Abstract
Background/Objective: In a previous report of HIV-infected patients with fat redistribution, we found that recombinant human growth hormone (rhGH) therapy reduced visceral adipose tissue (VAT) but increased insulin resistance, and that the addition of rosiglitazone reversed the negative effects of rhGH on insulin sensitivity. In this study, we sought to determine the effects of rhGH and rosiglitazone therapy on an array of inflammatory and fibrinolytic markers.
Methods: 72 patients with HIV-associated abdominal obesity and insulin resistance were randomized to treatment with rhGH, rosiglitazone, the combination of rhGH and rosiglitazone, or placebo for 12 weeks. Subjects with plasma and serum samples available at weeks 0 (n = 63) and 12 (n = 46–48) were assessed for adiponectin, C-reactive protein, homocysteine, interleukin-1, interleukin-6, tumor necrosis factor alpha, interferon gamma, fibrinogen, plasminogen activator inhibitor-1 antigen, and tissue plasminogen activator antigen.
Results: Treatment with both rosiglitazone alone and the combination of rosiglitazone and rhGH for 12 weeks resulted in significant increases in adiponectin levels from baseline. Adiponectin levels did not change significantly in the rhGH arm alone . There were no significant changes in the other biomarkers among the different treatment groups.
Discussion: In this study of HIV-infected patients with altered fat distribution, treatment with rosiglitazone had beneficial effects on adiponectin concentrations, an effect that was also seen with a combination of rosiglitazone and rhGH. RhGH administration alone, however, did not demonstrate any significant impact on adiponectin levels despite reductions in VAT.
Introduction
HIV-associated visceral fat accumulation is associated with an increased risk of metabolic disturbances and cardiovascular disease (CVD).Citation1 Various inflammatory and fibrinolytic markers have been identified as potential surrogate predictors of cardiovascular risk in animal models or the general populationCitation2–13 and, to a lesser degree, in HIV-infected patients.Citation14–18 Several studies have demonstrated elevations in these biomarkers, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and plasminogen activator inhibitor-1 (PAI-1) antigen, in HIV-infected patients with altered fat distribution often in association with insulin resistance.Citation19–21 The adipokine adiponectin is of particular interest as epidemiological studies have identified hypoadiponectinemia as an independent risk factor for CVD in the general population.Citation2,3 In the HIV population, adiponectin levels are reduced in patients with fat redistribution and inversely correlated with abdominal visceral fat mass.Citation22 Several drugs including recombinant human growth hormone (rhGH) have been shown to reduce visceral fat in HIV-infected patients,Citation23–25 but little is known about growth hormone’s influence on adiponectin and other cardiovascular biomarkers.
We previously reported the results of a randomized, placebo-controlled study examining the effects of rhGH and rosiglitazone on visceral adipose tissue (VAT) and insulin sensitivity in HIV-infected patients with abdominal fat accumulation.Citation26 In our primary study, treatment with rhGH for 12 weeks decreased visceral adiposity but increased insulin resistance; the addition of rosiglitazone successfully attenuated the effects on insulin resistance without abrogating the reduction in visceral fat. Herein, we report the results of a substudy aimed at exploring the effects of rhGH and rosiglitazone therapy on the secondary outcomes of adiponectin and other inflammatory and fibrinolytic markers selected because of known associations with visceral adiposity and/or CVD risk.
Methods
Study design
The current substudy is from a randomized, double-blind, placebo-controlled, multicenter trial using a 2 × 2 factorial design. Eligible subjects were randomized in a 1:1:1:1 ratio to receive rhGH 3 mg daily, rosiglitazone 4 mg twice daily, combination rhGH + rosiglitazone, or double placebo treatment for 12 weeks. The primary endpoint of the main study was change in insulin sensitivity index (SI) assessed by frequently sampled intravenous glucose tolerance test (FSIVGTT). Key secondary endpoints included changes in VAT and subcutaneous adipose tissue (SAT) volumes by magnetic resonance imaging from baseline to week 12, as previously published.Citation26 In this substudy, stored blood samples of study participants were assayed for changes from baseline to week 12 in the inflammatory and fibrinolytic markers of interest.
Participants
Eligible subjects were 18–65 years old with documented HIV-1 infection and on stable antiretroviral medications. Subjects were referred by their clinicians or recruited via flyers and print ads. The study was conducted at the General Clinic Research Centers of Weill Cornell Medical College, Columbia University School of Medicine, and St. Luke’s-Roosevelt Hospital Center, all in New York City. A subsite of St. Luke’s-Roosevelt Hospital Center, AIDS Community Research Initiative of America, recruited and enrolled participants and conducted outpatient assessments. Subjects had to meet established anthropometric criteria for excess abdominal fat consisting of both waist circumference >88.2 cm for men and 75.3 for women, and waist:hip ratio of ≥0.95 for men and ≥0.90 for women.Citation27,28 They also had to have evidence of insulin resistance based on a quantitative insulin sensitivity check indexCitation29 ≤0.33. In the primary study, 77 subjects were enrolled and randomized, of whom 72 initiated study drugs. The 63 subjects included in this secondary analysis were those with available frozen serum and plasma at study entry for performance of the biomarker assays.
Ethics
The study protocol was approved by the institutional review boards of all participating study sites. All subjects provided written informed consent.
Laboratory methods
Fibrinogen assay was performed at the Biomarkers Core Laboratory of the Irving Institute for Clinical and Translational Research of Columbia University Medical Center. Fibrinogen was measured in an automated clot-rate assay based upon the original method of Clauss using the ST4 instrument (Diagnostica Stago) with an inter-assay coefficient of variation of 2.9%. Serum PAI-1 antigen, tPA antigen, adiponectin, IFN-γ, IL-1, IL-6, CRP, and homocysteine assays were performed by the General Core Laboratory of the Weill Cornell Medical College Clinical and Translational Science Center. Serum adiponectin and CRP were determined using a quantitative singleplex immunoassay; serum concentrations of IL-1β, IL-6, IFN-γ, and TNF-α with a quantitative 4-plex multiarray immunoassay (Meso Scale Discovery, Gaithersburg, MD); serum homocysteine with a quantitative enzyme immunoassay kit (Bio-Rad, Hercules, CA); tPA and PAI-1 with quantitative enzyme immunoassay kits (Diagnostica Stago, Parsippany, NJ). Manufacturer average intra-assay and inter-assay coefficients of variation were <10% for adiponectin, CRP, homocysteine, tPA, and PAI-1.
Statistical methods
Baseline data were summarized on all subjects with baseline data on at least one biomarker. Absolute changes from baseline to week 12 (i.e. change = week 12 minus baseline) were defined as the primary endpoint of interest, and absolute change was then compared between the treatment groups. Only patients who had complete data at both time points were included in the analyses of changes in biomarkers, and missing data were not imputed. Normality of error terms from one-way ANOVA was initially tested through the Kolmogorov–Smirnov test. Since the normality of error terms failed, the non-parametric Kruskal–Wallis test was used to compare absolute change between the treatment groups for each outcome of interest. The Dunnett-Hsu multiple comparison adjustment was used for all post-hoc pairwise comparisons to the double placebo arm (via pairwise Wilcoxon rank-sum tests), whenever the overall treatment group effect existed. Two-way ANOVA was explored to evaluate the interaction of rosiglitazone and rhGH. Median and interquartile range (IQR) of original scale data were reported for all analyses. All p-values were two-sided, and p < 0.05 was considered as statistical significance. Analyses were conducted with SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Table summarizes the characteristics of the patient population at study entry. Age, body composition, and metabolic parameters were similar between study groups.
Table 1 Baseline characteristics of study participants with data on at least one biomarker (Total N = 63)
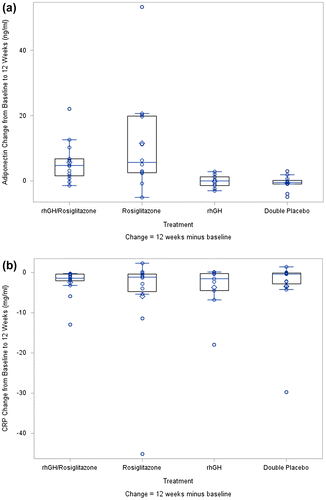
Table shows the change in inflammatory markers in the four study groups from baseline to 12 weeks. Change in adiponectin levels was significantly different between the four study groups (p < 0.0001), driven by greater increases in the rosiglitazone and dual therapy arms, each relative to the double placebo arm. Figure a illustrates boxplots of the absolute changes in adiponectin by study arm, highlighting the increases in the rosiglitazone-containing arms in contrast to the relatively stable rhGH alone and double placebo arms. Changes in other inflammatory and fibrinolytic markers did not differ significantly between the groups. By two-way ANOVA, there was no statistically significant interaction between rhGH and rosiglitazone with regard to any of the biomarkers. Specifically, the interaction between rhGH and rosiglitazone for adiponectin had a P-value of 0.99, indicating that concurrent use of rhGH did not affect the changes in adiponectin mediated by rosiglitazone. The change in adiponectin did not correlate with change in insulin SI (r = 0.08; p = 0.58) or change in VAT (r = –0.14; p = 0.37). As depicted in Figure b, CRP declined significantly within all four treatment arms from study entry to week 12 but did not differ across treatment arms.
Table 2 Changes in Biomarkers by Study Arm
Figure 1 (a) Change in Adiponectin by Study Arm. p = 0.0004 across treatment arms by Kruskal–Wallis test. Within arm changes: rhGH/rosiglitazone p = 0.0010; rosiglitazone p = 0.0073; rhGH p = 0.82; double placebo p = 0.27. Boxplots display the median (line inside box), first and third quartiles (lower and upper edges of box), mean (diamond), and whiskers (maximum and minimum observations aside from outliers). Open circles depict the individual data points. (b) Change in C-reactive Protein by Study Arm. p = 0.74 across treatment arms by Kruskal–Wallis test. Within arm changes: rhGH/rosiglitazone p = 0.0001; rosiglitazone p = 0.012; rhGH p = 0.0078; double placebo p = 0.014.
Discussion
In this study of HIV-infected patients with visceral adiposity, we found that adiponectin levels increased significantly with rosiglitazone and combination of rosiglitazone and rhGH treatment, whereas rhGH had no effect on adiponectin concentrations despite reductions in VAT. We did not find significant changes in CRP, fibrinogen, homocysteine, IFNγ, IL-1, IL-6, PAI-1, TNFα, or tPA levels between the study arms, though CRP declined within all four arms.
Adiponectin is an adipose tissue-derived regulatory protein that in the general population is inversely correlated with metabolic disturbances such as obesity, insulin resistance, type 2 diabetes, and CVD. In the HIV population, adiponectin has been found to correlate inversely with visceral adiposity, lipoatrophy, insulin resistance, and triglyceride levels.Citation22 It also appears to correlate with subclinical cardiac disease as assessed by coronary reserve flow in HIV-infected subjects.Citation30 More recently, a larger study found that HIV-infected men had lower levels of adiponectin compared to HIV-uninfected men, and that these levels were inversely associated with increased coronary stenosis on CT angiography.Citation31 These observations have led to the hypothesis that adiponectin could serve as a novel predictor of cardiovascular risk, as well as a potential target for pharmacologic intervention.
Although rhGH therapy elicited significant reductions in VAT, the improvement in adiponectin concentrations in our study was attributable to rosiglitazone action alone and not to rhGH-related effects. Our findings are consistent with another study in HIV-infected subjects with abdominal fat accumulation in which rhGH therapy did not result in any change in adiponectin levels compared to placebo, though a lower physiologic dose of rhGH was employed in that study.Citation32
Rosiglitazone is an insulin sensitizer that is known to raise adiponectin levels at the cellular levelCitation33 and in several studies of HIV-negative individuals with type 2 diabetes,Citation34 prediabetes,Citation35,36 and polycystic ovary syndrome.Citation37 In the HIV-infected population, our findings are consistent with another study that reported improvement in metabolic indices, including adiponectin, in HIV patients with lipoatrophy and hyperinsulinemia treated with rosiglitazone.Citation38 The improvement in adiponectin in the rosiglitazone-exposed groups could have been mediated by the drug’s effects on insulin resistance, though these exact mechanisms have yet to be elucidated. Surprisingly, we were not able to demonstrate a significant association between the change in adiponectin and change in VAT.
Notably, we found that CRP trended down longitudinally in all four study arms from baseline to week 12. A possible explanation for this could be improved compliance with lipid-lowering agents, taken by about half of study subjects, in the setting of being active participants in a study, though we have no data in this regard. Of note, CRP was an independent predictor of five-year mortality in the Study of Fat Redistribution and Metabolic Change in HIV infection (FRAM)Citation39 as well as in an older study of HIV-infected women.Citation40 Elevated CRP levels were also predictive of acute myocardial infarction in HIV-infected patients in a large database analysis from academic health centers in Boston,Citation16 though results from case-control studies have been conflicting.Citation14,41 Furthermore, CRP levels were predictive of progression of carotid intima-media thickness over 96 weeks in antiretroviral-naïve patients with HIV infection in a small, single center cohort.Citation15 The utility of CRP measurement in HIV-infected patients in a clinical setting, however, is uncertain.
We did not observe significant changes in fibrinogen, homocysteine, IFNγ, IL-1, IL-6, PAI-1, TNFα, or tPA level over the study period despite our a priori hypotheses that rhGH-induced reductions in VAT and rosiglitazone-induced improvements in insulin sensitivity would yield favorable changes in each biomarker. Our negative findings may have been due to limited statistical power in light of our small sample size. It is also possible that our specific pharmacologic interventions were not robust enough or of sustained duration to overcome all the complex metabolic derangements in HIV lipohypertrophy.
We acknowledge that our study is limited by its small sample size and relatively high variability of some of the laboratory assays. In addition, since completion of the study, both drugs have come under limitations. RhGH did not receive FDA approval for the indication of treatment of HIV-related abdominal fat accumulation. Instead, tesamorelin (an analog of growth-hormone releasing hormone) has emerged as the preferred agent for this indication. Moreover, recent studies with tesamorelin demonstrated improvements in adiponectin that correlated with reduction in VAT,Citation42 supporting the drug’s potential for concomitant cardiometabolic benefits. Rosiglitazone is less commonly used in clinical practice due to initial concern about increased cardiovascular events based on a 2007 meta-analysis;Citation43 however, re-analysis of the longer-term RECORD trial (Rosiglitazone Reevaluated for Cardiac Outcomes and Regulation of Glycemia in Diabetes) showing no significant elevated risk of heart attack or death compared to standard of care diabetes medications led to the removal of prior FDA restrictions in 2013.Citation44
In conclusion, our data indicate that rosiglitazone therapy is associated with improved adiponectin levels in patient with HIV-related abdominal fat accumulation and insulin resistance. Although rhGH therapy has favorable effects on body composition including VAT reduction, it does not appear to have any corresponding benefit on inflammatory or fibrinolytic markers. Instead, dual therapy with an insulin-sensitizing agent seems to be preferable to rhGH alone for HIV-related fat accumulation by abrogating the insulin resistance observed with rhGH and raising adiponectin levels. More studies are ultimately needed to find the optimal combination of therapies to manage altered fat redistribution and adiponectin dysregulation in HIV-infected individuals.
Ethics statement
The study protocol was approved by the institutional review boards of all participating study sites. All subjects provided written informed consent.
Registration
The study was registered at Clinicaltrials.gov (NCT00130286). http://www.clinicaltrials.gov/ct2/show/NCT00130286
Acknowledgments
The authors thank Franco Santiago for performance of biomarker assays.
Funding
This work was supported by the National Institutes of Health [grant number R01 DK065515], [grant number K24 AI078884], [grant number UL1 RR024996], [grant number M01 RR 00047], [grant number M01 RR 00645-27], [grant number UL1 TR000040-07], [grant number UL1 TR000457], [grant number P30 DK026687]. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. rhGH and rosiglitazone, with matching placebos, were generously donated by EMD Serono and GlaxoSmithKline, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure Statement
MJG served as a consultant to EMD Serono on two occasions, most recently in 2007. His institution (Weill Cornell Medical College) received research support from EMD Serono for a clinical trial of rhGH for which he was the local principal investigator from 2004–2005. DPK and EE received research support from EMD Serono to their institution (St. Luke’s-Roosevelt Hospital) for a substudy of the present study (not reported herein). EE served as a consultant to Thera Technologies in 2010, which licensed tesamorelin to EMD Serono.
References
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62.10.1056/NEJMra041811
- Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: A population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576.10.1210/jc.2006-1067
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. J Am Med Assoc. 2004;291:1730–1737.10.1001/jama.291.14.1730
- Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. J Am Med Assoc. 2005;294:1799–1809.
- Bostom AG, Silbershatz H, Rosenberg IH, Selhub J, D’Agostino RB, Wolf PA, et al. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly framingham men and women. Arch Intern Med. 1999;159:1077–1080.10.1001/archinte.159.10.1077
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772.10.1161/01.CIR.101.15.1767
- Ridker PM, Vaughan DE, Stampfer MJ, Manson JE, Hennekens CH. Endogenous tissue-type plasminogen activator and risk of myocardial infarction. Lancet. 1993;341:1165–1168.10.1016/0140-6736(93)90998-V
- Held C, Hjemdahl P, Rehnqvist N, Wallen NH, Bjorkander I, Eriksson SV, et al. Fibrinolytic variables and cardiovascular prognosisin patients with stable angina pectoris treated with verapamil or metoprolol: Results from the angina prognosis study in stockholm. Circulation. 1997;95:2380–2386.10.1161/01.CIR.95.10.2380
- van der Bom JG, de KP, Haverkate F, Bots ML, Meijer P, de Jong PT, et al. Tissue plasminogen activator and risk of myocardial infarction: The rotterdam study. Circulation. 1997;95:2623–2627.10.1161/01.CIR.95.12.2623
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: Results from the health ABC study. Circulation. 2003;108:2317–2322.10.1161/01.CIR.0000097109.90783.FC
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153.10.1161/01.CIR.101.18.2149
- Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006.10.1161/01.ATV.16.8.1000
- Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761.10.1172/JCI119465
- De Luca A, de Gaetano DK, Colafigli M, Cozzi-Lepri A, DeCurtis A, Gori A, et al. The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: A nested case-control study. BMC Infect Dis. 2013;13:414.
- Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65:340–344.10.1097/QAI.0000000000000063
- Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273.10.1097/QAI.0b013e3181a9992c
- Knudsen A, Katzenstein TL, Benfield T, Jorgensen NR, Kronborg G, Gerstoft J, et al. Plasma plasminogen activator inhibitor-1 predicts myocardial infarction in HIV-1-infected individuals. Acquir Immune Defic Syndr. 2014;28:1171–1179.10.1097/QAD.0000000000000247
- Shikuma CM, Barbour JD, Ndhlovu LC, Keating SM, Norris PJ, Budoff M, et al. Plasma monocyte chemoattractant protein-1 and tumor necrosis factor-α levels predict the presence of coronary artery calcium in HIV-infected individuals independent of traditional cardiovascular risk factors. AIDS Res Hum Retroviruses. 2014;30:142–146.10.1089/aid.2013.0183
- He G, Andersen O, Haugaard SB, Lihn AS, Pedersen SB, Madsbad S, Richelsen B. Plasminogen activator inhibitor type 1 (PAI-1) in plasma and adipose tissue in HIV-associated lipodystrophy syndrome. Implications of adipokines. Eur J Clin Invest. 2005;35:583–590.10.1111/eci.2005.35.issue-9
- Lihn AS, Richelsen B, Pedersen SB, Haugaard SB, Rathje GS, Madsbad S, Andersen O. Increased expression of TNF-α, IL-6, and IL-8 in HALS: Implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab. 2003;285:E1072–E1080.10.1152/ajpendo.00206.2003
- Johnson JA, Albu JB, Engelson ES, Fried SK, Inada Y, Ionescu G, Kotler DP. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2004;286:E261–E271.
- Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: Cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–4831.10.1210/jc.2003-030214
- Grunfeld C, Thompson M, Brown SJ, Richmond G, Lee D, Muurahainen N, Kotler DP. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12 week induction and 24-week maintenance therapy. J Acquir Immune Defic Syndr. 2007;45:286–297.
- Kotler DP, Muurahainen N, Grunfeld C, Wanke C, Thompson M, Saag M, et al. Effects of growth hormone on abnormal visceral adipose tissue accumulation and dyslipidemia in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:239–252.10.1097/00126334-200403010-00004
- Kotler DP, Muurahainen N, Grunfeld C, Wanke C, Thompson M, Saag M, et al. Effects of growth hormone on visceral adipose tissue and dyslipidemia in HIV, an erratum. J Acquir Immune Defic Syndr. 2006;43:378–380.10.1097/01.qai.0000243108.26136.66
- Glesby MJ, Albu J, Chiu YL, Ham K, Engelson E, He Q, et al. Recombinant human growth hormone and rosiglitazone for abdominal fat accumulation in HIV-infected patients with insulin resistance: A randomized, double-blind, placebo-controlled, factorial trial. PLoS ONE. 2013;8:e61160.10.1371/journal.pone.0061160
- Engelson ES, Kotler DP, Tan Y, Agin D, Wang J, Pierson RN Jr, Heymsfield SB. Fat distribution in HIV-infected patients reporting truncal enlargement quantified by whole-body magnetic resonance imaging. Am J Clin Nutr. 1999;69:162–169.
- Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness–a critical review. Int J Obes Relat Metab Disord. 1998;22:719–727.10.1038/sj.ijo.0800660
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410.10.1210/jcem.85.7.6661
- Bezante GP, Briatore L, Rollando D, Maggi D, Setti M, Ghio M, et al. Hypoadiponectinemia in lipodystrophic HIV individuals: A metabolic marker of subclinical cardiac damage. Nutr Metab Cardiovasc Dis. 2009;19:277–282.10.1016/j.numecd.2008.07.009
- Ketlogetswe KS, Post WS, Li X, Palella FJ Jr, Jacobson LP, Margolick JB, et al. Lower adiponectin is associated with subclinical cardiovascular disease among HIV-infected men. AIDS. 2014;28:901–909.10.1097/QAD.0000000000000186
- Lo J, You SM, Canavan B, Liebau J, Beltrani G, Koutkia P, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation. J Am Med Assoc. 2008;300:509–519.10.1001/jama.300.5.509
- Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: Comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54:3358–3370.10.2337/diabetes.54.12.3358
- Yang WS, Jeng CY, Wu TJ, Tanaka S, Funahashi T, Matsuzawa Y, et al. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25:376–380.10.2337/diacare.25.2.376
- Hung YJ, Lin SH, Pei D, Kuo SW, Hsieh CH, He CT, et al. Rosiglitazone improves insulin sensitivity in nonobese subjects with impaired glucose tolerance: The role of adiponectin and C-reactive protein. Metabolism. 2006;55:439–444.10.1016/j.metabol.2005.10.004
- Kim SG, Ryu OH, Kim HY, Lee KW, Seo JA, Kim NH, et al. Effect of rosiglitazone on plasma adiponectin levels and arterial stiffness in subjects with prediabetes or non-diabetic metabolic syndrome. Eur J Endocrinol. 2006;154:433–440.10.1530/eje.1.02100
- Majuri A, Santaniemi M, Rautio K, Kunnari A, Vartiainen J, Ruokonen A, et al. Rosiglitazone treatment increases plasma levels of adiponectin and decreases levels of resistin in overweight women with PCOS: a randomized placebo-controlled study. Eur J Endocrinol. 2007;156:263–269.10.1530/eje.1.02331
- Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy. Ann Intern Med. 2004;140:786–794.10.7326/0003-4819-140-10-200405180-00008
- Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: Analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322.10.1097/QAI.0b013e3181e66216
- Feldman JG, Goldwasser P, Holman S, DeHovitz J, Minkoff H. C-Reactive protein is an independent predictor of mortality in women with HIV-1 infection. J Acquir Immune Defic Syndr. 2003;32:210–214.10.1097/00126334-200302010-00014
- Westhorpe CL, Schneider HG, Dunne M, Middleton T, Sundararajan V, Spelman T, et al. C-reactive protein as a predictor of cardiovascular risk in HIV-infected individuals. Sex Health. 2014;11:580–582.10.1071/SH14130
- Stanley TL, Falutz J, Mamputu JC, Soulban G, Potvin D, Grinspoon SK. Effects of tesamorelin on inflammatory markers in HIV patients with excess abdominal fat: Relationship with visceral adipose reduction. AIDS. 2011;25:1281–1288.10.1097/QAD.0b013e328347f3f1
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471.10.1056/NEJMoa072761
- Mahaffey KW, Hafley G, Dickerson S, Burns S, Tourt-Uhlig S, White J, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J. 2013;166:240–249.10.1016/j.ahj.2013.05.004
