Abstract
Introduction: Pregnancy is an exclusion criteria in most clinical trials involving antiretroviral therapy (ART) and modern contraception methods are systematically proposed to women of childbearing age. Nevertheless pregnancies are often observed. Reproductive choices during clinical trials should be understood to adapt interventions to the level of risk for mother and baby safety. Our goal was to describe the reproductive behavior and pregnancy outcomes among HIV-infected women on second-line antiretroviral treatment enrolled in two clinical trials and to compare them with those of HIV-positive women in non-research settings.
Methods: The number and outcomes of pregnancies were recorded among 281 non menopausal women enrolled in the ANRS 12169-2LADY and ANRS 12286-MOBIDIP clinical trials in Cameroon, Senegal and Burkina Faso. All participants had agreed to use a least one contraceptive method (barrier or non-barrier) which was provided for free during the study. Data were collected through revision of pregnancy notification forms and by data extraction from the study database, regularly updated and checked during the study.
Results: Sixty-six women had 84 pregnancies between January 2010 and July 2015 resulting in a pregnancy rate of 8.0 per 100 women-years (WY) (95% CI 6.5–9.9) which is similar to the ones observed in cohort studies in Sub-Saharan Africa (varying from 2.5 to 9.4 pregnancies per 100 WY). Among 60 live births, 10 (16.6%) were born prematurely and 9 (15%) had a low birth weight. Sixteen miscarriages/stillbirths occurred (19.5%). This percentage is comparable to the one expected in the seronegative population which is reassuring for HIV-positive women considering pregnancy on ART. Only one minor birth defect was diagnosed. In univariate and multivariate analysis, miscarriages/stillbirths were not associated either with age, nadir of CD4 count, duration of ART, CD4 count, or viral load at the beginning of pregnancy.
Conclusion: HIV-positive women participating in clinical trials conducted in Sub-Saharan Africa tend to get pregnant as often as seropositive women who received medical care in non-research settings. It is therefore essential to adopt a pragmatic approach by re-evaluating the relevance of the criteria for exclusion of pregnant women according to the risk associated with exposure and to seek more effective and innovating contraceptive strategies when using potentially teratogenic molecules.
Introduction
Sub-Saharan Africa has the highest fertility rate of any world region with an average of 4.7 children born per woman.Citation1 In couples affected by HIV, desire for pregnancy and contraceptive needs are complex issues. In Sub-Saharan Africa, where the social value of childbearing is high,Citation2 studies investigating maternity desires have shown that diagnosis of HIV infection was not followed by a decrease in childbearing desire and that family planning counseling was not successfully attended.Citation3 Nevertheless, before the highly active antiretroviral therapy (HAART) era, HIV infection was associated with a significant reduction in fertility. A review of six studies in sub-Saharan Africa concluded that untreated HIV-infected women have between 25 and 40% lower fertility than HIV-uninfected women.Citation4 Mechanisms through which fertility is impaired by HIV are not fully understood and are probably a combination of biologicalCitation5 behavioral and social factors.Citation6 Some authors founded that the generalization of HAART has been followed by an increase in fertility, as shown in a study published in 2010 based on the MTCT-Plus cohort, in which the incidence of pregnancy was significantly higher among women receiving antiretroviral therapy (ART) compared to untreated women (9 per 100 person-years (PY) vs. 6.5 per 100 PY, HR: 1.7, 95% CI 1.2–2.5), though it did not reach that of the general populationCitation7 A high CD4 count was positively associated with increased fertility, as well as, like in the general population, young age, increased parity, low education, being married or in couple and absence of use of contraceptive methods.Citation7,8
In clinical trials involving antiretroviral drugs (ARV), pregnancy and breastfeeding are commonly considered exclusion criteria in order to prevent potential toxicity on embryo, fetus, or newborn. Yet more and more of these clinical trials are conducted in low-income countries where the majority of participants are women and desire for pregnancy remains high.Citation9 In the Adult Antiretroviral Treatment and Drug Resistance Study conducted in Botswana (“Tshepo study”), the authors reported a pregnancy rate of 7.9 per 100 PY, although all women had verbally agreed to defer childbearing for the duration of the three-year studyCitation10 However, information on reproductive behavior and pregnancy outcomes among HIV-infected women enrolled in clinical trials remains scarce in sub-Saharan Africa.
This study reports the reproductive behavior and pregnancy outcomes over 5 years of follow-up among 281 HIV-infected women on second-line ART enrolled in the ANRS 12169-2LADY and ANRS 12286-MOBIDIP clinical trials.
Methods
Study design and population
Between January 2010 and September 2012, 325 HIV-infected women failing first-line ART were enrolled in the 2LADY trial, of which 281 were non-menopausal. The 2LADY trial is a 48-week, randomized, open-label, multicenter, non-inferiority trial comparing efficacy and safety of three boosted protease inhibitor (PI)-based second-line antiretroviral combinations: tenofovir, emtricitabine (TDF/FTC), and Lopinavir (LPV/r) (current WHO standard regimen), TDF/FTC and Darunavir (DRV/r) and abacavir (ABC), didanosine (ddI) and LPV/r. It was conducted in three countries: the HIV services of the Central and Military Hospitals of Yaounde in Cameroon, the Clinical Research Centre and the Day Care Centre of the Fann Hospital of Dakar in Senegal, and the Day Care Hospital of the University Hospital of Bobo Dioulasso in Burkina Faso. At the beginning of 2014, while the 2Lady trial ended, a multicenter, randomized, superiority trial to evaluate efficacy of a mono or dual-therapy of protease inhibitors with or without lamivudine over a period of 96 weeks (MOBIDIP) was proposed to former participants of the 2LADY trial. It included 265 patients in Yaounde, Bobo Dioulasso, and Dakar between March 2014 and January 2015. The rest of the participants of the 2LADY trial were followed until December 2015 (2LADY long-term follow-up) in order to collect long-term efficacy and safety data on the three PI-based regimens.
Population characteristics have been described elsewhere.Citation11 All non menopausal women had a negative pregnancy test at enrollment in the 2LADY and the MOBIDIP trials and accepted to defer pregnancy for the study period. They received counseling before study enrollment regarding dual protection, using condoms and a non-barrier contraceptive method. Free hormonal contraceptive was provided to all willing women of childbearing age (medroxyprogesterone, one injection/3 months or levonorgestrel-releasing implant). In accordance with the study protocol, in case of a pregnancy, the HIV viral load (VL) was controlled 8–12 weeks before the expected date of delivery and ART was modified if VL was detectable.
Primary and secondary endpoints
The primary endpoint of this study was the occurrence of pregnancy during the trial follow-up. Pregnancy screening was based on a urine pregnancy test performed every 3 or 6 months. Overall pregnancy rate was calculated as the number of pregnancy by 100 women-years (WY). Overall fertility rate was defined as the number of live births per 100 WY (including 6 births which occurred after the 15 July 2015). Secondary end-points were: outcomes of pregnancy categorized into: miscarriage (pregnancy loss before 22 completed weeks of gestation (WG)), stillbirth (intrauterine death after 22 WG), extra uterine pregnancy, voluntary abortion, and live birth. Proportion of prematurity and low birth weight were also calculated. Gestational age was assessed according to the last menstrual period and by means of ultrasonographic findings when available. Prematurity was defined as a gestation of less than 37 weeks and low birth weight was a weight of less than 2.5 kg.Citation12
Data collection
In order to describe characteristics of the women who became pregnant and to identify factors associated with risk of pregnancy loss socioeconomic, clinical and biological data were recorded, including: age, marital status, education, profession, urban/rural area, disclosure of serological status with the partner/family/friends, co morbidity, body mass index, parity, time since HIV diagnosis, time on ART, CD4 cell count and VL at enrolment, closest to the beginning/ending of pregnancy (in a time window of 3 months), and ART received during pregnancy. Data were gathered by reviewing the medical records of all pregnant women, pregnancy diagnosis, and pregnancy outcome notification forms, the case report forms of 2LADY and MOBIDIP trials. Socioeconomic data were provided by the database of the “medico-economic evaluation of three second-line antiretroviral regimens in Africa (study associated with the 2LADY trial)” – ANRS 12231 (UMI 912/Inserm/IRD).
Statistical analysis
Baseline characteristics of the women were described in term of median and interquartile range for quantitative variables and in term of frequency and percentage for qualitative variables. Incidence rates of pregnancy event including recurrences were calculated per 100 women-years of follow-up with their 95% confidence interval (95% CI). Person-time at risk of pregnancy was calculated as follows: women who became pregnant during follow-up were censored upon the reported date of their last menstrual period and uncensored upon the date of delivery/diagnosis of miscarriage/abortion. Women who did not become pregnant were censored at the time of data collection, on the 15 July 2015 or last news. To identify factors associated with pregnancy outcomes, we used generalized linear mixed models including women identification number as a random effect to account for multiple successive pregnancies in some women. Variables retained in the multivariable analysis were those with a p-value for association with unproductive pregnancy (defined as miscarriage/stillbirth or extra uterine pregnancy) <0.20. Statistical analyses were realized using STATA software (version 14).
Ethical considerations
Written informed consent was obtained from all patients. Study protocols were approved by the appropriate ethic committees and health authorities and were conducted in accordance with the Declaration of Helsinki and Good Clinical Practices.
Results
Population characteristics and pregnancy rate
Between the 15 January 2010 and the 15 July 2015, 84 pregnancies occurred in 66 women during 1046.3 women-years (WY) of follow-up. Fifty-one women had 1 pregnancy, 12 had 2 pregnancies and 3 had 3 pregnancies. Baseline characteristics of pregnant women are detailed in Table . Median age was 31 [interquartile range (IQR) 28–34], 7 years younger than the median age in the global 2LADY study population. Median parity was 3 (including the index pregnancy, [IQR 2–4]). All women were asymptomatic at the time of study enrollment despite a low CD4 count (median 203 cells/mm3 [IQR 103–300]) and a median VL of 4.5 log10 [IQR 3.9–5.1]. In comparison with the other female participants, women who became pregnant were significantly younger (31year vs. 36year), more often married or in couple (57.6% vs. 36.9%), students (10.6% vs. 3.8%) or housewives (31.8% vs. 19%), had more often disclosed their serology status to their partners (95.5% vs. 31.7%), and had a shorter duration of known positive serology (5year vs. 6year) and ART before enrollment (3.5year vs. 4.2year). These last two results might be related to the younger age of the participants who became pregnant as it was not associated with any difference in median CD4 nadir, median CD4 count and median VL at enrollment. Sixty pregnancies (71.4%) occurred on Lopinavir and 27 (32.1%) on Darunavir (3 patients were switch from Darunavir to Lopinavir during pregnancy). At the beginning of pregnancy, median CD4 count was 383/mm3 [IQR 297–537] and 66.6% of the women had an undetectable VL (i.e. HIV VL < 50 copies/mL) and results were similar at the end of the pregnancy. Of note, 13 of the 66 women who became pregnant had received injections of medroxyprogesterone before pregnancy. Nevertheless, adherence to this contraception was poor as these women received in median only one dose (IQR 1–3). Seven women received medroxyprogesterone or a levonorgestrel-releasing implant after pregnancy.
Table 1 Characteristics of the 66 women who became pregnant and comparison with the other women enrolled in the 2LADY and MOBIDIP trials
The overall pregnancy rate was 8.03 per 100 WY (95% CI 6.5–9.9) (Table ). Twenty women (7.1%), including 2 pregnant women, were lost to follow-up at the time of the analysis. The highest pregnancy rate was reported in Senegal [10 pregnancies per 100 WY (95% CI 6.4–15.7) vs. 7.5 (95% CI 5.6–9.9) in Cameroon and 7.9 (95% CI 4.9–12.7) in Burkina Faso].
Table 2 Pregnancy and fertility rates
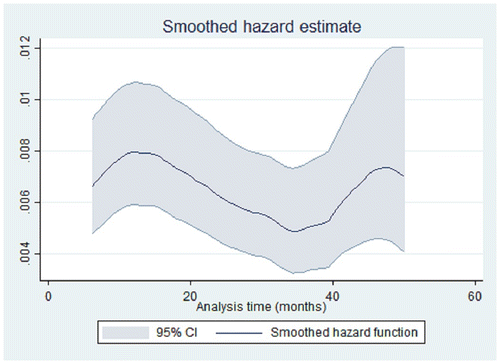
Median time between enrollment and the first pregnancy was 15.7 months [IQR 8.1-28.6]. Incidence of pregnancies was stable during the first two years then declined and peaked again after 4 years of follow-up (partially because of recurrent pregnancies) (Fig. ).
Pregnancy outcome
The 84 pregnancies resulted in 60 (73.2%) live births, 13 (15.8%) miscarriages, 3 (3.6%) stillbirths, 2 (2.4%) extra-uterine pregnancies, and 4 (4.8%) voluntary abortions (Table ). Two women were lost to follow-up during pregnancy. The general fertility rate was 5.73 live births per 100 WY (95% CI 4.45–7.39). The 60 live births (38 female and 22 male babies) were all singleton except for two. The median term was 38 WG (IQR 37–40) and the median birth weight was 2.9 kg (IQR 2.6–3.2). Ten children were born prematurely (16.6%) with a median term of 34 WG [IQR 31–34.5]. Nine children (15%) had a low birth weight, four of them being also premature. Only one birth defect was diagnosed (ankyloglossia) and among the 4 children who necessitated resuscitation care at birth, one died of respiratory distress. One woman died of bleeding at delivery.
Table 3 Pregnancy outcomes
In univariate analysis, unproductive pregnancy was not associated either with age, nadir of CD4 count, duration of ART, CD4 count, or VL at the beginning of pregnancy (Table ). There was a trend in an increasing proportion of miscarriages/stillbirths with exposure to Darunavir compared to Lopinavir (OR: 3.0 [1.0–9.0]). Multivariate analysis showed similar results (OR: 3.1 [0.9–10.1]).
Table 4 Factors associated with miscarriage/stillbirth (logistic regression) voluntary abortions excluded (n = 78)
Discussion
In this study, we estimated that the pregnancy rate among HIV-infected women after enrollment in a phase 3-randomized second-line ART trial at five clinical sites in three West and Central African countries was 8.03/100 WY (95% CI 6.5–9.9). There are limited data on the reproductive behavior of HIV-infected women in West and Central Africa and the few published studies are very heterogeneous in term of population sample, types of data collected, study period, and ART availability which makes comparisons difficult. In a 10-year cohort study from 1998 to 2011, Burgos et al. reported a pregnancy rate of 3.46/100 WY (95% CI 3.1–3.8) in Burkina Faso and of 2.51/100 WY (95% CI 1.0–4.0) in Senegal after ART initiationCitation14, while the global fertility rate in the general population was 20.7 births per 100 women in 2010 in Burkina Faso and 17.2 in Senegal in 2012.Citation15,16 The higher pregnancy rate we recorded might be due in part to methodological differences: notably, as pregnancy was diagnosed by a systematic urinary test, it might have allowed to detect a higher number of pregnancies resulting in spontaneous abortion. Indeed, the observed fertility rate of 5.73 live births per 100 WY (95% CI 4.45–7.39) is close to the one observed in the sub group of HIV-infected women aged between 30 and 34 living in the urban area in a population-based study conducted in 2004 in Cameroon (5.7 births per 100 WY (95% CI 2.3 –11.9)).Citation17 Although not exactly comparable because of different socio-demographic characteristics, our results are also similar to previous observations reported in East Africa: in an Ugandan HIV cohort study conducted from 2003 to 2006, an incident pregnancy rate of 8.2 per 100 PY was observed while, by comparison, the age-specific fertility rate for HIV-negative Ugandan women aged 35–39 was 19.5 births per 100 women.Citation18 Despite all these limitations, it is interesting to note that the reproductive behavior of HIV-infected women enrolled in clinical trials in sub-Saharan Africa appears to be similar to that of women in non-research settings.
Incidence of pregnancies was stable during the first two years of the study period then declined and peaked again after four years of follow-up: explanation for this trend is not clear. Continuity of contraception is difficult to evaluate. No other contraception than condom was reported at enrollment and the understanding of the effect of contraception is limited by the fact that we did not record condom use during the trial. Uptake of injectable hormonal contraception remained limited throughout the study and was not superior among women who did not become pregnant, although all female participants in the 2LADY and MOBIDIP trials had free access to contraception and received ongoing contraceptive counseling. It is interesting to note that overall adherence to the study regimens remained high in this population on second-line treatment with a presumed history of poor compliance to ART as evaluated in the parent studyCitation11 and that there were no difference in CD4 count and VL (which can reflect compliance to ART) between the women who became pregnant and the others. One of the main stated reasons for lack of adherence to contraceptives was the temporary menstrual disorders associated with injectable hormonal contraceptive. Another explanation might be that all women who had a desire of pregnancy had done so in the first 2 years as the second peak of pregnancies observed at the end of the study period was partly driven by second pregnancies. A final hypothesis could attribute the increase in pregnancy incidence to prolonged and effective ART although the impact of ART on fertility is still controversial. Several studies suggested that restoration of health status by ART is associated with increasing pregnancy desire and partial recovery of fertility particularly for those with a stable partner with whom they have disclosed their serological status, as observed in our study.Citation7,8 In a retrospective study in western Uganda, the incidence rate of pregnancy increased progressively over the years after ART initiation from 2.98 pregnancies per 100 PY in 2006 to 12.2 in 2010 (p < 0.001).Citation8 Yet in another study, also conducted in East Africa, the relationship between ART initiation and incident pregnancy seemed nuanced and substantially weaker than previously reported.Citation18
These observations underscore the need to redefine the criteria for interdiction of pregnancy during clinical trials. In the context of strategy/public health trials, using ARV drugs that have received regulatory approval in developed countries since many years without mention of a teratogenic risk in either animal studies or post marketing surveillance, exclusion of pregnant women or women planning to become pregnant should be questioned. The goal of these trials is to assess the effectiveness of antiretroviral combinations adapted to resource-limited settings, easier to take for patients and less expensive, in the context of more frequent co-morbidities and reduced biological monitoring. A generalized surveillance of pregnancies during these trials would strengthen the knowledge of potential drug effects in pregnancy as data are gathered under rigorous scientific conditions without endangering the health of the mother or the child. Moreover, in Sub-Saharan African cultures where social desirability of children is high and motherhood remains central to the feminine identity,Citation2 unjustified interdiction of pregnancy could hinder enrollment in clinical trials for women and exclude them from beneficial research.
On the other hand, the situation is different in the context of therapeutic trials testing molecules in early phase of development or for which the risk of teratogenicity has not been ruled out; in these cases, the high pregnancy rate observed in African settings is problematic and reveal a failure in contraceptive care. The fact that incidence of pregnancy is high since the beginning of the study suggests that importance of avoiding pregnancy during the study period and contraception information were not well understood or accepted by female participants since the very beginning of the trial. A qualitative study aiming to explore the factors contributing to high pregnancy rates among participants in two HIV clinical trials in Uganda showed that despite a consent processes appearing to be effective, there was a general lack of concern about the potential impact of the drug on pregnancy.Citation19 Researchers suggested exploration of alternative methods for evaluation of the understanding of informed consent, such as vignette or narratives, as proposed in HIV vaccine studies.Citation20 In addition, they recommended to review participant’s contraceptive needs and pregnancy desire after a certain period, as desire for children, partners and health status of participants may change over time. In the Ditrame Plus project in Abidjan, tailored contraceptive advices have led to a contraceptive utilization rate of more than 50% (whereas only 6% of women attending to the same maternal and health centers before the study were using contraceptives such as condoms or hormonal contraceptives).Citation21
Furthermore, it is particularly striking to note the occurrence of unwanted pregnancy resulting in voluntary abortion in a non-anecdotic frequency (5% in our study, 12.5% in the Tshepo study and up to 17.5% in the DART trial) in settings such as clinical trials where access to contraception has been facilitated.Citation10,22 These women had to face considerable risks and costs in attempting abortion in countries where this practice is illegal. Several African cohort studies have found a marked discordance between low desire for pregnancy and limited family planning use in women infected by HIV.Citation23 Despite several researches aiming to identify potential barriers limiting the use of efficient contraceptive methods,Citation24,25 these observations point out that integration of contraceptive services in clinical trials still needs optimization. Providing contraceptive services free of charge is clearly not sufficient as observed in the Tshepo Study and in ours, however it seems more effective when provided at the study clinical site rather than in a family planning clinic. Researchers may also need to consider lesser used female contraceptive options like diaphragm or female condoms. Efforts should be intensified in women presenting several risks factors of getting pregnant like, as found in our study and others, young age, being married or in couple, being student or housewife, and having disclosed one’s serology status with their partner. Of note, serology disclosure with family or friends was not associated with pregnancy in our study.
Despite a lower pregnancy rate, the rate of miscarriage/stillbirth among women who conceived in our study was not higher than the one expected in the seronegative population. Most of the pregnancy losses in our study occurred during the first trimester of pregnancy. This is in accordance with previous findings reported in the Women’s Interagency HIV Study after HAART initiation in USCitation26 and in the DART trial in Sub-Saharan Africa.Citation22 We didn’t find any association between these pregnancy outcomes and either age, disease status at diagnosis (estimated by the lowest CD4 count) or at the beginning of pregnancy (estimated by CD4 count and VL measurement), or duration of ART. The trend of a higher risk of unfavorable pregnancy outcome in women exposed to Darunavir may be due to the low number of recorded events (18 pregnancy losses, voluntary abortion excluded) and should be investigated by further studies. However, to our knowledge, there are no published data linking individual ARV medications or classes of ARV medications to spontaneous abortion. Some authors reported increased rates of premature deliveries and low birth weight among HAART-treated women, especially with boosted-PI regimensCitation27 but the involved physiopathologic mechanisms are probably different from those leading to early pregnancy losses. Of note, the observed prematurity and low birth weight rates of 14.8 and 12.9% respectively, are lower than the rates reported in women on PI–based ART in some of the studies conducted in African settings.Citation28,29 It is important that African data from strategy clinical trials like ours continue to be gathered and reported to the Antiretroviral Pregnancy Registry in order to provide early notification of any major adverse effect of ARV drugs associated with a prenatal exposure.
Conclusion
In Sub-Saharan Africa, inclusion in a biomedical trial is not followed by a decrease in fertility rate among women on ART, despite their initial consent to delay any childbearing project for the duration of the study. Researchers and doctors need to take this into account when planning trials in such regions with high fertility rates. According to the level of risk of investigational ARV drugs during pregnancy, interdiction or permission to get pregnant should be discussed in ethics committees, with a better integration of family planning counseling and increased contraception options in case of formal interdiction. Furthermore, the reported rate of miscarriage/stillbirth in this study is not different from those reported in seronegative women, which is reassuring for treated HIV-positive women considering pregnancy.
Contributors
SA, CL, LS, and CJ conceived and designed the study; SA, CA, and DBP analysed the data; SA, CL CA, and LS wrote the article; ZJ, DM, TR, and MNM collected the data and revised the article, KSS and DE obtained the funding and revised the article.
Funding
This works was supported by Département Soutien et Formation, Institut de Recherche pour le Développement (FR) and ANRS Agence Nationale de Recherches sur le Sida et les Hepatites Virales [grant number 12169/12286].
References
- Bongaarts J, Casterline J. Fertility transition: is sub-saharan Africa different? Popul Dev Rev. 2013;38(Suppl. 1):153–168.10.1111/padr.2013.38.issue-s1
- Ferry B, Desgrées du Loû A. Sexualité et procréation confrontées au sida dans les pays du Sud [Sexuality and Procreation Facing AIDS in Developing Countries]. Paris: Les collections du CEPED; 2006.
- Nebié Y, Meda N, Leroy V, et al. Sexual and reproductive life of women informed of their HIV seropositivity: a prospective cohort study in Burkina Faso. J Acquir Immune Defic Syndr. 2001;28(4):367–372.
- Zaba B, Gregson S. Measuring the impact of HIV on fertility in Africa. AIDS Lond Engl. 1998;12(Suppl. 1):S41–50.
- Ross A, Van der Paal L, Lubega R, et al. HIV-1 disease progression and fertility: the incidence of recognized pregnancy and pregnancy outcome in Uganda. AIDS. 2004;18(5):799–804.10.1097/00002030-200403260-00012
- Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105(8):836–848.10.1111/bjo.1998.105.issue-8
- Myer L, Carter RJ, Katyal M, et al. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in sub-saharan Africa: a cohort study. PLoS Med. 2010;7(2):e1000229.10.1371/journal.pmed.1000229
- Kabami J, Turyakira E, Biraro S, Bajunirwe F. Increasing incidence of pregnancy among women receiving HIV care and treatment at a large urban facility in western Uganda. Reprod. Health. 2014;11:81.10.1186/1742-4755-11-81
- Gac SL, Coutherut J, Desclaux A, et al. Réalités et enjeux de la participation des femmes dans les essais cliniques sur les antirétroviraux: expérience au Sénégal [Realities and challenges in the participation of women in ART clinical trials: experience in Senegal]. 2011;219–32.
- Bussmann H, Wester CW, Wester CN, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007;45(3):269–73.
- Ciaffi L, Koulla-Shiro S, Sawadogo A, et al. Efficacy and safety of three second-line antiretroviral regimens in HIV-infected patients in Africa. AIDS. 2015;29(12):1473–1481.10.1097/QAD.0000000000000709
- Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. The Lancet. 2014;384(9946):857–868.10.1016/S0140-6736(14)60932-6
- Burgos-Soto J, Balestre E, Minga A, et al. Incidence of pregnancy after antiretroviral therapy initiation and associated factors in 8 West African countries. J Acquir Immune Defic Syndr. 2014;67(2):e45–e54.10.1097/QAI.0000000000000279
- Institut National de la Statistique et de la Démographie (INSD) [Burkina Faso] et ICF International. Enquête Démographique et de Santé et à Indicateurs Multiples (EDSBF-MICS IV) [Multiple indicators cluster demographic and health survey]. Calverton, MD: INSD et ICF International; 2010.
- Agence Nationale de la Statistique et de la Démographie (ANSD) [Sénégal], et ICF International. Enquête Démographique et de Santé Continue (EDS-Continue 2012–2013) [Demographic and Health Survey]. Calverton, MD: ANSD et ICF International; 2012–2013.
- Kongnyuy EJ, Wiysonge CS. Association between fertility and HIV status: what implications for HIV estimates? BMC Public Health. 2008;8:309.10.1186/1471-2458-8-309
- Homsy J, Bunnell R, Moore D, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS One. 2009;4(1):e4149.10.1371/journal.pone.0004149
- Untangling the relationship between antiretroviral therapy use and incident pregnancy: a marginal structural model analysis using data From 47,313 PubMed – NCBI [Internet]. [cited 2016 Jun 26]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=untangling+the+relationship+between+antiretroviral+therapy+use+and+incident+pregnancy+%3A+a+marginal+structural+model+analysis
- Ssali A, Namukwaya S, Bufumbo L, et al. Pregnancy in HIV clinical trials in sub saharan Africa: failure of consent or contraception? PLoS One. 2013;8(9):e73556.10.1371/journal.pone.0073556
- Lindegger G, Milford C, Slack C, et al. Beyond the checklist: assessing understanding for HIV vaccine trial participation in South Africa. J Acquir Immune Defic Syndr. 2006;43(5):560–566.
- Brou H, Viho I, Djohan G, et al. Contraceptive use and incidence of pregnancy among women after HIV testing in Abidjan, Ivory Coast. Rev Dépidémiologie Santé Publique [Epidemiology and public health]. 2009;57(2):77–86.10.1016/j.respe.2008.12.011
- Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9(5):e1001217.10.1371/journal.pmed.1001217
- King R, Khana K, Nakayiwa S, et al. ‘Pregnancy comes accidentally – like it did with me’: reproductive decisions among women on ART and their partners in rural Uganda. BMC Public Health. 2011;11:530.10.1186/1471-2458-11-530
- Cates W, Steiner MJ. Dual protection against unintended pregnancy and sexually transmitted infections: what is the best contraceptive approach? Sex Transm Dis. 2002;29(3):168–174.10.1097/00007435-200203000-00007
- Anglewicz P, Clark S. The effect of marriage and HIV risks on condom use acceptability in rural Malawi. Soc Sci Med. 2013;97(97):29–40.10.1016/j.socscimed.2013.06.024
- Massad LS, Springer G, Jacobson L, et al. Pregnancy rates and predictors of conception, miscarriage and abortion in US women with HIV. AIDS. 2004;18(2):281–286.10.1097/00002030-200401230-00018
- Sibiude J, Warszawski J, Tubiana R, et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis. 2012;54(9):1348–1360.10.1093/cid/cis198
- Powis KM, Kitch D, Ogwu A, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204(4):506–514.10.1093/infdis/jir307
- Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294.10.1056/NEJMoa0907736