Abstract
Objective: To evaluate whether treatment with 100,000 IU/month (equivalent to 3200 IU/day) of cholecalciferol and 1 g/day of dietary calcium supplementation in HIV patients following different cART regimens yields normal levels of vitamin D3 and PTH as well as whether changes in bone mineral density are clinically significant.
Methods: Consecutive HIV patients following different cART regimens received 100,000 IU/month (equivalent to 3200 IU/day) of cholecalciferol and 1 g/day of dietary calcium supplementation. The participants underwent BMD assessment via dual energy X-ray absorptiometry of the spine and hip at baseline (T0) and after 24 months (T1). Levels of 25(OH) vitamin D3 and parathyroid hormone (PTH) were assessed at T0 and T1. Quantitative variables were assessed with a paired t-test, independent t-test or analysis of variance, as appropriate. A chi-squared analysis was used to assess the association between qualitative variables. A p-value <0.05 was considered significant. Patients were divided into three groups depending on the cART regimen.
Results: A total of 79 patients were included (40 males, 51% and 39 females, 49%), with a mean age of 46.6 (SD ±11.2) years, a baseline CD4 count of 649 cells/µl and a mean 25 hydroxycholecalciferol (25(OH) D3) value of 25 + 10 ng/ml. After 24 months, the 25(OH) D3 increased to 40 + 11 ng/ml. The initial BMDs at T0 were estimated as 0.919 (±0.27) and 0.867 (±0.14) g/cm2 at the spine and hip, respectively. After 24 months, the BMD was 0.933 (±0.15) g/cm2 at the spine and 0.857 (±0.14) g/cm2 at the hip. Based on a BMD change exceeding 3%, a worsening was observed in 23% of patients at the spine and 27% at the hip, whereas stability or improvement was demonstrated in 77% of patients at the spine and 73% at the hip.
Subgrouping patients based on antiretroviral therapy indicated that, at T1, there was a statistically significant increase in vitamin D3 concentration in all patients, while PTH concentration was not significantly reduced in patients taking tenofovir or efavirenz. BMD stability or improvement was demonstrated in 77% of patients at the spine and 73% at the hip after 24 months.
The multivariate analysis confirms a decrease in vitamin D3 and an increase in PTH levels in smokers, as well higher vitamin D3 concentrations in males and lower spine BMDs in menopausal females.
Conclusion: The proposed protocol of cholecalciferol and dietary calcium supplementation is safe and valid for correcting vitamin D abnormalities in almost all patients as well as reducing PTH levels in a high percentage of patients; however, it is not sufficient for normalization, particularly in patients exposed to tenofovir or efavirenz. At the spine, no significant BMD change was found in any of the therapy groups. At the hip, our data confirm a modest negative effect on bone mass caused by tenofovir and efavirenz.
Background
An alteration of vitamin D status is frequent in patients with human immunodeficiency virus (HIV) infection that can lead to bone loss and osteoporosis.Citation1
Multiple studies showed that individuals affected by HIV demonstrate a higher incidence of osteopenia and osteoporosis than the general population.Citation2–8
A reduction in bone mineral density (BMD), ranging from 33 to 58%, and osteoporosis, ranging from 2 to 13%, was reported in HIV patients. HIV-infected patients present a lower BMD and a higher risk of bone fracture than those of the general population, with an incidence ratio for all fractures of 1.58 (95% CI 1.25–2).Citation9,Citation10
Bone loss fluctuating between 2 and 6% yearly is present during the early years after beginning cART, with an evident depletion after 96 weeks of cART treatment that continues steadily until an average of 7.5 years, which is different from that observed in HIV patients not receiving Cart.Citation11,Citation12
This phenomenon is explained by multiple factors. HIV patients are equally or even more highly exposed to traditional risk factors, such as smoking, alcohol, lifestyle factors, sedentary lifestyle (particularly in advanced disease), some classes of drugs, and concomitant infectious diseases.Citation13,Citation14 Moreover, due to the increase in the average age of patients, osteopenia and osteoporosis linked to menopause and senescence are also observed.Citation15
Studies on 25(OH) vitamin D3 concentrations in subjects with HIV have reported conflicting data.Citation16,Citation17
An important role is played by cART; however, some categories of antiretroviral drugs, in particular, tenofovir disoproxil fumarate (TDF), efavirenz (EFV), and protease inhibitors (PIs), markedly influence vitamin D3 metabolism, renal function and the balance between osteoclasts and osteoblasts.Citation18–22 It is also important to emphasize that, in HIV patients, BMD is already reduced by 34% at the time of diagnosis prior to the beginning of cART.Citation23
TDF, a nucleoside reverse transcriptase inhibitor (NRTI), has shown a strong tendency to accelerate bone loss and diminish BMD. A decrease in serum 1,25(OH) vitamin D3 with a concomitant increase in parathyroid hormone (PTH)-binding globulin was shown during TDF treatment.Citation18 Therefore, it is hypothesized that TDF facilitates bone loss by interfering with vitamin D3 metabolism;Citation18–21 however, the effect of TDF on PTH and BMD may be different depending on the 25(OH)D3 levels.Citation24
Therapy based on EFV, a non-nucleoside reverse transcriptase inhibitor (NNRTI), is associated with the induction of cytochrome P450, thus enhancing the catabolism of both 25(OH) D3 and 1,25(OH) vitamin D3 and reducing the concentration of circulating 25(OH) vitamin D3.Citation25 The administration of TDF and EFV in association with another NRTI, emtricitabine (FTC), represents one of the most effective treatments to control viral replication.Citation26,Citation27 Protease inhibitors reduce the activity of the enzymes (25-hydroxylase and 1 alpha-hydroxylase) responsible for the hydroxylation of vitamin D3 to 25(OH)D3 and to 1,25(OH)D3.Citation28
To improve vitamin D3 status, cholecalciferol supplementation is often suggested.
In normal populations, the guidelines focused on vitamin D bone effects recommend a target 25(OH) D3 concentration of 30 ng/ml/75 nmol/L (with vitamin D supplementation ranging between 400 and 800 IU/day).Citation29–31 Moreover, the guidelines for vitamin D pleiotropic effects, such as influencing cell proliferation, muscle performance, energy metabolism and bone strength, independent of its actions on calcium absorption, recommend a target 25(OH) D3 concentration above 30 ng/ml/75 nmol/L (with vitamin D3 supplementation ranging between 400 and 2000 IU/day), with the preferred range of 40–60 ng/ml/100–150 nmol/L (with vitamin D3 supplementation ranging from 1500–2000 IU/day.Citation29–31
The choice of dose depends on the age, body weight, dietary habits, and latitude of residence (sun exposition).
In patients taking medications that affect vitamin D metabolism, including medications to treat AIDS/HIV, a higher dose was also recommended (two to three times higher).Citation29,Citation32
As vitamin D3 supplementation alone is unlikely to reduce fracture risk, the combination of calcium supplementation with vitamin D3 supplementation reduces the risk of total fractures, including hip fracture.Citation31,Citation33,Citation34
In a short-term study (48 weeks) of HIV patients, treatment with cholecalciferol (4000 IU daily) and calcium supplementation (1000 mg of calcium carbonate) was effective at limiting the bone loss normally observed with TDF-EVF-FTC triple therapy, particularly within the neck of the femur.Citation35
Calcium supplementation can have harmful side effects as well, such as cardiovascular, renal, and gastrointestinal effects.Citation36
In the present study, we examined the effects of cholecalciferol (100,000 IU monthly, equivalent to 3200 IU daily) and dietary calcium supplementation (1000 mg daily, of which 700 mg was ingested by oral intake of calcium-rich water) in a sample of HIV patients following a cART regimen and evaluated the vitamin D3 status and BMD modifications after 24 months.
Methods
A retrospective review of prospectively collected data was performed on patients affected with HIV-type 1 on cART therapy, routinely monitored at the Department of Internal Medicine, University Hospital of Cagliari, between 2013 and 2015. The study received formal approval by the research ethics committee.
The exclusion criteria included subjects with confirmed neoplasia, recent steroid or chemotherapy treatments, clinically active thyroid disease, recreational drug or alcohol abuse, history of fragility fracture, hyperparathyroidism, or nephrolithiasis, weight >130 kg (limit of the dual energy X-ray absorptiometry (DXA) scanner), and pregnancy or breastfeeding.
None of the patients had taken drugs affecting bone metabolism or had consumed any calcium or cholecalciferol supplements prior to the beginning of the study. All participants received cholecalciferol (100,000 IU monthly, equivalent to 3200 IU per day, for oral administration after the main meal) plus dietary calcium supplementation (1 g daily). Additionally, 4000 IU/day is the Institute of Medicine (IOM), Food and Nutrition Board’s current tolerable upper intake level.Citation37
All patients received a cART regimen that remained unmodified throughout the entire study period as follows: 47 patients were treated with tenofovir alone or tenofovir plus protease inhibitors, 18 were treated with protease inhibitors, and 14 were treated with tenofovir plus efavirenz.
The data collected included patient characteristics, such as age, gender, body mass index, duration of HIV infection, previous fractures, smoking, and alcohol consumption as well as the risk for fragility fractures. The biochemical assessment performed at baseline (T0) and at follow-up (T1) included the measurement of serum calcium, phosphate, creatinine, 25(OH)D3, intact PTH levels (ELISA), HIV RNA copies/ml (real time PCR), and CD4 cell count (cells/µl, by flow cytometry).
All patients underwent baseline (T0) and follow-up (T1) DXA evaluation focused on the spine (L1–L4) and total hip using a QDR 4500 osteodensitometer (Hologic, Bedford, MA, USA). BMD was automatically calculated from the bone area (cm2) and bone mineral content (g) and expressed in absolute terms (g/cm2). Patients were classified as having normal BMD, osteopenia or osteoporosis based on T-scores using the WHO classification.Citation38 The frequency of osteoporosis at either measurement site (spine or hip) was based on the lowest T-score (≤–2.5) as proposed by the International Society for Clinical Densitometry.Citation39
At follow-up, patients were classified as stable/improved or worsened according to a BMD change exceeding 3%.
Statistical analysis was performed using SPSS software (version 21.0; IBM, Armonk, NY, USA). Quantitative variable analyses were performed using two-tailed paired t-tests, independent sample t-tests, and analysis of variance (ANOVA), as appropriate. Correlations between quantitative variables were analyzed using Spearman’s coefficient. Associations between categorical variables were determined by chi squared analysis. A p-value <0.05 was considered significant.
Results
A total of 79 Caucasian patients (40 men and 39 women) with HIV infection and 6 months stable viremia were enrolled.
The overall data for the 79 patients at T0 and T1 are reported in . Cholecalciferol (100,000 IU monthly, equivalent to 3200 IU per day) plus dietary calcium supplementation (1000 mg daily, of which 700 mg was ingested by oral intake of calcium-rich water) were taken by all patients until T1.
Table 1 T0 and T1 parameters of examined patients
Serum calcium, phosphate, and creatinine levels were in the normal ranges, both at T0 and T1.
At T0, 39% and 51% of patients presented normal bone mass at the spine and hip, respectively (). No fracture episodes occurred during the study. At basal levels, 39.2% of patients (31/79) showed vitamin D3 ≤ 20 ng/ml, 30.4% of patients (24/79) showed vitamin D3 between 20 and 30 ng/ml and 30.4% of patients (24/79) showed vitamin D3 > 30 ng/ml (). After 24 months of cholecalciferol and calcium supplementation, 8% of patients (6/79) showed vitamin D3 between 20 and 30 ng/ml and 92% of patients (73/79) showed vitamin D3 > 30 ng/ml ().
Based on the basal levels of 25(OH)vitamin D3, patients were divided into three groups. show data at T0 and T1 for the examined parameters. A significant difference was found for PTH concentration (p = 0.003), which was reduced at T1 in patients with basal vitamin D3 between 20 and 30 ng/ml; for vitamin D3 concentration (p = 0.02), which was increased at T1 in patients with basal vitamin D3 > 30 ng/ml; and in BMD at the hip (p = 0.001), which was reduced at T1 in patients with basal vitamin D3 < 20 ng/ml.
Table 2a T0 and T1 parameters of examined patients grouped according to basal vitamin D3 levels
Table 2b T0 and T1 BMD of examined patients grouped according to basal vitamin D3 levels
Based on a BMD increase or loss percentage exceeding 3%, some worsening was observed at the spine in 18 patients (22.8%), whereas stability or improvement was demonstrated in 61 individuals (77.2%) ().
Figure 1. T0 and T1 bone mass changes (thresholds of statistical significance >3%) in examined patients.
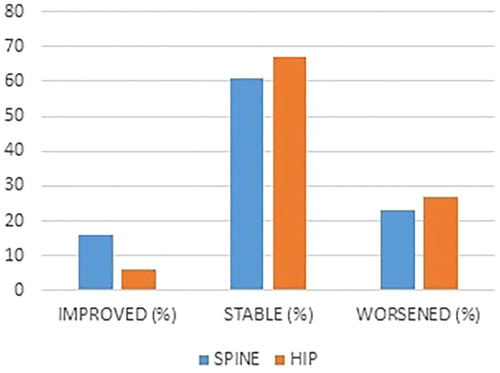
Based on a BMD increase or loss percentage exceeding 3%, some worsening was also observed at the hip in 21 patients (26.6%), whereas stability or improvement was demonstrated in 58 individuals (73.4%) ().
To better clarify the effect of therapy, we examined three groups () according to the type of cART regimen as follows: group 1 (47 patients taking tenofovir alone or with protease inhibitors or integrase inhibitors), group 2 (18 patients taking protease inhibitors alone), and group 3 (14 patients taking tenofovir with efavirenz).
Table 3a T0 and T1 parameters of examined patients grouped according to therapy
Table 3b T0 and T1 BMD of examined patients grouped according to therapy
At T1, all groups showed a statistically significant increase in vitamin D3 concentration, while the PTH concentration was significantly reduced only in group 2 (no significant decrease in PTH concentration was found in patients taking tenofovir + efavirenz).
No difference in BMD at the spine was found between therapy groups. BMD at the hip was significantly reduced at T1 in patients taking tenofovir alone or tenofovir + protease inhibitors (p = 0.005) and in patients taking tenofovir + efavirenz (p = 0.04).
ANOVA showed a statistically significant difference at T1 between group 1 (tenofovir alone or with protease inhibitors) and group 2 (protease inhibitors alone) for the vitamin D3 concentration (group 1, 43 ± 11 ng/ml and group 2, 35 ± 7 ng/ml, p = 0.04) and PTH concentration (group 1, 49 ± 16 pg/ml and group 2, 39 ± 14, p = 0.01). Moreover a significant difference at T1 was found between group 2 (protease inhibitors alone) and group 3 (tenofovir with efavirenz) in PTH reduction (group 2, 38 ± 14 and group 3, 49 ± 18, p = 0.05).
shows the percentage of normal, osteopenic and osteoporotic patients at basal and follow up.
Table 4 T0 and T1 percentages of bone status in examined patients
The multivariate analysis for age, gender, smoking status, alcohol consumption, diabetes, coinfections, and menopausal status showed a reduction of vitamin D3 levels (smokers 23 ± 8 and no smokers 29 ± 13, p = 0.02) and an increase in PTH levels (smokers 72 ± 19 and no smokers 63 ± 20, p = 0.05) in smokers, as well a higher vitamin D3 concentration in males (28 ± 13 in males and 22 ± 9 in females p = 0.04). Females in menopause (13% of females, 5/39) showed a reduction in T0 BMD at the spine (menopause 0.771 ± 0.100 and no menopause 0.970 ± 0.129, p = 0.02).
Discussion and conclusion
Vitamin D is essential for optimal bone health. The effects of vitamin D deficiency in HIV are largely unknown, and few published RCTs have investigated the effect of vitamin D and calcium supplementation on the improvement of bone health in the adult HIV population and HIV-infected youth population.Citation35,Citation40–43
The novelty of our study lies in the administration of cholecalciferol together with the administration of food calcium.
Multiple studies have shown the limited influence on bone mass and fracture risk s with only vitamin D3 or D2 treatment in the population. However, when supplementation with vitamin D3 is associated with calcium supplementation, both BMD improvement and fracture risk reduction appear, primarily in the deficient and elderly population.Citation44 In this population, the effects on BMD and fracture risk become evident using cholecalciferol dosages higher than 700 IU/day and calcium supplementation of at least 500 mg/day.Citation44
In our study, the frequency of patients with hypovitaminosis D was quite high, with 30,4% of patients presenting as insufficient, and 39,2% were frankly deficient at time T0. Patients showed excellent adherence to the cholecalciferol schedule of treatment.
There is no consensus on daily calcium requirements; however, major guidelines recommend calcium supplements in doses of 1000–1200 mg/day for the treatment and prevention of osteoporosis, and most people take calcium supplements.Citation45
Recent concerns about the safety of such supplements led experts to recommend increasing calcium intake through food rather than by supplements, which safer as it does not cause the appearance of calcemic peaks that favor vascular calcifications and the formation of kidney stones. A meta-analysis of the 28,000 subjects available across all trials of calcium with or without vitamin D, demonstrated a 24% increase in the risk of myocardial infarction (p = 0.004) and a 15% increase in the risk of stroke (p = 0.055).Citation36 Moreover, this approach may influence compliance because it may be associated with gastrointestinal side-effects.
A recent meta-analysis of 59 studies in older adults (aged > 50) showed that increases in BMD were similar in trials of dietary sources of calcium and calcium supplements.Citation34
In our study, adherence to dietary supplementation with calcium was satisfactory in all subjects, unlike reported other studies in which there is a reduction of at least 10% in people taking calcium supplements.Citation46
No patient showed signs of cholecalciferol or calcium toxicity, and no patient showed calcium concentrations above 10.3 mg/dl, kidney stones or gastrointestinal side-effects.
In our study, treatment was suboptimal in a small percentage of patients (7.6%), although it ensured vitamin levels above 25 ng/ml in all patients. Additionally, 7.6% of patients who presented values between 25 and 30 ng/ml were deficient at T0. These results are considered satisfactory.
No patients showed 25 OH vitamin D3 concentrations above the normal range (100 ng/ml) after 24 months of treatment. The highest value was 67.7 ng/ml.
In our study, PTH blood concentrations showed a significant reduction after 24 months (67.5 ± 20.1 at T1 vs. 46.4 ± 16.3 at T0, p < 0.001).
In HIV-infected patients, the protocol of cholecalciferol and calcium supplementation normalized vitamin D3 levels in a high proportion of patients (92.4% > 30 ng/ml and 100% > 25 ng/ml), but it was less effective in correcting hyperparathyroidism (PTH <65 pg/ml in 88.6%), and 9 of the 79 patients (11.4%) showed PTH values >65 pg/ml but always lower than 96 pg/ml.
In our study, independent factors for not achieving the PTH objective were tenofovir and efavirenz. Treatment with tenofovir and efavirenz may play a central role in preventing the normalization of PTH concentrations as demonstrated by our results in group 1 and group 3.
Contrasting results are reported in the literature regarding the quantification of BMD reduction following cART in HIV patients, although a universal consensus exists that some drugs are associated with bone loss within the first 24 months after beginning cART.Citation47,Citation48 In a recent metanalysis of 37 longitudinal studies conducted on HIV patients,Citation9 the results differed significantly at different centers and according to the time that cART was started. Patients who were not receiving cART at baseline showed a BMD reduction at the hip one or two years after starting cART; however, patients who were already being treated with cART demonstrated either a stabilization or even an increase in the hip BMD. The authors suggest that the BMD increase observed in such patients is attributable to the weight gain observed after several months (more than two years, in most cases) in cART-responsive individuals.
Only a few studies examined the osseous depletion associated with cART, but they did not evaluate the BMD variations for periods longer than 48 weeks. Treatment with cholecalciferol (4000 IU daily) coupled with calcium supplementation (calcium carbonate 1000 mg daily) is effective for reducing the bone loss that ensues following the triple administration of TDF-FCT-EFV. This outcome was particularly pronounced in assessment of the hip BMD. The bone loss at 48 weeks was limited in up to 50% of patients, which demonstrates the effectiveness of the early administration of cholecalciferol and calcium to prevent bone loss and fracture risk.Citation35 In another study, supplementation with 300,000 IU of cholecalciferol decreased markers of bone turnover and significantly increased 25(OH)D3 levels after 3 months.Citation49
In a recent trial performed on HIV-infected youth with serum 25(OH) vitamin D3 concentrations <30 ng/ml, 3 different monthly cholecalciferol doses (18,000, 60,000, or 120,000 IU/month, equivalent to 600, 2000, or 4000 IU/day, respectively) led to improvements in BMD after 12 months, but only the high-dose arm showed significant decreases in bone turnover markers.Citation40
Our data confirmed a reduction of bone mass and clear osteoporosis at T0 at the spine and at hip. After 24 months of treatment, the BMD showed no significant changes. It was observed that 3/32 osteopenic patients, who became osteoporotic after 24 months, presented at least two risk factors (among alcohol, smoking, and C virus infection).
One of the 14 osteoporotic patients who became osteopenic after 24 months did not present any of the risk factors studied. The patient, who presented normal BMD after 24 months, consumed alcoholic beverages during the 24 months of study but did not have other risk factors.
No significant reduction of the mean spine BMD was found between T1 and T0, whereas the mean hip BMD was significantly reduced in 31 patients with 25 OH vitamin D3 < 20 ng/ml at T0 (p < 0.001)
After 24 months, 77.2 and 73.4% of the patients showed stabilization (changes < ± 3%) or an increase (changes >3%) in bone mass at the spine and hip, respectively.
Subgrouping of our patients based on 25 OH vitamin D3 at T0 showed that 77,4% of patients who presented 25 OH D3 deficit (39%) or insufficiency (30%) at T0 showed stabilization (61%) or an increase (16%) in bone mass at the spine after 24 months.
Subgrouping of our patients based on 25 OH vitamin D3 at T0 also showed that 77,4 of patients who presented 25 OH D3 deficit (0%) or insufficiency (30%) at T0 showed stabilization (71%) in bone mass at the hip after 24 months.
Moreover, 75% and 87.5% of patients who presented 25 OH D3 in the normal range at T0 did not show a BMD reduction in bone mass at the spine and hip, respectively.
Subgrouping patients based on antiretroviral therapy indicated no significant difference in vitamin D3, PTH and BMD between therapy groups at T0.
At T1, all groups showed a statistically significant increase in the vitamin D3 concentration, while the PTH concentration was significantly reduced only in group 2 (no significant decrease in PTH concentration was found in patients taking tenofovir or efavirenz).
Because low vitamin D was associated with PTH increases, it is likely that vitamin D3 repletion may reduce the risk of PTH increase. Hyperparathyroidism may be multifactorial in HIV infected patients. Tenofovir and efavirenz may be independent factors for not achieving a PTH reduction in 11.4% of patients in our study. The mechanism by which TDF produces hyperparathyroidism is unclear, and a previous study suggests that increased hydroxylation rates and tubular phosphate losses, which drive calcium preservation, and possibly altered bone metabolism, are dependent on the vitamin D status.Citation20,Citation21 Efavirenz is associated with a decrease in 25(OH)D levels, and efavirenz also induces cytochrome P450 enzymes involved in vitamin D metabolism and may accelerate the catabolism of 25(OH)D3 and 1,25(OH) 2D3.Citation22
At the spine, no significant BMD change was found in any of the therapy groups. At the hip, our data confirm a modest negative effect on the bone mass produced by tenofovir and efavirenz. Conversely, no increase in bone loss was shown in patients who used PIs.
The multivariate analysis for age, gender, smoke, alcohol consumption, diabetes, coinfections, and menopausal status confirms a reduction of vitamin D3 and an increase in PTH levels in smokers, as well a higher vitamin D3 concentration in males and a lower spine BMD in menopausal females. Moreover, the increased loss of bone mass in the smoking population emphasizes the importance of smoking cessation policies for HIV-positive individuals.
A long duration of cART does not seem to influence bone loss in patients using cholecalciferol. None of the patients suffered from fracture episodes.
When we examined the changes in bone mass by evaluating the thresholds of statistical significance (>3%), we determined that 77 and 73% of the patients showed a stabilization or an increase in bone mass at the spine and hip, respectively, after 24 months. Grant et al.Citation23 reported a constant bone mass reduction in HIV patients over a treatment period of 7.5 years, and the most pronounced effect was observed in the first 96 weeks of cART treatment. Our study shows that the proposed treatment is effective during the first period of cART therapy because a high percentage of patients maintained or increased bone mass, not only in the spine but also in the hip.
Subgrouping of our patients based on antiretroviral therapy indicated that 94.4 and 72.2% of patients who used PIs (group 2) showed stabilization or an increase in bone mass at the spine and hip, respectively. These data confirm a modest negative effect on the bone mass produced by PIs. Conversely, an increase in bone loss was shown in patients who used TDF or EFV.
Our data confirmed a reduction of bone mass and clear osteoporosis in HIV patients. A unique feature of this study is the monthly administration of cholecalciferol together with calcium intake exclusively by diet.
The proposed protocol for cholecalciferol and calcium supplementation is safe and valid for correcting vitamin D3 abnormalities in almost all patients and for reducing PTH levels in a high percentage of patients; however, this is not sufficient for normalization, particularly in patients exposed to tenofovir or efavirenz.
No significant reduction of the spine BMD was found between T1 and T0, whereas the hip BMD was slightly reduced.
Due to the high compliance observed in our patients receiving the bone mass preservation regimen and based on the obtained results and the cost-effectiveness of the protocol, we suggest that it is appropriate to recommend a monthly administration of 100,000 IU of cholecalciferol along with dietary supplementation of 1000 mg of calcium daily in all patients receiving cART.
Limitations of the present study are that vitamin D seasonal variations were not considered. Moreover, the main limitations are related to the retrospective, observational design of the current analyses and the small and heterogeneous number of patients included.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Eckard AR, McConsey GA. Vitamin D deficiency and altered bone mineral metabolism in HIV-infected individuals. Curr HIV/AIDS Rep. 2014;11(3):263–270.
- Arnsten JH, Freeman R, Howard AA, et al. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection AIDS. 2007;21(5):617–623.
- Arnsten JH, Freeman R, Howard AA, et al. HIV infection and bone mineral density in middle-aged women. Clin Infect Dis. 2006;42(7):1014–1020.
- Amorosa V, Tebas P. Bone disease and HIV infection. Clin Infect Dis. 2006;42(1):108–114.
- Amiel C, Ostertag A, Slama L, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2004;19(3):402–409.
- Dolan SE, Huang JS, Killilea KM, et al. Reduced bone density in HIV-infected women. AIDS. 2004;18(3):475–483.
- Mulligan K, Harris DR, Emmanuel P, et al. Low bone mass in behaviorally HIV-infected young men on antiretroviral therapy: adolescent trials network study 021B. Clin Infect Dis. 2012;55(3):461–468.
- Yin M, Dobkin J, Brudney K, et al. Bone mass and mineral metabolism in HIV + postmenopausal women. Osteoporos Int. 2005;16(11):1345–1352.
- Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174.
- Shiau S, Broun EC, Arpadi SM, et al. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS. 2013; 27(12):1949–1957.
- Assoumou L, Katlama C, Viard JP, et al. Changes in bone mineral density over a 2-year period in HIV-1-infected men under combined antiretroviral therapy with osteopenia. AIDS. 2013;27(15):2425–2430.
- Brown TT, McComsey GA, King MS, et al. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561.
- Short CE, Shaw SG, Fisher MJ, et al. Prevalence of and risk factors for osteoporosis and fracture among a male HIV-infected population in the UK. Int J STD AIDS. 2014;25(2):113–121.
- Hileman CO, Labbato DE, Storer NJ, et al. Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS. 2014; 28(12):1759–1767.
- Kooij KW, Wit FW, Bisschop PH, et al. Low bone mineral density in patients with well-suppressed HIV infection: association with body weight, smoking, and prior advanced HIV disease. J Infect Dis. 2015;211(4):539–548.
- Adeyemi OM, Agniel D, et al. Vitamin D deficiency in HIV-infected and HIV-uninfected women in the United States. J Acquir Immune Defic Syndr. 2011;57(3):197–204.
- Lambert AA, Drummond MB, et al. Risk factors for vitamin D deficiency among HIV-infected and uninfected injection drug users. PLoS ONE. 2014;9(4).
- Grigsby IF, Pham L, Mansky LM, et al. Tenofovir-associated bone density loss. Ther Clin Risk Manag. 2010;6:41–47.
- Childs KE, Fishman SL, Constable C, et al. Short communication: Inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS research and human retroviruses. 2010;26(8):855–859.
- Havens PL, Kiser JJ, Stephensen CB, et al. Association of higher plasma vitamin D binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: cause of a functional vitamin D deficiency? Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 063 Study Team. Antimicrob Agents Chemother. 2013;57(11):5619–5628.
- Havens PL, Stephensen CB, Hazra R, et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions 063 study team. Clin Infect Dis. 2012;54(7):1013–1025.
- Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther (Lond.). 2010;15(3):425–429.
- Grant PM, Kitch D, McComsey GA, et al. Long-term bone mineral density changes in antiretroviral-treated HIV-infected individuals. J Infect Dis. 2016;214(4):607–611.
- Klassen KM, Kimlin MG, Fairley CK, et al. Associations between vitamin D metabolites, antiretroviral therapy and bone mineral density in people with HIV. Osteoporos Int. 2016;27(5):1737–1745.
- Wohl DA, Orkin C, Doroana M, et al. Change in vitamin D levels and risk of severe vitamin D deficiency over 48 weeks among HIV-1-infected, treatment-naive adults receiving rilpivirine or efavirenz in a Phase III trial (ECHO). Antivir Ther. 2014;19(2):191–200.
- McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids clinical trials group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–1801.
- Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972.
- Cervero M, Agud JL, Torres R, et al. Higher vitamin D levels in HIV-infected out-patients on treatment with boosted protease inhibitor monotherapy. HIV Med. 2013; 14(9):556–562.
- Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab.
- Pludowski P, Karczmarewicz E, Bayer M, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013;64(4):319–327.
- Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. The Cochrane Collaboration. 2016 Published by JohnWiley & Sons, Ltd Cochrane Database Syst. Rev. 2009, CD000227. doi:10.1002/14651858.
- Grant WB, Wimalawansa SJ, Holick MF, et al. Emphasizing the health benefits of vitamin D for those with neurodevelopmental disorders and intellectual disabilities. Nutrients 2015;7(3):1538–1564.
- Silk LN, Greene DA and Baker MK. The effect of calcium or calcium and vitamin d supplementation on bone mineral density in healthy males: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab. 2015;25(5):510–524
- Tai V, Leung W, Grey A, et al. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015;351:h4183.
- Overton ET, Chan ES, Brown TT, et al. High-dose vitamin D and calcium attenuates bone loss with antiretroviral therapy initiation. Ann Intern Intern Med. 2015;162(12):815–824.
- Reid Ian R. Osteoporosis treatment: focus on safety. European Journal of Internal Medicine 2013: 24(8):691–697.
- Ross AC, Taylor CL, Yaktine AL, et al. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press (US); 2011.
- Kanis JA. On behalf of the World Health Organization Scientific Group. Technical report. Assessment of osteoporosis at the primary health care level. WHO Collaborating Centre for Metabolic Bone Diseases. 2007 Assessment of osteoporosis at the primary helalth-care level. Printed by the University of Sheffield.
- Hans DH, Downs RWJ, Duboeuf F, et al. Skeletal sites for osteoporosis diagnosis: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9(1):15–21.
- Eckard AR, O’Riordan MA, Rosebush JC et al. Effects of vitamin D supplementation on bone mineral density and bone markers in HIV-infected youth. Acquir Immune Defic Syndr. 2017;15;76(5).
- Lerma-Chippirraz E, Güerri-Fernández R, et al. Validation protocol of vitamin D supplementation in patients with HIV-infection. European AIDS Clinical Society EACS Guidelines AIDS Res Treat. 2016; Hindawi Publishing Corporation AIDS Research and Treatment. Vol 2016
- Havens PL, Mulligan K, Hazra R, et al. Serum 25-hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV-1 infection. J Clin Endocrinol Metab. 2012;97:4004–4013.
- Arpadi SM, McMahon DJ, Abrams EJ, et al. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. Am J Clin Nutr. 2012;95:678–685.
- Bischoff-Ferrari HA, Dawson-Hughes B, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. Br Med J (Clinical research ed).2009:339b3692.
- Ross AC, Manson JE, Abrams SA, The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96 (1): 53–58.
- Grant A M, Avenell A, Campbell M K, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebocontrolled trial. Lancet .2005:365:162–168.
- Bruera D, Luna N, David DO, et al. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003;17(13):1917–1923.
- Carr A, Grund B, Neuhaus J, et al. Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):137–146.
- Piso RJ, Rothen M, Rothen JP, et al. Per oral substitution with 300000 IU vitamin D (Cholecalciferol) reduces bone turnover markers in HIV-infected patients. BMC Infect Dis. 2013;13:577. doi10.1186/1471-2334-13-577.
