between the TAC animals treated with allopurinol compared with febuxostat or vehicle requires further confirmative investigation. Different biological effects between these two agents are not unexpected because of differences in their chemical structures, selectivity, and mode of XO inhibition.[ Citation 11 , Citation 12 ] Febuxostat is different from allopurinol in that it is a nonpurine molecule and does not inhibit other enzymes in purine and pyrimidine metabolism pathways.[ Citation 11 ] Moreover, unlike allopurinol, which is a substrate of XO (suicide XO inhibitor) and a prodrug for the active metabolite oxypurinol, febuxostat directly inhibits XO simply by obstructing substrate binding and this inhibition is not influenced by the redox status of the cofactor.[ Citation 12 ]
Acknowledgments
This study was sponsored by Takeda Global Research & Development Center, Inc., Deerfield IL, USA.
The authors are grateful to Karen Asin, Ph.D. (Takeda) for her critical and thorough reviews of the manuscript during its preparation.
J. W. was a contractor for TAP Pharmaceutical Products, Inc. (now Takeda Global Research & Development Center, Inc.) during this study and is now an independent consultant.
REFERENCES
- Ekelund , U. E. , Harrison , R. W. , Shokek , O. , Thakkar , R. N. , Tunin , R. S. , Senzaki , H. , Kass , D. A. , Marbán , E. and Hare , J. M. 1999 . Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure . Circ. Res. , 85 : 437 – 445 .
- Cappola , T. P. , Kass , D. A. , Nelson , G. S. , Berger , R. D. , Rosas , G. O. , Kobeissi , Z. A. , Marbán , E. and Hare , J. M. 2001 . Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy . Circulation. , 104 : 2407 – 2411 .
- Amado , L. C. , Saliaris , A. P. , Raju , S. V. , Lehrke , S. , St John , M. , Xie , J. , Stewart , G. , Fitton , T. , Minhas , K. M. , Brawn , J. and Hare , J. M. 2005 . Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure . J. Mol. Cell. Cardiol. , 39 : 531 – 536 .
- Stull , L. B. , Leppo , M. K. , Szweda , L. , Gao , W. D. and Marban , E. 2004 . Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy . Circ. Res. , 12 : 1005 – 1011 .
- Engberding , N. , Spiekermann , S. , Schaefer , A. , Heineke , A. , Wiencke , A. , Muller , M. , Fuchs , M. , Hilfiker-Kleiner , D. , Hornig , B. , Drexler , H. and Landmesser , U. 2004 . Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? . Circulation. , 12 : 2175 – 2179 .
- Naumova , A. V. , Chacko , V. P. , Ouwerkerk , R. , Stull , L. , Marban , E. and Weiss , R. G. 2006 . Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts . Am. J. Physiol. , 290 : H837 – H843 .
- Xu , X. , Hu , X. , Lu , Z. , Zhang , P. , Zhao , L. , Wessale , J. L. , Bache , R. J. and Chen , Y. 2008 . Xanthine oxidase inhibition with febuxostat attenuates systolic overload-induced left ventricular hypertrophy and dysfunction in mice . J. Card. Fail. , 14 : 746 – 753 .
- Lu , Z. , Fassett , J. , Xu , X. , Hu , X. , Zhu , G. , French , J. , Zhang , P. , Schnermann , J. , Bache , R. J. and Chen , Y. 2008 . Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle . Circulation. , 118 : 1713 – 1721 .
- Zhang , P. , Xu , X. , Hu , X. , van Deel , E. D. , Zhu , G. and Chen , Y. 2007 . Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure . Circ Res. , 100 : 1089 – 1098 .
- Zhao , L. , Roche , B. M. , Wessale , J. L. , Kijtawornrat , A. , Lolly , J. L. , Shemanski , D. and Hamlin , R. L. 2008 . Chronic xanthine oxidase inhibition following myocardial infarction in rabbits: effects of early versus delayed treatment . Life. Sci. , 82 : 495 – 502 .
- Takano , Y. , Hase-Ayoki , K. , Horiuchi , H. , Zhao , L. , Kasahara , Y. , Knodo , S. and Becker , M. A. 2005 . Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase . Life. Sci. , 76 : 1835 – 1847 .
- Okamoto , K. , Eger , B. T. , Nishino , T. , Kondo , S. , Pai , E. F. and Nishino , T. 2003 . An extremely potent inhibitor of xanthine oxidoreductase: Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition . J. Biol. Chem. , 278 : 1848 – 1855 .