Abstract
Background: Practical issues, including cost, hinder implementing virologic monitoring of patients on antiretroviral therapy (ART) in resource-limited settings. We evaluated factors that might guide monitoring frequency and efforts to prevent treatment failure after initial virologic suppression.
Methods: Participants were the 911 HIV-infected antiretroviral-naïve adults with CD4 count <300 cells/μL who started efavirenz-based ART in the international A5175/PEARLS trial and achieved HIV-1 RNA <1000 copies/mL at 24 weeks. Participant report of ART adherence was evaluated using a structured questionnaire in monthly interviews. Adherence and readily available clinical and laboratory measures were evaluated as predictors of late virologic failure (late VF: confirmed HIV-1 RNA ≥1000 copies/mL after 24 weeks).
Results: During median follow-up of 3.5 years, 82/911 participants (9%) experienced late VF. Of 516 participants reporting missed doses during the first 24 weeks of ART, 55 (11%) experienced late VF, compared with 27 (7%) of 395 participants reporting no missed doses (hazard ratio: 1.73; 95% CI: 1.08, 2.73). This difference persisted in multivariable analysis, in which lower pre-ART hemoglobin and absence of Grade ≥3 laboratory results prior to week 24 were also associated with higher risk of late VF.
Discussion: In this clinical trial, the late VF rate after successful suppression was very low. If achievable in routine clinical practice, virologic monitoring involving infrequent (e.g. annual) measurements might be considered; the implications of this for development of resistance need evaluating. Patients reporting missed doses early after ART initiation, despite achieving initial suppression, might require more frequent measurement and/or strategies for promoting adherence.
Introduction
Guidelines for antiretroviral treatment (ART) for HIV-infected patients recommend monitoring response to ART and the diagnosis of treatment failure using measurements of viral load.Citation1–3 Current United States guidelines recommend frequent monitoring first to establish an initial virologic response after starting or changing ART, and then every three to four months to confirm continuous suppression, with possible reduction to every six months for adherent stable patients whose viral load has been suppressed for more than two years.Citation3 In contrast, current World Health Organization (WHO) guidelines recommend viral load measurements at six months after starting ART and then at least every 12 months as the monitoring approach to detect virologic failure (defined as two consecutive HIV-1 RNA measurements greater than 1000 copies/mL).Citation1,2 Even with the reduced frequency in WHO guidelines, practical issues including high cost have resulted in variable implementation of virologic monitoring in many resource-limited settings.
Virologic suppression is achieved in the large majority of patients within six months of first starting ART. Virologic monitoring after initial suppression aims at detecting virologic failure prior to accumulation of multiple resistance mutations that may compromise future treatment options, and prior to progression of HIV disease. Allocation of resources could be optimized if monitoring after initial suppression could be effectively targeted to patients at greater risk for virologic failure. A systematic review concluded that WHO clinical and immunologic criteria were insensitive for this purpose.Citation4 Other studies have evaluated scoring systems based on more extensive immunologic, clinical, laboratory and adherence evaluations with modest improvements.Citation5–8
In this study, we use data from participants in a large clinical trial conducted in diverse geographical locations (ACTG A5175/PEARLSCitation9) in a post hoc analysis to evaluate factors during the first six months of ART, particularly a participant’s self-reported adherence to ART, that might predict treatment failure during three or more years after initial successful virologic suppression on efavirenz(EFV)-based ART. Such factors might then help identify patient subgroups at higher risk for treatment failure subsequent to initial suppression in which more frequent viral load monitoring and/or efforts to prevent treatment failure might be best used. This extends previous evaluation of adherence in the PEARLS study which focused on predicting virologic failure early during ART, so predominantly reflecting failure to achieve initial virologic suppression.Citation10
Methods
The AIDS Clinical Trials Group (ACTG) Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS; ClinicalTrials.gov NCT00084136) study was a phase IV, prospective, randomized, open-label evaluation of the efficacy of two once-daily EFV-based antiretroviral regimens and an atazanavir-based regimen for the initial treatment of HIV-1-infected individuals from nine countries: Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, United States, and Zimbabwe. The study design and primary outcomes have been previously reported.Citation9 In brief, participants were ≥18 years old, had documented HIV-1 infection, had received no more than seven days of cumulative prior ART (prior use of single-dose nevirapine or zidovudine for any duration to prevent mother-to-child transmission of HIV was allowed), and had a CD4 cell count <300 cells/μL within 90 days prior to entry into the study. The eligibility criteria also included hemoglobin ≥7.5 g/dL. Written informed consent was obtained from all participants, and the human experimentation guidelines of the United States Department of Health and Human Services were followed. The study was approved by local Ethics Committees at each participating institution.
The results presented here are restricted to participants who were randomized to receive one of the two EFV-based regimens: lamivudine and zidovudine with EFV (3TC/ZDV + EFV), or emtricitabine and tenofovir with EFV (FTC/TDF + EFV). This was because the Data and Safety Monitoring Board recommended stopping treatment with the atazanavir-based regimen due to inferiority and this regimen is not recommended for use in clinical practice. As the focus of our study was to evaluate treatment failure following initial response to ART, we further restricted the study population to participants who had a plasma HIV-1 RNA measurement <1000 copies/mL at 24 weeks after starting ART with no virologic failure or AIDS-defining event during the first 24 weeks of ART. Changes in one or more drugs at or prior to week 24 did not lead to exclusion from the study population.
Plasma HIV-1 RNA was measured prior to starting ART, and then at weeks four and eight and every eight weeks thereafter through the end of study follow-up. A standard questionnaireCitation10,11 was used to obtain a participant’s self-report of adherence to ART at weeks two and four and then every four weeks through to week 24 after starting ART, and every eight weeks thereafter. This questionnaire included multiple questions about whether any dose of each drug was missed on each of the prior three days, during the past weekend, or during the previous two weeks. In addition, participants were asked when they last missed taking any of their anti-HIV medications, if at all. Adherence counseling by trained site personnel was conducted prior to starting ART using a checklist of discussion points, and as needed at follow-up visits at the discretion of the site investigator.
For this report, the outcome of interest is “late” virologic failure, defined as two successive measurements of plasma HIV-1 RNA ≥1000 copies/mL, with the first measurement more than 24 weeks after starting ART, so following the week 24 HIV-1 RNA measurement which had to be <1000 copies/mL. As possible predictors of late virologic failure, we considered variables which would often be routinely available in clinical practice prior to starting ART or obtained during the first 24 weeks of ART: demographic (age, sex, country); pre-ART disease status (CD4 count, log10 HIV-1 RNA and clinical status including prior or active AIDS or tuberculosis [TB], hepatitis B infection, Karnofsky Score, weight, and body mass index [BMI]); pre-ART laboratory test results (creatinine clearance, albumin, hemoglobin; and platelet, absolute neutrophil, absolute lymphocyte and white blood cell counts); changes in disease status from pre-ART to week 24 (change in CD4 count, HIV-1 RNA at week 24 classified as ≤400 vs. 401–999 copies/mL, change in weight, and change in BMI); initial ART regimen (TDF/FTC + EFV or ZDV/3TC + EFV); ART changes, Grade 3 or 4 laboratory test results and Grade 3 or 4 signs and symptoms during the first 24 weeks of treatment (graded according to the DAIDS Toxicity TablesCitation12); and participant’s self-reported adherence to ART (categorized as report of missed vs. no missed doses at any of the evaluations through to 24 weeks after starting ART).
Statistical analysis used proportional hazards models to evaluate possible predictors of late virologic failure. Censoring of follow-up was at the last available HIV-1 RNA measurement. Age and laboratory measurements were included as continuous variables; linearity assumptions appeared reasonable based on standard graphical and residual plots. Variables that achieved p < 0.10 in univariable analysis were considered for inclusion in multivariable analysis using stepwise variable selection with p < 0.10 required for variable retention in the model and p > 0.10 for removal. The models were extended to check for effect modification (interaction) between included variables and randomized treatment.
Results
Of the 1045 participants randomized to receive TDF/FTC + EFV or ZDV/3TC + EFV, 911 (87%) had plasma HIV-1 RNA <1000 copies/mL at week 24 and were included in our study population. The reasons for excluding the other 134 participants were: virologic failure at or prior to week 24 (n = 64 participants), plasma HIV-1 RNA >1000 copies at week 24 (n = 18), new AIDS-defining event prior to week 24 (n = 3), death prior to week 24 (n = 11), loss to follow-up at or before week 24 (n = 28), and no plasma HIV-1 RNA measurement at week 24 (n = 10). Table summarizes characteristics of the 911 participants prior to starting ART and after 24 weeks of ART.
Table 1 Characteristics of the study population (participants achieving plasma HIV-1 RNA < 1000 copies/mL at 24 weeks after starting efavirenz-based antiretroviral therapy)
At the time of closure of follow-up of the randomized trial, participants had been followed for a median of 184 weeks (3.5 years) from starting ART. During this time, 82 (9%) of the 911 participants experienced late virologic failure. Among the remaining 829 participants, 7% were lost to follow-up and 3% died without experiencing late virologic failure. Figure , panel A, shows the cumulative incidence of late virologic failure. The late virologic failure rate appeared slightly higher (3%) during the period from 24 to 48 weeks after starting ART, but was reasonably constant thereafter (about 3% per year).
Figure 1 Cumulative percentage of participants experiencing late virologic failure by time from start of antiretroviral therapy (ART): all participants (A); by participant report of missed vs. no missed doses during first 24 weeks of ART (B); by per-ART hemoglobin level (C); and by whether or not participant had a grade 3 or 4 laboratory abnormality during the first 24 weeks of ART.
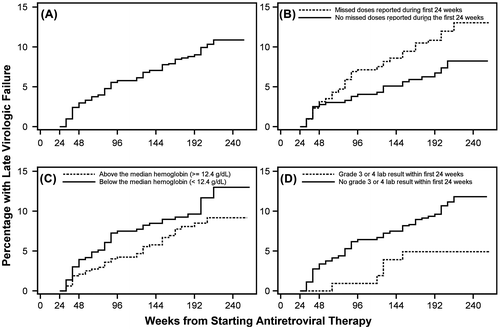
Participants who reported missing doses during the first 24 weeks of ART had significantly higher risk of late virologic failure compared to participants who reported no missed doses (p = 0.023; Fig. , panel B). Specifically, 55 (10.7%) of the 516 participants reporting missed doses experienced late virologic failure compared with 27 (6.8%) of the 395 participants reporting no missed doses. The absolute difference between participants reporting missed vs. no missed doses appeared to increase during the four years of follow-up after starting ART.
Among the other possible predictors of late virologic failure evaluated (see Methods), lower pre-ART hemoglobin (p = 0.025), lower pre-ART absolute neutrophil count (p = 0.044) and higher pre-ART white blood cell count (p = 0.026) were also significantly associated with higher risk of late virologic failure. Associations for higher risk with older age and with higher pre-ART albumin level, and for lower risk among participants with a Grade ≥3 laboratory test result during the first 24 weeks of ART also met the statistical criterion (p < 0.10) for consideration for inclusion in the multivariable analysis. There were no significant associations with demographic variables (age, sex, country) or disease status variables including plasma HIV-1 RNA at baseline or week 24 (the latter categorized as ≤400 vs. 401–999 copies/mL) and CD4 count at baseline or week 24. In addition, the proportion of participants experiencing late virologic failure was not significantly different between the two randomized regimens (42 of 417 [9.2%] for 3TC/ZDV + EFV vs. 40 of 412 [8.9%] for FTC/TDF + EFV; hazard ratio: 1.05, 95% confidence interval: 0.68–1.62).
In multivariable analysis, participant report of missed doses and lower pre-ART hemoglobin were significantly associated with increased risk of late virologic failure, while a grade ≥3 laboratory test result during the first 24 weeks of ART was significantly associated with lower risk (Table ). For each of these three factors, the unadjusted hazard ratios (from univariable analysis) and the adjusted hazard ratios (from multivariable analysis) were very similar suggesting minimal confounding of these associations. In particular, participant report of missed vs. no missed doses was associated with 72 and 73% increases in risk of late virologic failure in unadjusted and adjusted analysis. Panels B, C and D of Fig. show the cumulative incidence of late virologic failure by time for these three factors (categorizing, for illustration, pre-ART hemoglobin level as above or below the median level of 12.4 g/dL). There was no significant evidence that these associations varied between the two EFV-based randomized treatments. Very similar results were obtained in a sensitivity analysis which was restricted to participants who remained on their randomized ART at week 24. In addition, the associations with self-reported adherence and with pre-ART hemoglobin were very similar in an analysis restricted to participants who did not have a grade ≥3 laboratory test result during the first 24 weeks of ART.
Table 2 Results from univariable and multivariable analyses for participant characteristics predicting late virologic failure that were included in the multivariable model (n = 911, including 82 with late virologic failure)
Table provides a summary of how the model results might be used to suggest a range of simple decision rules for identifying a subgroup of participants at higher risk of late virologic failure in which more frequent monitoring and/or efforts to prevent failure might be best used. Again, for simplicity of illustration, pre-ART hemoglobin level is categorized as above or below the median level of 12.4 g/dL. For example, the multivariable model might suggest the subgroup of participants who reported missed doses, had a pre-ART hemoglobin (less than the median of 12.4 mg/dL is used in the table), and did not have a Grade 3+ laboratory result during the first 24 weeks of ART. For this subgroup, shown in the first row of the table, 195 (21%) of the 911 participants met all three criteria including 30 (37%) of the 82 participants who experienced late virologic failure during follow-up (the 37% is equivalent to the sensitivity of the decision criterion). In this subgroup, 30 (15.4%) of the 195 participants experienced late virologic failure. As would be expected, as the criteria are relaxed so increasing the size of the subgroup identified, the sensitivity increases.
Table 3 Performance of simple algorithms for identifying participants at risk of late virologic failure based on information available at 24 weeks
Discussion
In this multinational clinical trial setting, the rate of late virologic failure after successful virologic suppression on EFV-containing ART was low, about 3% per year. A similarly low rate has been reported among patients initiated on public sector EFV- or nevirapine-based ART in South Africa.Citation13 If such low rates are achievable more generally in clinical practice and for other regimens, then less frequent virologic monitoring such as annual monitoring (as suggested in current WHO guidelines) might be reasonable. For example, if the rate of late virologic failure is 3% per year, then annual monitoring would delay detection of virologic failure by 6 months for 1.5% of patients each year but at the expense of twice as much virologic monitoring. The major downside of less frequent monitoring is the increased risk of viral resistance mutations leading to decreased future treatment options and increased risks of disease progression and transmission of resistant virus.Citation14,15 The risks associated with cost savings in less frequent virologic monitoring then need balancing against the potential benefits of having more resources available to increase drug availability, adherence to treatment, and retention in care. Further exploration of these competing issues is needed, building on cost effectiveness modeling of ART monitoring strategies in the resource limited setting.Citation16
Motivated by the idea that more intensive virologic monitoring might be best used among patients at increased risk of late virologic failure, we evaluated a variety of possible predictors of late virologic failure that are routinely available in clinical practice. However, we found few variables that were statistically significant predictors in multivariable analysis. Of note, despite achieving plasma HIV-1 RNA <1000 copies/mL after 24 weeks of ART, study participants who reported missed doses during the first 24 weeks appeared to have an ongoing increased risk of late virologic failure over several years (Fig. , panel B). This confirms findings from a smaller study undertaken in South Africa using a similar questionnaire to obtain patient-reported adherence.Citation17 Having increased frequency of virologic monitoring for those that report missed doses might therefore be reasonable. In addition, this finding emphasizes the need for ongoing adherence support systems for patients who report missed doses despite achieving initial virologic suppression as a possible approach for reducing late virologic failure rates.
Lower pre-ART hemoglobin level was associated with higher risk of late virologic failure despite the fact that the large majority of study participants had normal levels (median: 12.4 g/dL; inter-quartile range: 11.2–13.8 g/dL) . The mechanism for this is unclear. While it could reflect increased risk for ART- and/or disease-related adverse events leading to poorer adherence or treatment interruptions around ART changes, the effect persisted in multivariable analysis adjusted for adherence and persisted in analysis restricted to participants who did not change ART. Other studies suggest associations of hemoglobin with virologic failure: greater decreases in hemoglobin, though not pre-ART hemoglobin levels, were predictive of late virologic failure in a case-control study of patients initiating ART in South Africa;Citation7 and hemoglobin decreases were associated with virologic failure in a cross-sectional study among Cambodian patients on first-line ART.Citation5 In addition, lower pre-ART hemoglobin levels have been associated with increased mortality among patients starting ART in sub-Saharan Africa.Citation18 Hemoglobin levels were not collected in the database during follow-up in the A5175 trial (except in the presence of Grade ≥3 levels) so we are not able to explore further the importance of changes in hemoglobin for predicting late virologic failure.
Our study also found that participants who experienced a Grade 3 or higher laboratory test result during the first 24 weeks of ART were at lower risk of late virologic failure. This finding persisted in a sensitivity analysis restricted to participants who did not have a change in their ART during the first 24 weeks and so the association is not explained by ART change. This finding was not expected and might reflect chance given the large number of associations evaluated. Alternatively, laboratory test abnormalities might reflect higher drug concentrations within study participants for pharmacokinetic reasons or better adherence not captured by a participant’s self-report, which might also then be associated with more potent virologic suppression.
Based on the three variables included in the multivariable model, we evaluated simple criteria for identifying a subgroup of study participants at higher risk of late virologic failure (Table ). In clinical practice, such a subgroup might be identified for more frequent virologic monitoring or adherence-based intervention. A key finding, also apparent in other studies,Citation5–8 is that to achieve high sensitivity, that is to identify a subgroup which included the large majority of participants who experienced late virologic failure, the subgroup also included a large majority of participants. For example, the subgroup of participants who reported missed doses or who had a pre-ART hemoglobin <12.4 mg/dL included 82% of participants who experienced late virologic failure (sensitivity of 82%) but also included 78% of participants. Of note in this example, the observed sensitivity was also little different from what would be achieved by taking a random sample of 78% of participants. These findings provide support for a strategy of virologic monitoring for all patients rather than restricting monitoring to a particular subgroup. The risk factors for late virologic failure that were identified might, however, be used to identify a smaller subgroup for more frequent virologic monitoring. For example, 39% of participants who experienced late virologic failure had lower pre-ART hemoglobin levels and reported missed doses during the first 24 weeks of ART (Table ) suggesting that more frequent monitoring in this subgroup might be useful. The cost effectiveness of increased monitoring in selected patient subgroups needs exploration.Citation16
Our study has some strengths and limitations. Strengths include the prospective follow-up with real-time measurement of plasma HIV-1 RNA at two month intervals over a median 3.5 year period, low rate of loss to follow-up,Citation9 and diverse geographical settings which may increase the generalizability of results. Provision of adherence counseling at study entry using an evidence-based approach,Citation19,20 and as needed during follow-up (at the discretion of the site investigator), was intended to mimic how adherence counseling is often provided during routine care in antiretroviral treatment programs. However, the study used data from a clinical trial with clinical and laboratory assessments at more frequent intervals than in usual practice, as well as completion of a questionnaire by study participants about missed doses which might itself reinforce adherence. It is possible that the rate of late virologic failure may be higher in routine clinical practice when clinic visits may be less frequent and so provide fewer opportunities to identify and resolve adherence issues. It is also possible that participants in a clinical trial differ from the general patient population and may be retained in care more readily. In addition, even though 82 participants experienced late virologic failure, the precision to estimate associations is somewhat limited. For example, the confidence interval for the hazard ratio comparing late virologic failure for 3TC/ZDV + EFV vs. FTC/TDF + EFV was from 0.68 to 1.62, so modest true differences between regimens, for example due to twice- vs. once-daily dosing, cannot be ruled out despite the fact that the observed rates were very similar. Finally, while we identified factors that were associated with increased risk of late virologic failure, it would be important to confirm these findings in general patient populations and to validate any algorithm for selecting a subgroup for increased frequency of monitoring also in general clinical practice.
Conclusions
In conclusion, our study showed an ongoing but low rate of late virologic failure over several years of follow-up (about 3% per year) among HIV-infected adults in diverse geographical locations who achieved plasma HIV-1 RNA <1000 copies/mL at 24 weeks after starting EFV-based ART. For patients achieving initial virologic suppression, if such a low rate is achievable in general clinical practice, then less frequent virologic monitoring might be reasonable; however the implications of this for the accumulation of resistance mutations and cost effectiveness of ART programs should be evaluated further in clinical practice. Patients reporting missed doses early after ART initiation despite achieving virologic suppression were at increased risk of virologic failure over several years and so might be targeted for more frequent measurement and/or more intensive strategies for promoting adherence.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under [grant numbers UM1 AI068634], [UM1 AI068636], and [UM1 AI106701].
Adherence to ethics
Written informed consent was obtained from all participants, and the human experimentation guidelines of the United States Department of Health and Human Services were followed. The study was approved by local Ethics Committees at each participating institution in the A5175/PEARLS study.
Clinical trials registration number
ClinicalTrials.gov NCT00084136
Acknowledgments
The authors thank the study participants and site and ACTG staff who conducted the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and Gilead Sciences provided study drugs and Gilead Sciences provided funding to purchase a study drug that was not otherwise available. Representatives of these companies participated as study team members, but did not participate in data collection or analysis, or in the preparation of this manuscript or the decision to publish. Dr Campbell reports having received money from Gilead for participation on an advisory board. Dr La Rosa reports being an employee of Merck Sharp and Dohme Peru. Dr Flanigan reports having stock ownership in Abbot, Bristol-Myers Squibb, Gilead Sciences and Glaxo SmithKline. The other authors do not have any potential conflicts of interest to declare. This work was presented in part at the Conference on Retroviruses and Opportunistic Infections: Abstract number 539, 3–6 March 2014, Boston, MA, USA.
References
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Geneva: World Health Organization; 2013. http://www.who.int/hiv/pub/guidelines/arv2013/en. Accessed March 30, 2016.
- What’s new in monitoring. Fact sheet: HIV treatment and care. Geneva: World Health Organization; 2015. http://www.who.int/hiv/pub/arv/arv2015-monitoring-factsheet/en/. Accessed March 30, 2016.
- Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed March 30, 2016.
- Rutherford GW, Anglemyer A, Easterbrook PJ, et al. Predicting treatment failure in adults and children on antiretroviral therapy. AIDS. 2014;28(Suppl 2):S161–S169.10.1097/QAD.0000000000000236
- Lynen L, An S, Koole O, et al. An algorithm to optimize viral load testing in HIV-positive patients with suspected first-line antiretroviral therapy failure in Cambodia. J Acquired Immune Defic Syndr. 2009;52(1):40–48.10.1097/QAI.0b013e3181af6705
- Abouyannis M, Menten J, Kiragga A, et al. Development and validation of systems for rational use of viral load testing in adults receiving first-line ART in sub-Saharan Africa. AIDS. 2011;25(13):1627–1635.10.1097/QAD.0b013e328349a414
- Evans DH, Fox MP, Maskew M, et al. CD4 criteria improves the sensitivity of a clinical algorithm developed to identify viral failure in HIV-positive patients on antiretroviral therapy. J Int AIDS Soc. 2014;17:19139.
- van Griensven J, Phan V, Thai S, Koole O, Lynen L. Simplified clinical prediction scores to target viral load testing in adults with suspected first line treatment failure in Phnom Penh, Cambodia. PLoS ONE. 2014;9(2):e87879.10.1371/journal.pone.0087879
- Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: A randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9(8):e1001290.10.1371/journal.pmed.1001290
- Safren SA, Biello KB, Smeaton L, et al. Psychosocial predictors of non-adherence and treatment failure in a large scale multi-national trial of antiretroviral therapy for HIV: Data from the ACTG A5175/PEARLS Trial. PLoS ONE. 2014;9(8):e104178.10.1371/journal.pone.0104178
- Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG Adherence Instruments. AIDS Care. 2000;12(3):255–266.10.1080/09540120050042891
- Division of AIDS table for grading the severity of adult and pediatric adverse events: December, 2004. Bethesda, MD: NIH/NIAID/DAIDS; 2004. http://www.hptn.org/web%20documents/HPTN046/SSP/Appendices/AppendixE-ToxicityTables_DAIDS_AE_GradingTable_FinalDec2004.pdf. Accessed November 20, 2015.
- Fox MP, Cutsem G, Giddy J, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquired Immune Defic Syndr. 2012;60:428–437.10.1097/QAI.0b013e3182557785
- Barth RE, Aitken SC, Tempelman H, et al. Accumulation of drug resistance and loss of treatment options precede commonly used criteria for treatment failure in HIV-1 subtype-C-infected patients. Antiviral Ther. 2012;17:377–386.
- Cozzi-Lepri A, Paredes R, Philips AN, et al. The rate of accumulation of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance in patients kept on a virologically failing regimen containing an NNRTI. HIV Med. 2012;13:62–72.
- Working group on modelling of antiretroviral therapy monitoring strategies in sub-Saharan Africa. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature. 2015;528:S68–S76.
- Ford N, Darder M, Spelman T, Maclean E, Mills E, Boulle A. Early adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: Five-year follow up of an observational cohort. PLoS ONE. 2010;5(5):e10460.10.1371/journal.pone.0010460
- Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second‐line regimens in antiretroviral‐treated patients in sub‐Saharan African sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48(1):115–122.10.1086/596722
- Safren SA, Otto MW, Worth J. Life-steps: Applying cognitive behavioral therapy to HIV medication adherence. Cognitive Behav Pract. 1999;6:332–341.10.1016/S1077-7229(99)80052-2
- Safren SA, Otto MW, Worth J, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–1162.10.1016/S0005-7967(00)00091-7
