Abstract
Background: The efficacy and safety of a single tablet regimen (STR) of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) was analyzed in Phase 3 clinical trials in antiretroviral therapy (ART)-naive and ART-experienced Asian participants infected with human immunodeficiency virus (HIV)-1 through 96 or 144 weeks.
Objective: In Asian population requiring treatment, it is imperative to have data specific to this group, particularly as there is a general concern that Asians with lower body weight have increased risk of tenofovir disoproxil fumarate (TDF)-related renal dysfunction.
Methods: Studies -104 and 111 were randomized, double-blind, placebo-controlled, 144-week studies conducted in ART-naive participants, comparing E/C/F/TAF versus E/C/F/TDF. Study 109 was a randomized, open-label, 96-week study conducted in virologically suppressed, ART-experienced participants, who switched to E/C/F/TAF from ritonavir/cobicistat-boosted atazanavir ATV+(RTV or COBI) + F/TDF regimens, from non-nucleoside reverse transcriptase inhibitors (NNRTI) + F/TDF regimens, or from E/C/F/TDF. Study 112 was a single arm, open-label, 144-week study conducted in HIV suppressed, ART-experienced participants with mild-moderate renal impairment, who switched to E/C/F/TAF.
Results: Asian participants in these studies had sustained efficacy safety and tolerability. In Study 104/111, Asian participants achieved 93% virologic suppression on TAF vs 88% on TDF at week 144. At baseline, there were numerically more Asians with median CD4 counts < 200 cells/uL and VL > 100,000 c/mL. In Study 109, 95% of Asians on TAF vs 86% on TDF maintained virologic suppression at week 96. Lastly, in Study 112, 91% maintained virologic suppression at week 144. There were no discontinuations due to renal AE, no cases of PRT or Fanconi syndrome in any of the studies.
Introduction
UNAIDS estimated 5.1 million people were living with human immunodeficiency virus (HIV) in Asia and the Pacific region in 2016, with 300,000 new infections per year, making the region the second highest for HIV prevalence in the world.Citation1 Progress has been made on the impact of HIV infection, which saw a decline of 13% in new infections from 2010 to 2016. However, new infections are on the rise in some countriesCitation2 and in key affected populations.Citation3 Given that healthcare providers in Asia and the Pacific region are managing these patients, we feel that it is important to provide data mirroring this patient population to help guide clinical practice.
At the time the study was initiated, the single tablet regimen (STR) containing E/C/F/TAF is recommended in the US Department of Health and Human Services (DHHS) and the European acquired immunodeficiency syndrome (AIDS) Clinical Society (EACS)Citation4,Citation5 guidelines for initial HIV therapy. Two identical Phase 3, randomized, double-blind, placebo-controlled clinical trials in antiretroviral therapy (ART)-naïve adults infected with HIV-1, E/C/F/TAF demonstrated non-inferior efficacy at weeks 48 and 96, and at week 144 was superior to E/C/F/TDF (Study 104 and 111). In addition, there was significantly less impact on renal biomarkers and bone mineral density (BMD), including no cases of renal and bone adverse events (AEs) leading to discontinuation.Citation6–8 Study 109 examined the efficacy, safety and tolerability of switching to E/C/F/TAF from ATV+(RTV or COBI) + F/TDF, from NNRTI + F/TDF or from E/C/F/TDF regimens, in virologically suppressed (VS; HIV-1 RNA <50 copies/mL), ART-experienced adults participants.Citation9,Citation10 At weeks 48 and 96, Study 109 demonstrated that switching to E/C/F/TAF from an F/TDF + 3rd agent regimen was associated with significantly higher rates of virologic success, as well as improvements in renal biomarkers and significant increases in spine and hip BMD. Study 112 examined the efficacy, safety and tolerability of switching to E/C/F/TAF in VS adult participants with estimated glomerular filtration rate (eGFR) of 30–69 mL/min.Citation11,Citation12 Through week 144, switching to E/C/F/TAF maintained high rates of viral suppression while renal function remained stable with improvements in multiple measures of proteinuria. There were no cases of proximal renal tubulopathy (PRT) or Fanconi syndrome in these participants. There were significant gains in spine and hip BMD through week 144 after switching to E/C/F/TAF from a TDF-containing regimen. In addition, these findings were also observed in participants with the lowest baseline eGFR (30–39 mL/min), diabetes, and in women.Citation13–15
In Asian population requiring treatment, it is imperative to have data specific to this group, particularly as there is a general concern that Asians with lower body weight have increased risk of TDF-related renal dysfunction.Citation16 Here, we report data from sub-analyses of E/C/F/TAF on efficacy and safety in Asian adults enrolled in Studies 104/111, 112, and 109.
Methods
Study design and participants
Details on design, inclusion criteria, and methodology of the trials for the overall study population have been previously reported.Citation6–12 Briefly, in the pooled analysis of two ART-naïve studies (Studies 104 and 111), 1,733 participants were randomized to E/C/F/TAF (n = 866) or E/C/F/TDF (n = 867). In ART-experienced, VS participants (Study 109) 1,436 participants were randomized 2:1 to switch to E/C/F/TAF (n = 959) or continue their current regimen of F/TDF + a 3rd agent (n = 477). And in VS participants with mild-moderate renal impairment (Study 112), 242 participants switched to E/C/F/TAF. At study entry, participants self-identified (on an enrollment questionnaire) as Asian or non-Asian (White, Black, American Indian/Alaskan, native Hawaiian/Pacific Islander). These studies were not stratified by Asian versus non-Asian race. In these four clinical trials, 308 Asian participants consisting of 180 from Studies 104 and 111 (E/C/F/TAF, n = 91; E/C/F/TDF, n = 89), 94 from Study 109 (E/C/F/TAF, n = 59 vs. F/TDF + 3rd agent, n = 35), and 34 from Study 112 (E/C/F/TAF, n = 34) were included in this sub-analysis of the E/C/F/TAF data.Citation17–19
Statistical analysis
Key endpoints for these post-hoc Asian sub-analyses included: a proportion in each group with plasma HIV-1 RNA <50 copies/mL as defined by U.S. FDA snapshot algorithm; changes in CD4 cell count; AEs and discontinuations due to AEs; changes in eGFR and quantitative proteinuria; and changes in spine and hip BMD. AEs were coded with the Medical Dictionary for Regulatory Activities. The Fisher exact test was used to compare differences for AEs and Wilcoxon rank-sum test to compare differences for continuous laboratory test results.
Results
Asian participants (%) on E/C/F/TAF in the ART-naïve (Study104/111 (11%) pooled) and ART-experienced (Study 109 (6%) and 112 (14%)) had a median age 30, 39, and 55 years, were mostly male 55, 69, and 74% with a median eGFR (Cockcroft-Gault) of 109, 94, and 44 mL/min, respectively. At baseline, there were numerically more Asians with median CD4 counts < 200 cells/uL and VL > 100,000 c/mL ().
Table 1 Demographics.
Overall, Asians on E/C/F/TAF had similar high rates of virologic success (HIV-RNA <50 copies/mL) and comparable immunological outcomes as non-Asians. At week 144, in ART-naïve Asians, virologic suppression rates were 93% vs 88% (p = 0.061) (95% CI: −1.5 to 18%) compared to non-Asians with 83% vs 79% for E/C/F/TAF and E/C/F/TDF, respectively There were no Asians in either arm with HIV-1 RNA ≥ 50 copies/mL. However, 5% non-Asians on E/C/F/TAF and 4% on E/C/F/TDF had HIV-1 RNA ≥50 copies/mL (). Mean CD4+ T cell count increase from baseline was 297 vs. 278 cells/mm3 (p = 0.68) for Asians on E/C/F/TAF and E/C/F/TDF, respectively.
Figure 1 (a) Study 104/111: ART-Naïve at 144. (b) Study 109: Virologically suppressed at week 96. *P-values were estimated from the CMH test stratified by the prior treatment regimen (STB, ATR, or boosted ATV + TVD). (c) Study 112: Virologically Suppressed with renal impairment at Week 144.
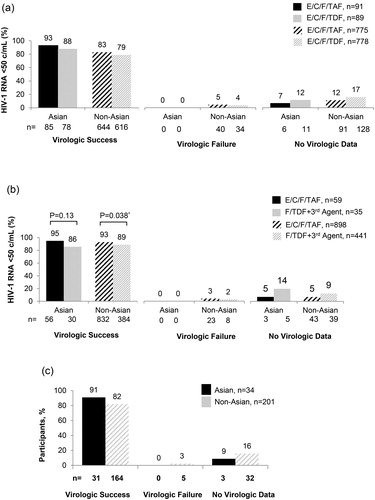
In ART-experienced Asians (Study 109), virologic suppression was maintained through week 96 in 95% vs 86% (p = 0.13) (95% CI: −5.3 to 23.8%), while non-Asians were 93% vs 89% (p = 0.038) for E/C/F/TAF and F/TDF-containing regimens, respectively. There were no Asians in either arm, compared to 3% non-Asians on E/C/F/TAF and 2% on F/TDF + 3rd agent, who had HIV-1 RNA ≥50 copies/mL (). Mean CD4+ T cell count increase from baseline through week 96 was 46 vs. −15 cells/mm3 (p = 0.067) for Asians on E/C/F/TAF and F/TDF-containing regimens, respectively. In ART-experienced Asians with mild-moderate renal impairment (Study 112), 91% maintained viral suppression after switching to E/C/F/TAF, through 144 weeks compared to 82% of non-Asians. There were no Asians, compared to 3% non-Asians, who had HIV-1 RNA ≥ 50 copies/mL (). Mean CD4+ T cell count increase from baseline was 18 cells/mm3.
Safety and tolerability of E/C/F/TAF in both ART-naïve and ART-experienced Asian participants were similar to that of non-Asian participants on E/C/F/TAF in all four studies. In study 104/111, through 144 weeks, among Asians on E/C/F/TAF vs E/C/F/TDF, common AEs were nasopharyngitis (23% vs 14%), upper respiratory tract infection (20% vs. 15%), nausea (14% vs. 18%), and diarrhea (13% vs. 14%). Grade 3 or 4 AEs occurred with similar frequency at 2% in each arm. AEs leading to discontinuations were similar between both arms (1%, n = 1 vs. 2%, n = 2, respectively). There were no discontinuations due to renal AEs in either treatment arm; however there were 12 renal AE discontinuations among non-Asians on E/C/F/TDF.
In Study 109, the most common AE among Asians on E/C/F/TAF or F/TDF-containing regimens was upper respiratory tract infection (12% vs. 9%, respectively). Grade 3 or 4 AEs were 8% on E/C/F/TAF and 9% on F/TDF-containing regimens. No Asians on E/C/F/TAF discontinued study drug due to any AEs vs. 2 on F/TDF-containing regimens (one due to Fanconi syndrome). For the non-Asians, there were 2 participants on E/C/F/TAF and 4 on F/TDF-containing regimens that discontinued due to renal AEs. In study 112, Asians with mild-moderate renal impairment, the most common AE on E/C/F/TAF through week 144 was dizziness (15%). Grade 3 or 4 AEs occurred in 26% (n = 9) of Asians and 20% (n = 42) in non-Asians. There were no Asians who discontinued study drug due to any AEs, whereas there were 12 non-Asians, who discontinued (previously reportedCitation12), details of discontinuations in non-Asians are further addressed in the “Discussion” section.
The median changes in eGFR from baseline through 144 were similar to those observed in non-Asians on F/TDF-containing regimens. In ART-naïve Asian participants, those on E/C/F/TAF had similar changes in eGFR from baseline to week 144 compared to those on E/C/F/TDF (−9 vs. −10 mL/min). In VS Asian participants, switching to E/C/F/TAF through week 96 resulted in an increase in eGFR of 3.1 mL/min vs a decrease of −4.2 mL/min for those continuing on F/TDF + 3rd agent (p = 0.04). In Asians with mild-moderate renal impairment, eGFR remained stable from baseline through 144 weeks.
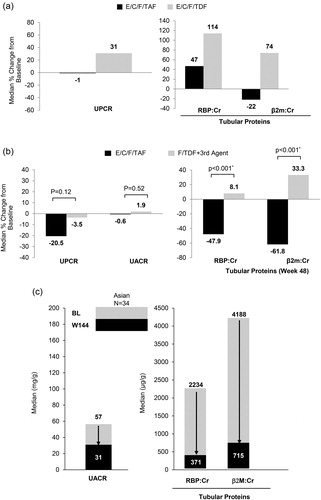
The renal safety of TAF-based regimens has been demonstrated in numerous multinational studies with long-term (3 years) data in the overall study population.Citation6–12 The significant improvement in renal biomarkers (urine protein to creatinine ratio [UPCR], urine albumin to creatinine ratio [UACR], urine retinol binding protein to creatinine ratio [RBP:CR] and urine beta-2-microglobulin to creatinine ratio [β2M:CR]) among Asian participants were similar to non-Asians. In ART-naïve Asians on E/C/F/TAF (study 104/111) there were decreases or smaller increases in UPCR and tubular proteins compared to participants on E/C/F/TDF (). Asians who were VS and switched to E/C/F/TAF (study 109) had a decrease in proteinuria (UPCR and UACR) and tubular proteins (RBP:Cr and β2M:Cr) while those who continued on F/TDF-containing regimens had increases or minor decreases in all renal biomarkers (). In Asians with mild-moderate renal impairment (study 112), those who switched to E/C/F/TAF had decreases in all renal biomarkers () with no PRT or Fanconi syndrome.
Figure 2 (a) Changes in proteinuria in ART-Naïve Asian at week 144. (b) Changes in proteinuria in virologically suppressed Asian at Week 96. *P-values were estimated using the Van Elteren test stratified by the prior treatment regimen (STB, ATR, or boosted ATV + TVD). (c) Quantitative proteinuria at baseline and Week 144 in mild-moderate renal impaired Asian.
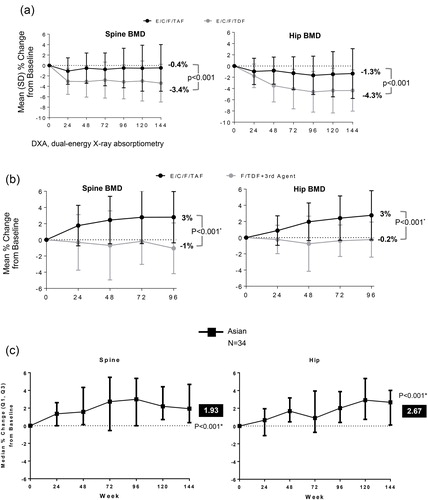
In addition to the renal safety seen with TAF-based regimens, significant improvements in BMD have been demonstrated in these multinational studies. Both Asian and non-Asian participants in the naïve and switch studies had improvements. In Study 104/111, ART-naïve Asians on E/C/F/TAF compared to E/C/F/TDF had significantly less decrease in mean % change in BMD from baseline through week 144 for spine (−0.5% vs −3.4%, respectively) and hip (−1.3% vs −4.4%, respectively) (). In Study 109, VS Asians who switched to E/C/F/TAF through 96 weeks had a significant increase of 3% in spine and hip BMD, while those who remained on their current F/TDF-containing regimens had a slight decreased in BMD of the spine and hip (−1 and −0.2%, respectively) (). Furthermore, switching to E/C/F/TAF among Asians with mild-moderate renal impairment had a significant BMD improvement of approximately 2% in the spine and 3% in the hip through week 144 ().
Figure 3 (a) Change in bone mineral density in ART-Naïve Asian through week 144. (b) Change in bone mineral density in virologically suppressed Asian week 96. *P-values were estimated from the analysis of variance model including treatment and prior treatment regimen as fixed effects. (c) Change in bone mineral density in mild-moderate renal impaired Asian through Week 144. *P-values estimated for the change from baseline was based on 2-sided Wilcoxon signed rank test.
Lipid outcomes among Asians were similar to non-Asians. Both treatment-naïve and treatment-experienced participants who were on a TAF-based regimen experienced a small, but statistically significant, increase in all fasting lipid parameters [total cholesterol (TC), low density lipoprotein (LDL), triglyceride (TG), and high-density lipoprotein (HDL)] when compared to those on F/TDF-containing regimens, however the TC:HDL ratio remained unchanged in the treatment naïve population and showed a statistically significant increase in Study 109 overall population.
Discussion
Tenofovir prodrugs are preferred NRTIs for initial therapy based on their favorable efficacy and safety data in randomized clinical trials and are used widely in clinical practice.Citation4 However, TDF has been associated with renal toxicity (including cases of Fanconi syndrome),Citation20,Citation21 which can unpredictably occur in patients with normal or low eGFR and limits its use in those with renal impairment. In addition, the use of TDF has been associated with a greater loss of BMD when compared to other NRTI options, and has been shown in at least one study to increase fracture risk.Citation22–24 Given that people with HIV are living longer, there is an unmet medical need for an NRTI backbone option that provides potent antiviral activity comparable to TDF but with an improved safety profile.
TAF is a tenofovir prodrug that minimizes tenofovir plasma levels resulting in fewer off-target side effects, such as renal and bone toxicity, compared to TDF. In two trials of treatment-naïve adults with HIV-1 infection, a 10 mg oral dose of TAF in FTC/TAF + EVG/COBI (administered as an STR) resulted in >90% lower concentration of TFV in plasma as compared to a 300 mg oral dose of TDF in FTC/TDF + EVG/COBI.Citation6,Citation25
In the article, we presented the results for four different studies. They are for naïve (104 and 111), switch (109) and renal impairment (112) populations, respectively. Since the populations are very different for the underlying disease status clinically, we would not pool data from the four studies together. In this case, we think a multi-center/multi-study modeling to adjust for the effect of study would not be needed.
We reviewed the efficacy, safety and tolerability of E/C/F/TAF in treatment naïve and VS, treatment-experienced Asian adults living with HIV. In ART-naïve studies, Asians on E/C/F/TAF achieved a numerically higher rate of virologic suppression at week 144 compared to Asians on E/C/F/TDF and there were no Asians who had an HIV-1 RNA ≥50 copies/mL. Participants in Study 109 maintained high rates of virologic suppression, and the difference between non-Asians on E/C/F/TAF vs. F/TDF-containing regimens was statistically significant. Asians on E/C/F/TAF maintained a numerically higher rate of virologic suppression than Asians on F/TDF-containing regimens, but did not achieve statistical significance due to small sample size. There were no Asians on E/C/F/TAF that had an HIV-1 RNA ≥ 50 copies/mL at week 96.
The difference in baseline median CD4 cell counts seen in study 104/111 (higher in non-Asians) may be explained due to Asians presenting at later stages of their HIV infection. Moreover, there was a higher proportion of Asians with CD4 cell count less than 200 cells/mm3, 15% compared to non-Asians, 13%. Despite initiating therapy at a later stage of HIV infection, Asians on E/C/F/TAF still had an increase in CD4 cell count from baseline of 297 cells/mm3 at week 144.
The overall safety and tolerability of E/C/F/TAF in both ART-naïve and ART-experienced Asians were similar to non-Asians. The overall AEs, grade 3 or 4 AEs, and AEs leading to discontinuation were low in both Asians and non-Asians. Of note, there were no renal AE discontinuations among ART-naïve Asians. In study 104/111 there was only one discontinuation due to any AE among the Asians on E/C/F/TAF which was a hemorrhagic stroke deemed not related to study drug by the primary investigator (PI). Among the non-Asians on E/C/F/TDF, there were 12 renal AE discontinuations: renal tubular disorder (3), renal failure (2), glomerular filtration rate decreased (1), nephropathy (1), Fanconi syndrome acquired and glycosuria (1), proteinuria (1), SCr increased (2), and bladder spasm (1), which has been presented.Citation8 In Study 109, there were no Asians taking E/C/F/TAF discontinued study drug for any reasons, compared to 2 discontinuations in the F/TDF-containing regimen arm, one was due to Fanconi syndrome, which was drug related. Among the non-Asians in Study 109, there were 2 renal discontinuations on the E/C/F/TAF arm: acute kidney injury and tubulo-interstitial nephritis, both not related to study drug. For the non-Asians on F/TDF-containing regimens, there were 4 renal discontinuations: elevated serum creatinine (2), chronic kidney disease (1), and renal colic (1), all were related to study drug. Lastly, in Study 112, there were no renal discontinuations among the Asians, which included two Asians with a history of TDF-associated Fanconi syndrome who were successfully switched to E/C/F/TAF without recurrence of tubulopathy through 144 weeks. The main limitation of this sub-analysis is the relatively small number of Asian participants (308), who accounted for 9% of the overall population of the participants enrolled in the ART-naïve and ART-experienced studies. This restricted the definitive assessment of the efficacy, safety and tolerability of E/C/F/TAF in Asian participants, and may limit generalization of the results.
Conclusions
Asian participants in clinical studies of treatment naïve and VS demonstrated that starting with, or switching to, E/C/F/TAF obtained or maintained high non-inferior rates of virologic suppression, with few AEs leading to discontinuation. In Asians living with HIV, long term (96 and 144 week) efficacy, safety and tolerability results support starting or switching to E/C/F/TAF.
Notes on contributors
Yeon-Sook Kim, MD practices at Chungnam National University, Daejeon, South Korea. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide.
Shinichi Oka, MD practices at National Center for Global Health and Medicine Hospital, Tokyo, Japan. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Ploenchan Chetchotisakd, MD practices at Khon Kaen University, Khon Kaen, Thailand. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Amanda Clarke, MD practices at Brighton and Sussex University Hospitals NHS Trust, Brighton, UK. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Khuanchai Supparatpinyo, MD practices at Chiang Mai University, Chiang Mai, Thailand. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Anchalee Avihingsanon, MD practices at Thai Red Cross AIDS Research Centre, Bangkok, Thailand. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Winai Ratanasuwan, MD practices at Siriraj Hospital, Mahidol University, Bangkok, Thailand. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Sasisopin Kiertiburanakul, MD practices at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Kiat Ruxrungtham, MD practices at Thai Red Cross AIDS Research Centre (HIV-NAT). Primary investigator for elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide. Not receiving funding from Gilead Sciences.
Do Tran, SangYoun Yang, Susan Guo, YaPei Liu, Moupali Das, Damian McColl, Roberto Corales, Chris Nguyen are employee at Gilead Sciences.
Acknowledgments
The authors would like to acknowledge and thank the patients who participated in these studies, as well as the site and study management staff whose effort made this study possible. All investigators and sites that participated in these studies have previously published their respective results.
References
- UNAIDS (2017) Data Book.
- UNAIDS (2017) ‘Ending AIDS: Progress towards the 90-90-90 Targets’.
- UNAIDS (2013) ‘HIV in Asia and the Pacific.
- Department for Health and Human Services (DHHS). Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. March 2018. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0
- European AIDS Clinical Society (EACS). Guidelines Version 9.0, October 2017. Available at http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html
- Sax PE, Wohl DA, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615.
- Wohl DA, Oka S, Clumeck N, et al. A randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial hiv-1 treatment: week 96 results. J Acquir Immune Defic Syndr 2016;72:58–64.
- Arribas JR, Thompson M, Sax PE, et al. Brief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment: week 144 results. J Acquir Immune Defic Syndr. 2017;75(2):211–218.
- Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52.
- DeJesus E, Haas B, Segal-Maurer S, et al. Superior efficacy and improved renal and bone safety after switching from a tenofovir disoproxil fumarate regimen to a tenofovir alafenamide-based regimen through 96 weeks of treatment. AIDS Res Hum Retroviruses. 2016. 34(4):337–342.
- Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr. 2016;71(5):530–537.
- Post F, Tebas P, Clarke A, et al. Longer-term safety of tenofovir alafenamide in renal impairment. HIV Med 2016;17:15.
- Stein DK, Pozniak A, Gupta S, et al. Tenofovir alafenamide in participants with diabetes and renal impairment: renal safety through 96 weeks. American Society Microbe 2016. Oral presentation.
- McDonald C, Khalsa A, Kerkar S, et al. Efficacy and Safety of Tenofovir Alafenamide in HIV-Infected Women with Renal Impairment: 96-Week Results. American Society Microbe 2016. Oral presentation.
- Brown TT, Yin MT, Gupta S, et al. Switching from TDF To TAF in HIV-infected adults with low BMD: A pooled analysis. CROI 2017. Poster 683.
- Nishijima T, Komatsu H, Gatanaga H, et al. Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PLoS One. 2011;6(7):e22661.
- Kim Y-S, Chin BS, Oka S, et al. Longer-term efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in treatment − Naïve Asian Adults. KoSAids 2016. Poster P-31.
- Kim Y-S, Chetchotisakd P, Supparatpinyo K, et al. Efficacy and safety of switching from a tenofovir disoproxil fumarate-based regimen to co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in HIV-suppressed Asian adults. KoSAids 2016. Poster P-30.
- Chin BS, Avihingsanon A, Chetchotisakd P, et al. Efficacy and safety of co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in virologically suppressed Asian adults with renal impairment. KoSAids 2016. Poster P-28.
- Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57:773–780.
- Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55:49–57.
- Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–972.
- McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801.
- Tungsiripat M, Kitch D, Glesby MJ, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS. 2010;24:1781–1784.
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455.
