Abstract
Objective: HIV/HCV-coinfected patients and hepatitis C virus (HCV) monoinfected subjects are thought to respond equally to direct-acting antiviral (DAA)-based therapy despite the lack of data derived from clinical trials. This study is aimed to evaluate the impact of HIV coinfection on the response to DAA-based treatment against HCV infection in the clinical practice.
Patients and Methods: In a prospective multicohort study, patients who initiated DAA-based therapy at the Infectious Disease Units of 33 hospitals throughout Spain were included. The primary efficacy outcome variables were the achievement of sustained virologic response 12 weeks after the scheduled end of therapy date (SVR12).
Results: A total of 908 individuals had reached the SVR12 evaluation time-point, 426 (46.9%) were HIV/HCV-coinfected, and 472 (52%) received interferon (IFN)-free therapy. In an intention-to-treat analysis, SVR12 rates in subjects with and without HIV-coinfection were 55.3% (94/170 patients) versus 67.3% (179/266 subjects; p = 0.012) for IFN-based treatment and 86.3% (221/256 subjects) versus 94.9% (205/216 patients, p = 0.002) for IFN-free regimens. Relapse after end-of-treatment response to IFN-free therapy was observed in 3/208 (1.4%) HCV-monoinfected subjects and 10/231 (4.4%) HIV/HCV-coinfected individuals (p = 0.075). In a multivariate analysis adjusted for age, sex, transmission route, body-mass index, HCV genotype, and cirrhosis, the absence of HIV-coinfection (adjusted odds ratio: 3.367; 95% confidence interval: 1.15-9.854; p = 0.027) was independently associated with SVR12 to IFN-free therapy.
Conclusions: HIV-coinfection is associated with worse response to DAA-based therapy against HCV infection. In patients receiving IFN-free therapy, this fact seems to be mainly driven by a higher rate of relapses among HIV-coinfected subjects.
Introduction
Infection with HIV has a negative impact on the natural history of hepatitis C virus (HCV)-induced liver disease,Citation1 which is not fully compensated by antiretroviral therapy.Citation2 Likewise, rates of sustained virologic response (SVR) to treatment against HCV infection with pegylated interferon plus ribavirin (PR) are lower in HIV/HCV coinfection as compared to HCV monoinfection, especially among difficult-to-treat patients. In this context, data derived from different clinical trials gave reason to assume that response rates to dual therapy with PR were lower for the HIV/HCV-coinfected population, which could be confirmed in a large comparative real-life study.Citation3
Figure 1 Response to therapy including one or more direct-acting antiviral. (A) Triple therapy based on one direct-acting antiviral in combination with pegylated interferon plus ribavirin. *Calculated for those who had reached end-of-treatment response [HIV (–): n = 200; HIV (+): n = 105]; (B) Interferon-free therapy including at least two direct-acting antivirals with or without ribavirin. *Calculated for those who had reached end-of-treatment response [HIV (–): n = 208; HIV (+): n = 231]. Dark bars: HCV-monoinfected patients; light bars: HIV/HCV-coinfected patients; SVR12: Sustained virologic response 12 weeks after scheduled end-of-therapy. AE: adverse events; LTFU: lost to follow-up
![Figure 1 Response to therapy including one or more direct-acting antiviral. (A) Triple therapy based on one direct-acting antiviral in combination with pegylated interferon plus ribavirin. *Calculated for those who had reached end-of-treatment response [HIV (–): n = 200; HIV (+): n = 105]; (B) Interferon-free therapy including at least two direct-acting antivirals with or without ribavirin. *Calculated for those who had reached end-of-treatment response [HIV (–): n = 208; HIV (+): n = 231]. Dark bars: HCV-monoinfected patients; light bars: HIV/HCV-coinfected patients; SVR12: Sustained virologic response 12 weeks after scheduled end-of-therapy. AE: adverse events; LTFU: lost to follow-up](/cms/asset/9ce09478-e840-4c2b-bd25-7095dc1237c3/yhct_a_1330801_f0001_b.gif)
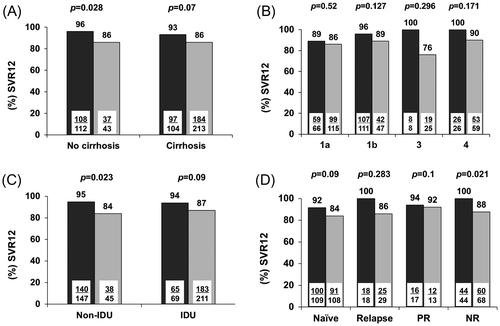
Figure 2 Rates of sustained virologic response 12 weeks after scheduled end-of-therapy (SVR12) to interferon-free, all oral treatment regimens including at least two direct-acting antivirals with or without ribavirin according to (A) baseline cirrhosis, (B) HCV genotype, (C) prior injecting drug use (IDU) and (D) response to previous therapy with pegylated interferon plus ribavirin in an intention-to-treat approach. Dark bars: HCV-monoinfected patients; light bars: HIV/HCV-coinfected patients; SVR12: Sustained virologic response 12 weeks after scheduled end-of-therapy. PR: partial response; NR: null response
With the arrival of the firstly approved direct-acting antivirals (DAA), the impact of HIV-infection on treatment response may have changed. In fact, it is widely assumed that response rates of HIV/HCV-coinfected patients are equal to HCV-monoinfected subjects.Citation4–8 This conclusion was drawn by comparing different clinical trials, as well as data derived from real-life studiesCitation9–12. In these, HIV/HCV-coinfected and HCV-monoinfected patients in most cases attended different clinical units and were cared for by different physicians, thus receiving non-homogeneous clinical management. The assumption is manifested in Spanish national, as well as international guidelines,Citation4, 5 which stopped differentiating between HCV-monoinfection and HIV/HCV-coinfection. Thus, the current recommendation is that treatment should be managed disregarding their coinfection status. However, adequately designed comparative studies are warranted in order to evaluate whether those treatment strategies that could imply a higher risk of failure, such as shortening therapy or ribavirin free combinations, are equally valid for monoinfected and coinfected patients. Furthermore, real-life data obtained regarding this topic are of special interest, since less adherence, as well as a patient supervision less strict than conducted in clinical trials, could be the key to the detection of differences in real life that may not be identified in clinical trials.
Therefore, the aim of this study was to evaluate the impact of HIV coinfection on the response to DAA-based treatment against HCV infection in the clinical practice.
Patients and methods
Study design and population
In a prospective multicohort study, HIV/HCV-coinfected patients (HEPAVIR-DAA Cohort; ClinicalTrials.gov ID: NCT02057003) and HCV-monoinfected individuals (GEHEP-MONO Cohort; ClinicalTrials.gov ID: NCT02333292) who initiated any DAA-based therapy at the Infectious Diseases units of 33 hospitals throughout Spain since October 2011 were included. All 33 centers which participated in both cohorts and candidates for IFN-free treatment were uniformly selected according to the criteria of a nation-wide program for HCV therapy.Citation13 According to the protocol, clinical visits are conducted at least at baseline, at treatment weeks 4, 12, and every 12 weeks thereafter, if applicable, as well as 12 weeks and 24 weeks after the scheduled end of treatment. Hematological, biochemical, and clinical determinations are carried out at each visit. Those subjects who had reached the scheduled time point for assessing SVR 12 weeks after completing therapy (SVR12) were included in the present study.
Regimens, treatment groups, and definition of response
All DAA-containing regimens that have been approved in Spain and thus have been available in the clinical practice are considered for this analysis if they included at least two DAA or one DAA in combination with PR. These regimens consisted of i) triple-drug regimens including pegylated interferon (IFN) alfa-2a or alfa-2b plus oral ribavirin (RBV) in combination with either the NS3/4A protease inhibitor (PI) boceprevir (BOC), telaprevir (TVR) or simeprevir (SMV), or the NS5B inhibitor sofosbuvir (SOF), or ii) IFN-free combinations of either SOF/SMV, SOF/daclatasvir (DCV), SOF/ledipasvir (LED), ritonavir-boosted paritaprevir (PTV/r) plus ombitasvir (OBT/r; collectively referred to as 2D) or PTV/r/OBT plus dasabuvir (collectively referred to as 3D). Previous response to dual therapy including PR was categorized as described elsewhere.Citation14 Coadministration of RBV to IFN-free regimens, futility rules for the IFN-based regimens, as well as treatment durations were in accordance with Spanish national and international guidelines.Citation4, 5 End-of-treatment response (ETR) was defined as undetectable HCV RNA at the scheduled end of therapy. SVR12 was defined as undetectable HCV RNA 12 weeks after the scheduled end of therapy. Non-response to IFN-based regimens was considered when predefined stopping rules were fulfilled.Citation4, 5 Viral breakthrough was considered when HCV RNA was detectable following undetectability during therapy. Detectable HCV RNA at the SVR12 evaluation time point after achieving ETR was considered as relapse. Adverse events were managed according to guidelines, and, ultimately, according to the criteria of the caring physician.
Laboratory determinations and definition of cirrhosis
Plasma HCV RNA levels were determined as described elsewhere.Citation14 The presence of cirrhosis was evaluated according to liver biopsy, applying the Scheuer Index.Citation15 If liver biopsy was not available, a baseline liver stiffness value equal to or above 12.5 kPa as determined by transient elastometry (Fibroscan, Echosense, Paris, France) was considered as cirrhosis, according to the criteria used in most clinical trials where DAA have been evaluated.
Statistical analysis
The primary efficacy endpoint was the achievement of SVR12 using an intention-to-treat approach. An on-treatment approach was conducted as secondary sensitivity analysis for SVR12. Here, those patients who discontinued therapy due to adverse events, those who voluntarily dropped out and those who were not evaluable were excluded. Categorical variables were expressed as number (percentage) and continuous variables were expressed as median (quartile 1-quartile 3). The frequencies of subjects with the primary endpoints according to HIV coinfection were assessed. The Student’s t-test or the Mann-Whitney U-test were carried out for comparisons of continuous variables, when applicable, while the χ2 test or the Fisher’s exact test was used for the analysis of categorical variables. Subsequently, those factors that were associated with SVR12 with a p < 0.2 in the univariate analysis, as well as other known predictors of SVR, were entered in a multivariate logistic regression analysis, adjusting for age and sex. Statistical analysis was performed using the SPSS statistical software package release 23.0 (IBM, Chicago, IL, USA).
Ethical aspects
The study was designed and performed according to the Helsinki declaration and was approved by the Ethics Committee of the Valme University Hospital (Seville, Spain). All patients gave their written informed consent before being included in the study.
Results
Patient characteristics and regimens
Of the 1411 subjects included in the cohorts, 908 (63%) individuals had reached the scheduled SVR12 evaluation time point at the moment of analysis (November 2015) and were included in this study. In the overall population, 426 (46.9%) patients were HIV/HCV-coinfected. Baseline characteristics are listed in Table , while the numbers of patients who received specific regimens are shown in Table . RBV was coadministered in 109 (51%) of the HCV-monoinfected versus 118 (46%) of the HIV/HCV-coinfected individuals (p = 0.344) among those who received IFN-free therapy. Among those HIV/HCV-coinfected patients who received IFN-based or IFN-free therapy, CD4 cell counts (Q1-Q3) were 551 (367-772) cells/mL and 438 (240-722) cells/mL, while baseline plasma HIV RNA was undetectable in 159 (93.5%) subjects and 243 (94.9%) individuals, respectively.
Table 1 Baseline characteristics of the study populations
Table 2 Treatment regimens initiated according to HIV infection status
Response to therapy
In the intention to treat analysis, SVR12 rates in subjects with and without HIV-coinfection were 55.3% (94/170 patients) versus 67.3% (179/266 subjects; p = 0.012) for IFN-based treatment and, 86.3% (221/256 subjects) versus 94.9% (205/216 patients, p = 0.002) for IFN-free regimens, respectively. ETR was attained by 305 (70%) patients of those patients who received IFN-based therapy and 208 (93%) subjects of those who were treated with IFN-free regimens. SVR12 rates and other treatment outcomes after therapy including IFN, as well as IFN-free all oral regimens, are shown in Figure 1A and 1B, respectively. After stratifying the cirrhotic patients of the IFN-free group for the baseline LS, the proportions of HCV-monoinfected versus HIV/HCV-coinfected subjects who achieved SVR12 were 147/152 (96.7%) versus 98/111 (88.3%; p = 0.008) among those with an LS < 21 kPa. Among those who presented an LS > 21 kPa, 51/56 (91.1%) versus 113/131 (86.3%; p = 0.359) presented SVR12. Likewise, among cirrhotic patients with a baseline Child-Pugh-Turcotte (CPT) score A, 84/90 (93.3%) monoinfected versus 149/166 (89.8%) HIV/HCV-coinfected patients showed SVR12 (p = 0.663), while among those with a CPT score B or C, 8/9 (88.9%) HCV-monoinfected versus 32/42 (76.2%) coinfected individuals reached SVR12 (p = 0.34). After stratifying for sex, corresponding figures were 137/146 (93.8%) versus 181/206 (87.9%; p = 0.062) among male subjects and 68/70 (97.1%) versus 40/50 (80%; p = 0.002) among female individuals, respectively. The impact of HIV coinfection on SVR12 after stratifying for other baseline parameters is presented in Figure 2. Four (0.8%) patients discontinued therapy due to adverse events in the IFN-free group; all of them were HIV-coinfected. Table displays characteristics of those individuals who did not achieve SVR12.
Table 3 Characteristics of the patients who failed to achieve sustained virologic response to interferon-free regimens according to HIV coinfection status
In an on-treatment analysis of those individuals who received IFN-free therapy, 205 (98.6%) HCV-monoinfected patients versus 221 (94.8%) HIV/HCV-coinfected individuals achieved SVR12 (p = 0.032) and none versus 2 (0.9%) individuals showed viral breakthrough (p = 0.5). Among those patients who presented ETR, 3 (1.4%) HCV-monoinfected versus 10 (4.4%) HIV/HCV-coinfected subjects experienced a relapse (p = 0.075). Ten out of the 13 (77%) patients who relapsed presented detectable HCV-RNA in week 4 post-treatment, while in the remaining three, viremia was not analyzed until week 12 post-treatment, where they showed detectable HCV-RNA. In all 13 patients, risk factors for HCV infection during the period before viral rebound could be excluded, as these subjects had stopped using drug before treatment and did so during the immediate follow-up period. Additionally, reinfection could be excluded by genotype determination and/or phylogenetic analyses in all five patients who had this data available.
Predictors of response
In the group receiving IFN-based therapy, a multivariate analysis adjusted for age, sex, IL28B, and HCV genotype, presence of baseline cirrhosis, previous therapy and the body-mass index was conducted. Here, absence of HIV coinfection was independently associated with SVR12 (adjusted odds ratio: 2.742; 95% confidence interval: 1.424-5.279; p = 0.003). For IFN-free therapy, lack of HIV infection was also an independent predictor of SVR12 (adjusted odds ratio: 3.367; 95% confidence interval: 3.367 (1.15-9.854); p = 0.027). Detailed univariate and multivariate analysis of potential predictors of response in this subset are depicted in Table .
Table 4 Univariate and multivariate analysis to assess predictors of sustained virologic response 12 weeks after the scheduled end of therapy (SVR12) to all-oral, interferon-free regimens including at least two direct-acting antivirals in an intention-to-treat analysis
Discussion
The present study shows that patients with HIV-coinfection respond worse to DAA-based therapy. While in patients who were treated with interferon-based combinations this was due to a lower rate of primary non-response, with all oral DAA including combinations this difference is mainly driven by a higher frequency of relapses among HIV/HCV-coinfected subjects.
It has been demonstrated that response to traditional dual therapy comprising PR is lower in the HIV/HCV-coinfected population when directly compared with HCV-monoinfection within the same clinical setting.Citation3 Comparative data on IFN-based triple therapy including a NS3/4A PI is only available in a population receiving telaprevir, where only 33 patients were HIV infected.Citation16 Likewise, comparative data for all-oral, IFN-free regimens are scarce. Trials conducted in HIV/HCV-coinfected patients yield SVR rates that oscillate around 95%,Citation17–20 thus, similar to response rates obtained from monoinfection trials with a similar design.Citation21–23 However, the accuracy of comparing these results is limited due to possible differences in patient characteristics. Limited data on the impact of HIV on treatment response within one single trial are only available from phase 2 studies with grazoprevir plus elbasvir,Citation24 and comparative real-life data include low numbers of patients.Citation25 In the herein presented study, an independent impact of the presence of HIV coinfection on response to DAA-based regimens, with or without IFN, was detected in a large population in which patients were treated within the same centers by the same physicians, following a single protocol.
There are a number of possible reasons why HIV-coinfected patients may show lower response rates to IFN-free therapy as compared to HCV-monoinfected patients. The main cause could be a higher proportion of cirrhotics in the coinfected population, a predictor of worse response to IFN-free regimens.Citation18, 21 Likewise, the coinfected population featured a higher proportion of males and HCV genotype 1a infections. However, in analyses stratified for potential factors that impact on response, including the presence of cirrhosis and HCV genotype, HIV infection maintained its impact on SVR. Furthermore, adjusting for these covariates in the logistic regression model, HIV infection was identified as independent predictor of not achieving SVR. It is however to note that the larger representation of unfavorable factors for SVR12 among the HIV coinfected patients may cause that multivariate techniques might be insufficient to account for the differences observed. Clearly, randomized clinical trials to elucidate this issue are warranted. Although the rate of voluntary drop-outs tended to be higher in HIV-infected patients and the proportion of prior injecting drug users was higher among the HIV-coinfected individuals without SVR12 as compared to the monoinfected patients, it is also unlikely that differences in adherence underlay the distinct response rates. The vast majority of the HIV-infected patients presented undetectable HIV-RNA, demonstrating high adherence in this population. Therefore, it may be reasonable to hypothesize that the cause of lower SVR rates to DAA-based therapy, as well as higher relapse rates, in HIV/HCV-coinfected subjects are based on the effect of HIV on the immune system and its response to HCV-infection.Citation26
The currently recommended treatment duration for diverse DAA combinations, as well as the use of RBV, is based on the probability of presenting SVR or relapse in each individualized patient. In this context, subjects with a lower probability to relapse are candidates for shortening specific treatments to up to 8 weeksCitation4, 5, 27 or for being treated with RBV-free regimens.Citation28, 29 Importantly, the present work demonstrates that HIV-coinfected patients are still at higher risk of relapse. Therefore, recommendations should not be directly extrapolated to the HIV/HCV-coinfected population until data derived from specific clinical trials including coinfected subjects are available. This is particularly important for regimens that could be associated with a higher probability of relapse, as it is the case for shorter treatment duration or the sparing of RBV in patients with advanced liver damage. Results from the ALLY-2 clinical trial conducted in coinfected patients and in which shortening therapy to 8 weeks was associated with higher relapse rates support the former conclusion.Citation18
This study has limitations. Firstly, due to its observational design, the study is susceptible to the inherent biases of this type of work. Still, a very high number of patients are included in the cohort, with a highly homogeneous follow-up and patient management. In addition, selection of patients for IFN-free treatment was done following the criteria of a nation-wide programme for HCV therapy.Citation13 This represents the main strength of the study and partly compensates the prior mentioned limitation. Secondly, since phylogenetic analyses at baseline and at the moment of reappearance of HCV-RNA could only be conducted in a limited number of patients who relapsed, theoretically, some cases considered as relapses could potentially have been reinfections. However, relapse could be confirmed in all five patients who underwent this analysis. Additionally, the recurrence of HCV-RNA occurred very early in the post-treatment follow-up. In our area, most of the HIV/HCV-coinfected patients treated against HCV infection are prior injecting drug users who receive treatment once they stopped drug abuse. In this population, a backslide to illicit parenteral drug use is unusual in the short-term, while the recent epidemics of reinfections occur in men who have sex with men. Because of this, in our area reinfections are uncommon and they are usually seen longer thereafter.Citation30 Therefore, reinfection can be reasonably discarded in these patients since they were likely not exposed to HCV before HCV-RNA re-emergence.Citation30
In summary, this study shows for the first time that HIV/HCV-coinfected patients show a lower rate of SVR to all-oral DAA combinations and that the likelihood of relapse is higher in this population. This finding should be taken into account when tailoring the composition and duration of HCV treatment in such a subset. Specifically, strategies with higher risk of relapse recommended on the basis of studies conducted in HCV monoinfected patients should be avoided in this population, unless clinical trials specifically performed in coinfected patients prove their efficacy.
Disclosure statement
K.N. has received lecture fees from Janssen-Cilag, Roche, Bristol-Meyers Squibb and Merck Sharp & Dohme and has received research support from Janssen-Cilag, Bristol-Meyers Squibb, Merck Sharp & Dohme, Gilead Sciences and Abbott Pharmaceuticals. A.R.J has received lecture fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, ViiV Healthcare and Roche. A.C. reports having received consultancy fees from Janssen-Cilag, Merck Sharp & Dohme, Bristol-Myers Squibb, Gilead Sciences, Pfizer and AbbVie. S.R.-B. has received consulting fees from Gilead Sciences, Janssen-Cilag, Abbvie, Bristol-Myers Squibb, Pfizer and Merck-Sharp & Dome. M.M. reports having received consulting fees from Gilead Sciences and Janssen-Cilag and lecture fees from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck-Sharp & Dome and ViiV Healthcare. J.A.P. reports having received consulting fees from Bristol-Myers Squibb, Abbvie, Gilead Sciences, Merck Sharp & Dohme, and Janssen-Cilag. He has received research support from ViiV Healthcare, Bristol-Myers Squibb, Abbvie, Gilead Sciences, Pfizer and Merck Sharpe & Dohme. He has received lecture fees from ViiV Healthcare, Bristol-Myers Squibb, Abbvie, Gilead Sciences, Merck Sharp & Dohme and Janssen-Cilag.
Funding
This work has been partially funded by the RD12/0017/0012 project as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación, the Fondo Europeo de Desarrollo Regional (FEDER) and the Consejería de Salud of the Junta de Andalucía (grant numbers AC-0095-2013 and PI-0492-2012). K.N. is the recipient of a Miguel Servet research grant from the Instituto de Salud Carlos III (grant number CP13/00187). A.R.-J. is the recipient of a post-doctoral perfection grant from the Consejería de Salud of the Junta de Andalucía (grant number RH-0024/2013). M.M. is the recipient of a grant from the Ministerio de Economía y Competitividad (PTA2014-09009-I). J.A.P. is the recipient of an intensification grant from the Instituto de Salud Carlos III (grant number Programa-I3SNS).
Notes
On behalf of the Grupo de Estudio de Hepatitis Vírica, of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica: GEHEP-SEIMC and Grupo de Estudio de Hepatitis Vírica, of the Sociedad Andaluza de Enfermedades Infecciosas y Microbiología Clínica: HEPAVIR/Red de Investigación en SIDA (RIS-HEP07).
References
- Macías J, Berenguer J, Japón MA, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50(4):1056–1063.10.1002/hep.23136
- Lo Re V 3rd, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160(6):69–79.
- Monje-Agudo P, Castro-Iglesias A, Rivero-Juárez A, et al. Impact of HIV infection on sustained virological response to treatment against hepatitis C virus with pegylated interferon plus ribavirin. Eur J Clin Microbiol Infect Dis. 2015;34(10):1929–1936.10.1007/s10096-015-2434-6
- Documento de consenso del Grupo Español para el Estudio de la Hepatitis (GEHEP) sobre el tratamiento de la hepatitis C. http://www.seimc.org/contenidos/gruposdeestudio/gehep/dcientificos/documentos/gehep-dc-2014-TratamientodelaHepatitisC_actualizacionJun2015.pdf. Accessed December 4, 2016.
- European Association for the Study of the Liver (EASL). EASL Recommendations on Treatment of Hepatitis C 2015. http://www.easl.eu/medias/cpg/HEPC-2015/Full-report.pdf. Accessed December 4, 2016.
- Rockstroh JK. Does HIV remain a risk factor for achieving sustained virologic response under direct acting antiviral-based modern hepatitis C virus therapy? Clin Infect Dis. 2014;59(12):1777–1778.10.1093/cid/ciu662
- Soriano V, Sherman KE, Rockstroh J, et al. Challenges and opportunities for hepatitis C drug development in HIV-hepatitis C virus-co-infected patients. AIDS. 2011;25(18):2197–2208.10.1097/QAD.0b013e32834bbb90
- Shafran D. HIV coinfected have similar SVR rates as HCV monoinfected with DAAs: it’s time to end segregation and integrate HIV patients into HCV trials. Clin Infect Dis. 2015;61(7):1127–1134.10.1093/cid/civ438
- Poordad F, McCone J Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–1206.10.1056/NEJMoa1010494
- Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.10.1056/NEJMoa1012912
- Cotte L, Braun J, Lascoux-Combe C, et al. Telaprevir for HIV/hepatitis C virus–coinfected patients failing treatment with pegylated interferon/ribavirin (ANRS HC26TelapreVIH): an open-label, single-arm, phase 2 trial. Clin Infect Dis 2014(12);59:1768–1776.10.1093/cid/ciu659
- Hézode C, Fontaine H, Dorival C, et al. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147(1):132–142.e4.10.1053/j.gastro.2014.03.051
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Therapeutic strategy for chronic hepatitis caused by hepatitis C virus in the National Health System: general recommendations and current treatment regimens. http://aeeh.es/wp-content/uploads/2015/04/a77478e2a1147600cb9979b5992281cb.pdf. Accessed December 4, 2016.
- Neukam K, Munteanu DI, Rivero-Juárez A, et al. Boceprevir or telaprevir based triple therapy against chronic hepatitis C in HIV coinfection: real-life safety and efficacy. PLoS One. 2015;10(4):e0125080.10.1371/journal.pone.0125080
- Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13(3):372–374.10.1016/0168-8278(91)90084-O
- Martel-Laferrière V, Brinkley S, Bichoupan K, et al. Virological response rates for telaprevir-based hepatitis C triple therapy in patients with and without HIV coinfection. HIV Med. 2014;15(2):108–115.10.1111/hiv.2014.15.issue-2
- Naggie S, Cooper C, Saag M, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med;373(8):705–713.
- Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):714–725.10.1056/NEJMoa1503153
- Osinusi A, Townsend K, Kohli A, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA. 2015;313(12):1232–1239.10.1001/jama.2015.1373
- Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313(12):1223–1231.10.1001/jama.2015.1328
- Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014(16);370:1483–1493.10.1056/NEJMoa1316366
- Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898.10.1056/NEJMoa1402454
- Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrosis. N Engl J Med. 2014;370(21):1973–1982.10.1056/NEJMoa1402869
- Sulkowski MS, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1087–1097.10.1016/S0140-6736(14)61793-1
- Milazzo L, Lai A, Calvi E, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med 2016; doi:10.1111/hiv.12429. [ Epub ahead of print].
- Goeser F, Glässner A, Kokordelis P, et al. HIV mono-infection is associated with an impaired anti-HCV activity of NK cells. AIDS. 2016;30(3):355–363.
- Kowdley K, Gordon SC, Reddy KJ, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888.10.1056/NEJMoa1402355
- Feld JJ, Moreno C, Trinh R, et al. Sustained virologic response of 100% in HCV genotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12weeks. J Hepatol. 2016;64(2):301–307.10.1016/j.jhep.2015.10.005
- Recommendations for testing, managing, and treating hepatitis C. Joint panel from the American Association of the Study of Liver Diseases and the Infectious Diseases Society of America. January 2014 http://www.hcvguidelines.org/. Accessed December 4, 2016.
- Pineda JA, Núñez-Torres R, Téllez F, et al. Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. J Infect. 2015;71(5):571–577.10.1016/j.jinf.2015.07.006
