Abstract
Background: VERxVE data showed non-inferior virologic efficacy with extended release nevirapine (NVP-XR) dosed 400 mg once daily (QD) versus immediate release nevirapine (NVP-IR) 200 mg twice daily in a double-blind, non-inferiority study in treatment-naïve HIV-1-positive patients.
Objective: To study the pharmacokinetics (PK) of the NVP formulations and identify possible associations with demographic factors.
Methods: Patients with viral load ≥1000 copies/mL and CD4+ count > 50– <400 cells/mm3 (males) and >50– <250 cells/mm3 (females) at screening received NVP-IR 200 mg QD during a 14-day lead-in and were then stratified by baseline viral load and randomized to NVP-XR or -IR. NVP trough concentrations at steady state (SS) (Cpre,ss,N) were measured up to week 48 for all participating patients. In a PK sub-study, SS parameters – AUC0–24, Cmax, Cmin, and peak-to-trough fluctuation were obtained and analyzed with relative bioavailability assessed at week 4 by plasma collection over 24 h.
Results: Trough concentrations were stable from week 4 to week 48 for all patients (n = 1011) with both formulations, with NVP-XR/IR ratios of 0.77–0.82. Overall, 49 patients completed the PK sub-study: 24 XR and 25 IR. NVP-XR showed less peak-to-trough fluctuation (34.5%) than IR (55.2%), and lower AUC0–24, Cmin, Cmax, and trough concentrations than IR. However, no effect of SS trough concentrations was found on the virologic response proportion at least up to 1000 ng/mL. No significant association was found between NVP PK and gender, race, and viral load.
Conclusion: These data suggest NVP-XR achieves lower but effective NVP exposure compared with NVP-IR.
Introduction
Non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy has been a popular first regimen of choice for treating human immunodeficiency virus (HIV) infection. Historically, the two most used NNRTIs are nevirapine immediate release (NVP-IR) and efavirenz.Citation1–3 NVP has a well-characterized efficacy, safety, and tolerability profileCitation4–6 and is currently prescribed as 200 mg once daily (QD) for the first 14 days and 200 mg twice daily (BID) subsequently.
Considering the substantial pill burden on patients, fixed-dose combinations are recommended as standard of care in HIV, particularly in treatment-naïve patients. Further, clinical studies have shown that treatment adherence is improved with QD versus BID dosing regimens.Citation7–9 A recent meta-analysis of 11 randomized controlled clinical trials reported that QD dosing regimens significantly improve adherence to antiretroviral therapy compared with more complex regimens.Citation10 Importantly, a previous pharmacokinetics (PK) study demonstrated that at steady state, exposure to NVP (area under the concentration–time curve from zero to 24 h [AUC0-24] was not significantly different with NVP-IR 400 mg QD versus 200 mg BID dosing regimen, but peak plasma NVP concentrations were higher (maximum concentration [Cmax]: 6.7 vs. 5.4 μg/mL) and trough concentrations were lower (minimum concentration [Cmin]: 2.9 vs. 3.7 μg/mL)Citation11 for the QD regimen. A QD extended release NVP formulation (NVP-XR) would allow dosing symmetry with QD nucleoside reverse transcriptase inhibitors (NRTIs), potentially increasing adherence and the resultant efficacy.
NVP has demonstrated absorption throughout the intestinal tract using the Enterion™ site-specific delivery capsule,Citation12 making it suitable for development as an XR formulation. A study evaluating slow release NVP 300 and 400 mg prototype tablet formulations demonstrated robust predictions of in vivo profiles based on in vitro dissolution profiles.Citation13 Subsequently, a study in HIV-1 patients investigating the PK characteristics and safety profile of 400 or 300 mg NVP-XR formulation tablets dosed QD found that both doses resulted in delayed absorption and lower peak plasma concentrations of NVP at steady state compared with NVP-IR BID, but importantly, similar trough plasma concentrations.Citation14 Based on these results, the NVP-XR 400 mg formulation was selected for further clinical investigations in the phase III VERxVE and TRANxITION trials.Citation14–16 The VERxVE trial compared the efficacy of treatment with 400 mg NVP-XR dosed QD versus 200-mg BID of the conventional IR formulation in 1011 patients.Citation15 Both NVP formulations were dosed in combination with NRTIs, emtricitabine and tenofovir, in an antiretroviral therapy-naïve study population with specific CD4+ cell count ranges for males and females as recommended for NVP treatment.Citation3,17 NVP-XR dosed QD was also shown to be non-inferior to NVP-IR dosed BID, with a similar safety and adverse event profile.Citation15 On the basis of these results, the NVP-XR formulation was approved in the United States, Canada, the European Union, and several other countries.
Despite previous research on the association of patient demographics with the PK of NNRTIs,Citation18–22 there is no clear consensus on whether demographic factors like race or gender are significant determinants of NVP concentrations.Citation19 As part of the VERxVE trial, NVP pre-dose trough concentrations trough concentrations (Ctrough) for all participating patients were collected in an optional PK sub-study assessing steady-state PK parameters. This article focuses on the PK data from the trial and determines PK profiles and Ctrough in relation to patient demographics and baseline characteristics, in order to identify possible associations.
Methods
Study design and patient population
VERxVECitation15 was a randomized, double-blind, double-dummy, active controlled trial which evaluated the PK of NVP-XR 400 mg QD in comparison to NVP-IR 200 mg BID, both in combination with tenofovir/emtricitabine. The trial was conducted in accordance with the International guidelines and regulations and all participants provided written informed consent.Citation15
Eligible patientsCitation15 were treated with NVP-IR 200 mg QD during a 14-day lead-in period, and were then stratified by viral load (VL) at baseline (≤100,000 vs. >100,000 copies/mL). Patients were randomized 1:1 within each stratum to receive NVP-XR 400 mg QD (plus placebo) or NVP-IR 200 mg BID (plus placebo), both combined with tenofovir 300 mg plus emtricitabine 200 mg QD for a treatment period of at least 48 weeks, with a planned study duration of 144 weeks.
Plasma sample collection
Morning trough (pre-dose) plasma samples were collected at each visit within 1 h before the next planned dose. For the optional PK sub-study only, intensive PK blood samples were collected at 0.5, 1, 2, 3, 4, 6, 8, 11.9, 12.5, 13, 14, 15, 17, and 24 h following the morning drug administration on Day 28. Patients were hospitalized overnight the day before Visit 4 (Day 27) and remained at the center until the last sample was collected.
Plasma NVP measurement
NVP concentrations in plasma samples were analyzed with a high-performance liquid chromatography tandem mass spectrometry assay validated over a concentration range of 25–10,000 ng/mL.Citation23
VL assay methodology
VL was measured by Covance Laboratory Services using the Roche Cobas® Amplicor Ultrasensitive HIV-1 test. This assay has a very wide range of quantification (48–10,000,000 copies/mL).
PK analysis
A non-compartmental analysis of steady-state PK parameters (Cmax, time to reach Cmax [tmax], Cmin, and AUC0-24) was carried out using WinNonlin v5.3 (Pharsight, CA, USA). Descriptive statistics were calculated for trough plasma concentrations for all patients, while concentration–time data were analyzed for the PK sub-study patients and PK parameters were analyzed for the sub-study cohort only. Relative bioavailability and Ctrough, reported as geometric means (GM), were assessed by comparing NVP-XR to NVP-IR.
Statistical analysis
For the PK sub-study, 60 randomized patients were to be entered at selected trial centers, which had experience with PK studies and were equipped in handling PK samples. Of these 60 patients randomized to the PK sub-study, 24 in the NVP-XR and 25 in the NVP-IR groups were included in the analysis.
Ctrough (Week 48 and GM of all weeks), AUC0–24, Cmin, and Cmax of the XR study formulation were compared with the IR standard formulation using the log-transformed values prior to fitting the data in an analysis of variance (ANOVA) model. The difference between the expected means for log(NVP-XR) − log(NVP-IR) were estimated according to the difference in the corresponding least square means (point estimate), and two-sided 90% confidence intervals were computed based on the t-distribution. These quantities were then back-transformed to the original scale to derive the GM and interval estimates between response under test and response under reference. Descriptive statistics for concentrations at specific time points were calculated only when at least two-third of the individuals had concentrations within the validated concentration range.
Results
Patient demographics
The VERxVE trial population consisted of 1626 screened patients, of whom 1068 received lead-in NVP constituting the treated set. Fifty-five patients discontinued during the lead-in phase. Of 1013 randomized patients, 1011 (NVP-XR: 505; NVP-IR: 506) were treated, which constituted the full analysis set (FAS). Baseline demographic data were similar between non-randomized and randomized patients, and between the NVP-XR and IR groups.Citation15 The extent of exposure to study medication was similar for both treatment groups.
Ctrough by gender, race, and baseline VL stratum
All GM Ctrough of patients included in the FAS exceeded 3000 ng/mL and were relatively stable for both formulations during the 48-week period. The NVP-XR Ctrough were ~20% lower than those for NVP-IR (Table ). The NVP-XR to NVP-IR Ctrough ratio was 0.77 at Week 48 and 0.82 for the GM of all weeks.
Table 1 Multiple dose NVP Ctrough (ng/mL) for NVP-XR 400 mg QD and NVP-IR 200 mg BID
Female patients in both the NVP-XR (n = 53) and NVP-IR (n = 48) groups had higher Ctrough compared to males (n = 273 and n = 323, respectively). Consequently, the NVP-XR to NVP-IR Ctrough ratios were slightly higher among female patients (Table ).
Table 2 Ctrough (ng/mL) of NVP-XR 400 mg QD and NVP-IR 200 mg BID by gender, race, and baseline VL strata
Black patients (NVP-XR, n = 68; NVP-IR, n = 72) showed higher Ctrough than white patients (NVP-XR, n = 294; NVP-IR, n = 234) in both the NVP-XR and NVP-IR treatment groups. Black patients tended to have greater variability in Ctrough in the NVP-IR group, with a difference of 1310 ng/mL [~30%] between the lowest and the highest concentrations over the 48 weeks for IR compared with a difference of 860 ng/mL for XR. Only a few Asian patients participated in both NVP-XR and NVP-IR treatment groups (15 and 13 patients, respectively), and Ctrough for these patients were similar to those of white patients in the NVP-IR group and tended to be higher than those of white patients in the NVP-XR group. The NVP-XR to NVP-IR ratios for black and white patients were mostly comparable (~0.77) (Table ).
There were no marked differences in Ctrough between patients in the two treatment groups based on baseline VL stratum. The NVP-XR to NVP-IR Ctrough ratios were also similar for both these patient groups (Table ).
PK sub-study
Forty-nine patients (NVP-XR: 25; NVP-IR: 24) completed the optional PK sub-study. Plasma concentration-time profiles of NVP on Day 28 showed that mean steady-state plasma concentrations of NVP after oral administration of NVP-XR 400 mg QD exhibited XR characteristics with less fluctuation and were generally lower than those with NVP-IR 200 mg BID (Figure ). The relative bioavailability of NVP-XR to NVP-IR based on the GM ratio was 0.77 for AUC0–24 and 0.69 for Cmax (Table ). This is not unexpected since a lower Cmax is expected for XR by formulation design, which amounted to 31% lower Cmax than that with the IR formulation, whereas the peak-to-trough fluctuation of NVP-XR was 37.5% lower than that with NVP-IR. Individual patient concentration–time profiles showed that the in vivo performance of the NVP-XR tablet formulation was consistently good during multiple dosing, with similar inter-patient variability compared with NVP-IR tablets (% coefficient of variance [%CV] for AUC0–24, XR: 29.5 and IR: 29.9).
Figure 1 Mean NVP plasma concentration–time profiles after oral administration of NVP-XR 400 mg QD and NVP-IR 200 mg BID on Day 28.
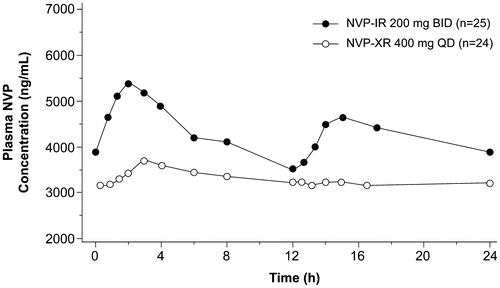
Table 3 Relative bioavailability and adjusted by-treatment GMs of PK sub-study parameters
Ctrough and efficacy
The effect of Ctrough on the proportion of sustained virologic response at Week 48 (defined by two consecutive VL < 50 copies/mL at least 2 weeks apart) was investigated using the GM of all available steady-state Ctrough from Week 4 to Week 48 for each patient. There appeared to be no effect of Ctrough on the virologic response proportion for patients in the ≥1000 ng/mL group who received NVP-XR 400 mg QD (Table ). The finding was consistent with use of the minimum steady-state Ctrough from Week 4 to Week 48; virologic response rates were comparable between patients in the 1000– <2000 ng/mL and ≥2000 ng/mL trough groups.
Table 4 Proportion of patients with virologic responseTable Footnotea at Week 48 by GM and minimum steady-state NVP Ctrough – Full analysis set
Discussion
The VERxVE trial demonstrated the non-inferior virologic efficacy and similar safety profile of NVP 400 mg QD, an XR formulation, compared with the standard BID dosing with the currently available IR formulation. An intensive PK sub-study, presented here, was conducted to evaluate further the in vivo performance of the NVP-XR tablets.
It was found that steady-state plasma concentrations of NVP-XR 400 mg QD demonstrated XR characteristics of the XR tablet formulation with comparatively lesser fluctuation and lower overall drug exposure (AUC0–24 and Cmax) in comparison to NVP-IR 200 mg BID. The results showed that NVP-XR tablets performed consistently and reproducibly in individual patients. The relative bioavailability of NVP-XR for steady-state AUC0–24 and Cmax was in good agreement with that found in the previous intensive PK study in HIV-1 patients, albeit Cmin was slightly lower in the current study (82.7 vs. 89.6%).Citation14 There was no indication of dose dumping when medication was taken with or without a meal. When NVP-XR tablets were administered with a high fat breakfast, the relative bioavailability of NVP was higher (~20%) compared with the fasted state. However, the extent of this increase was not clinically relevantCitation14; therefore, there were no food restrictions in the current study with respect to drug intake.Citation15
No relationship was noted between the trough NVP concentrations of either the XR or IR formulation and the proportion of patients with a virologic response at least up to a Ctrough of 1000 ng/mL. These results were consistent with those for the 2NN study,Citation24 a randomized open-label trial, where the efficacy of 400 mg QD of NVP-IR was compared to 200 mg BID of NVP-IR and to 600 mg QD of efavirenz. These regimens were administered with a fixed antiretroviral background over 48 weeks in treatment-naïve HIV-1 patients.
Several studies have investigated the association of patient demographics such as race, weight, and gender with the PK of NNRTIs. Body weight has been shown to influence plasma NNRTI concentration;Citation20 however, this effect is not consistently seen across studies.Citation18 Similarly, while clear associations have been reported between ethnicity and concentrations of antiretroviral therapies like NVP or efavirenz in some studies,Citation18,20 others have found no association between race and antiretroviral therapies.Citation21,22 In particular, one study by de Maat et al. reported no association between race and the PK of NVP, hence concluding that no race-dependent dose adaptations were required.Citation22 The present study adds to this body of literature, showing no association of NVP PK with race, as the relative trough exposure of NVP-XR to NVP-IR was consistent across the various races.
Although a number of antiretroviral PK studiesCitation19,25 report higher drug exposure in women than in men, there is no clear consensus on whether gender is a significant determinant of NVP concentrations.Citation19 This study reported higher Ctrough in females in both the NVP-XR and NVP-IR groups; however, the XR formulation had no impact on observed gender differences in the PK parameters analyzed. The relative trough exposure of NVP-XR to NVP-IR was also consistent across baseline VL strata.
Taken together, results of this study demonstrate that NVP-XR formulation achieves effective virologic response despite lower Ctrough than NVP-IR. NVP-XR, therefore, offers dosing convenience, reduces the pill burden of patients, and may result in increased adherence to antiretroviral therapy. Further, the lower peak-to-trough fluctuations associated with NVP-XR may contribute to increased tolerability. Patients already on NVP-IR can therefore switch to the XR formulation and may benefit from this transition. Eligible treatment-naïve patients may also benefit, despite the 14-day lead-in requirement with NVP-IR to reduce the frequency of hypersensitive reactions.
It must be noted, however, that the use of a parallel study design rather than a crossover design for the intensive PK sub-study may have resulted in limitations to the conclusions that can be drawn from the data. Additionally, the optional nature of the sub-study results in restrictions to the conclusions that can be drawn.
In conclusion, the PK results suggest that QD oral administration of the NVP-XR tablet formulation in HIV-1 patients with background antiretroviral therapy had favorable PK characteristics and was as effective as the IR formulation. Further, the results were not directly affected by demographic factors.
Funding
This study was sponsored and financed by Boehringer Ingelheim Pharmaceuticals, Inc. and funding for editorial assistance for the preparation of the manuscript was provided by Boehringer Ingelheim Pharmaceuticals, Inc.
Declaration of interest
J Gathe has received honoraria for consultancy work, support for travel to meetings, fees for participation in review activities, and payment for writing or reviewing manuscripts and lectures including service on speaker bureaus, from AbbVie, Janssen, Boehringer Ingelheim, Gilead, Merck, GlaxoSmithKline, ViiV Healthcare, Bristol Myers Squibb, and Tibotec. J Mallolas has board membership with and has received payment for lectures from Boehringer Ingelheim, Merck, Janssen, and Gilead. D Podzamczer and B Trottier have received consultancy fees, research grants, and/or payment for lectures from ViiV Healthcare, Pfizer, BMS, Abbott, AbbVie, Gilead, Janssen, and Merck. C Orrell has received an honorarium from Abbott and grants to her institution, Desmond Tutu HIV Foundation. G Knecht received an honorarium and payment for the development of educational presentations from Infektio Research.
W Zhang, JP Sabo, R Vinisko, M Drulak, and AM Quinson are employees of Boehringer Ingelheim. CL Yong was an employee of Boehringer Ingelheim at the time of the study and is currently retired.
Contributors
All the authors have contributed to conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and provided final approval of the manuscript.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance were provided by Michelle Rebello, PhD, of Cactus Communications and Ghzaleh Masnavi and Amineh Zafarani of Euro RSCG Life. Both were contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
The authors wish to thank the patients, investigators, clinicians, and nursing staff who participated in the trial. We thank co-investigator Dr. Maria Martinez-Rebollar for her contribution to the study.
References
- Kawalec P, Kryst J, Mikrut A, Pilc A. Nevirapine-based regimens in HIV-infected antiretroviral-naive patients: systematic review and meta-analysis of randomized controlled trials. PLoS ONE. Oct 2013;8(10):e76587.
- de Béthune MP. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years (1989–2009). Antiviral Res. Jan 2010;85(1):75–90.10.1016/j.antiviral.2009.09.008
- Jayaweera D, Dilanchian P. New therapeutic landscape of NNRTIs for treatment of HIV: a look at recent data. Expert Opin Pharmacother. Dec 2012;13(18):2601–2612.10.1517/14656566.2012.742506
- Merluzzi VJ, Hargrave KD, Labadia M, et al. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990;250(4986):1411–1413.10.1126/science.1701568
- Joly V, Yeni P. Non-nucleoside reverse transcriptase inhibitors. Ann Med Interne (Paris). Jun 2000;151(4):260–267.
- Waters L, John L, Nelson M. Non-nucleoside reverse transcriptase inhibitors: a review. Int J Clin Pract. Jan 2007;61(1):105–118.10.1111/j.1742-1241.2006.01146.x
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. Aug 2001;23(8):1296–1310.10.1016/S0149-2918(01)80109-0
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. Aug 2005;353(5):487–497.10.1056/NEJMra050100
- Nachega JB, Knowlton AR, Deluca A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults: a qualitative study. J Acquir Immune Defic Syndr. Dec 2006;43(Suppl 1):S127–S133.10.1097/01.qai.0000248349.25630.3d
- Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once‐daily antiretroviral regimens: a meta‐analysis. Clin Infect Dis. Feb 2009;48(4):484–488.10.1086/597429
- van Heeswijk RP, Veldkamp AI, Mulder JW, et al. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. AIDS. May 2000;14(8):F77–F82.10.1097/00002030-200005260-00001
- Macha S, Yong CL, MacGregor TR, et al. Assessment of nevirapine bioavailability from targeted sites in the human gastrointestinal tract. J Clin Pharmacol. Dec 2009;49(12):1417–1425.10.1177/0091270009344856
- Macha S, Yong CL, Darrington T, et al. In vitro-in vivo correlation for nevirapine extended release tablets. Biopharm Drug Dispos. Dec 2009;30(9):542–550.10.1002/bdd.691
- Battegay M, Arasteh K, Plettenberg A, et al. Bioavailability of extended-release nevirapine 400 and 300 mg in HIV-1: a multicenter, open-label study. Clin Ther. Sep 2011;33(9):1308–1320.10.1016/j.clinthera.2011.08.003
- Gathe J, Andrade-Villanueva J, Santiago S, et al. Efficacy and safety of nevirapine extended-release once daily versus nevirapine immediate-release twice-daily in treatment-naïve HIV-1-infected patients. Antivir Ther. 2011;16(5):759–769.10.3851/IMP1803
- Arasteh K, Ward K, Plettenberg A, et al. Twenty-four-week efficacy and safety of switching virologically suppressed HIV-1 infected patients from nevirapine immediate release 200 mg twice daily to nevirapine extended release 400 mg once daily (TRANxITION). HIV Med. Apr 2012;13(4):236–244.
- Gazzard BG, Anderson J, Babiker A, et al. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. Oct 2008;9(8):563–608.
- Burger D, van der Heiden I, la Porte C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. Feb 2006;61(2):148–154.10.1111/bcp.2006.61.issue-2
- Ofotokun I, Chuck SK, Hitti JE. Antiretroviral pharmacokinetic profile: a review of sex differences. Gend Med. Jun 2007;4(2):106–119.10.1016/S1550-8579(07)80025-8
- Stöhr W, Back D, Dunn D, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13(5):675–685.
- van der Leur MR, Burger DM, la Porte CJ, Koopmans PP. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther Drug Monit. Oct 2006;28(5):650–653.10.1097/01.ftd.0000245681.12092.d6
- de Maat MM, Nellen JF, Huitema AD, et al. Race is not associated with nevirapine pharmacokinetics. Ther Drug Monit. Aug 2004;26(4):456–458.10.1097/00007691-200408000-00018
- Rowland LS, MacGregor TR, Campbell SJ, Jenkins R, Pearsall AB, Morris JP. Quantitation of five nevirapine oxidative metabolites in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. Sep 2007;856(1–2):252–260.10.1016/j.jchromb.2007.06.007
- van Leth F, Kappelhoff BS, Johnson D, et al. Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses. Mar 2006;22(3):232–239.10.1089/aid.2006.22.232
- Floridia M, Giuliano M, Palmisano L, Vella S. Gender differences in the treatment of HIV infection. Pharmacol Res. Sep–Oct 2008;58(3–4):173–182.10.1016/j.phrs.2008.07.007
