Abstract
Background: The once-daily, single-tablet regimen darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) 800/150/200/10 mg is approved for the treatment of HIV-1 infection. The 48-week efficacy and safety of D/C/F/TAF versus darunavir/cobicistat + emtricitabine/tenofovir disoproxil fumarate (control) in treatment-naïve adults were demonstrated in the phase 3 AMBER study.
Objective: To describe AMBER outcomes across patient subgroups based on demographic and clinical characteristics at baseline.
Methods: AMBER patients had viral load (VL) ≥1000 copies/mL, CD4+ cell count >50 cells/µL, and genotypic susceptibility to darunavir, emtricitabine, and tenofovir. Primary endpoint was the proportion of patients with virologic response (VL <50 copies/mL; FDA snapshot). Safety was assessed by adverse events, estimated glomerular filtration rate (cystatin C; eGFRcystC), and bone mineral density. Outcomes were assessed by age (≤/>50 years), gender, race (black/non-black), baseline VL (≤/>100,000 copies/mL), baseline CD4+ cell count (</≥200 cells/µL), and baseline WHO clinical stage of HIV infection (1/2).
Results: For the 725 AMBER patients (D/C/F/TAF: 362; control: 363), virologic response rates at week 48 were similar with D/C/F/TAF (91%) and control (88%), and this was consistent across all subgroups. Adverse event rates were similar in both arms, although numerically higher among patients >50 years and women, relative to their comparator groups, regardless of treatment arm (notably, sample sizes were small for patients >50 years and women). Improvements in eGFRcystC and stable bone mineral density were observed with D/C/F/TAF overall, and results were generally consistent across subgroups.
Conclusions: For treatment-naïve patients in AMBER, initiating therapy with the D/C/F/TAF single-tablet regimen was an effective and well-tolerated option, regardless of demographic or clinical characteristics.
Trial registration: ClinicalTrials.gov identifier: NCT02431247.
Introduction
For treatment-naïve patients with human immunodeficiency virus (HIV)–1 infection, there are now a variety of antiretroviral (ARV) treatment options.Citation1,Citation2 The choice of which ARV regimen to initiate could be influenced by several factors, such as the presence of transmitted resistance-associated mutations (RAMs), the likelihood that the patient will be adherent to treatment, how far advanced the disease is, and concomitant use of other medications. For patients with polypharmacy, minimizing drug–drug interactions is a consideration; for example, patients may use cobicistat as a boosting agent instead of ritonavir.Citation2 Such patient characteristics could affect the efficacy and/or safety of a given ARV regimen, and the time from HIV-1 diagnosis to treatment initiation is another variable. During the primary HIV-1 infection period, transmission is especially likely and it is important that patients begin treatment as soon after infection as possible. In the case of rapid initiation models of care, for example, a regimen is initiated before certain information is known, such as the presence of RAMs and the propensity of a patient to adhere to therapy.Citation3–6
The oral, once-daily, single-tablet regimen (STR) darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) 800/150/200/10 mg is approved for the treatment of HIV-1 infection in the United States (US), Europe, and Canada. This STR is based on the protease inhibitor (PI) darunavir, which has demonstrated durable efficacy, a favorable safety profile, and a high genetic barrier to the development of resistance.Citation7,Citation8 A darunavir-based regimen is recommended in guidelines from the European AIDS Society and, in guidelines from the US Department of Health and Human Services (DHHS), it is recommended in clinical situations such as when resistance data are not available or treatment adherence is a concern (i.e., conditions in which a high barrier to resistance is important).Citation1,Citation2 Emtricitabine/TAF is an optimal nucleos(t)ide reverse transcriptase inhibitor backbone for ARV therapy, largely due to the improved renal and bone safety of TAF compared with the older tenofovir prodrug tenofovir disoproxil fumarate (TDF).Citation9–11 Moreover, as an STR, adherence with D/C/F/TAF may be higher than for a multi-tablet regimen.Citation12,Citation13
In the phase 3 AMBER study of treatment-naïve adults initiating ARV therapy, D/C/F/TAF was noninferior (10% margin) compared with a regimen consisting of darunavir/cobicistat + emtricitabine/TDF for virologic response at week 48.Citation14 Patients treated with D/C/F/TAF also had more favorable renal and bone safety profiles versus those who used darunavir/cobicistat + emtricitabine/TDF. Because treatment-naïve patients form a diverse population, we evaluated results from AMBER across subgroups of patients based on demographic characteristics and clinical characteristics at baseline.
Materials and methods
Study design
AMBER (ClinicalTrials.gov Identifier: NCT02431247) is a phase 3, randomized, multicenter, active-controlled, double-blind, placebo-controlled study to evaluate efficacy, resistance development, and safety in treatment-naïve patients with HIV-1 who initiate ARV therapy with the D/C/F/TAF STR versus darunavir/cobicistat (fixed-dose combination tablet) + emtricitabine/TDF. Detailed study methods have been described elsewhereCitation14 and are briefly summarized here.
Eligible patients were ARV treatment-naïve adults with HIV-1 infection. Additional inclusion criteria were HIV-1 RNA ≥1000 copies/mL, CD4+ cell count >50 cells/µl, and genotypic susceptibility to darunavir, tenofovir, and emtricitabine. Patients were randomized 1:1 to initiate D/C/F/TAF or darunavir/cobicistat + emtricitabine/TDF (control). Randomization was stratified by viral load (VL; ≤ or >100,000 copies/mL) and CD4+ cell count (< or ≥200 cells/µL) at screening. The treatment phase was 48 weeks, with an extension to week 96.
AMBER was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was reviewed and approved by an institutional review board or independent ethics committee. All patients provided written informed consent.
Analyses
The primary study endpoint was the proportion of patients with virologic response (HIV-1 RNA <50 copies/mL; US Food and Drug Administration [FDA] snapshot approach) at week 48. The difference (95% CI) between the D/C/F/TAF and control arms was calculated for subgroups using exact CIs; significance was not assessed for subgroup analyses. Post-baseline resistance was evaluated for patients with protocol-defined virologic failure (VF).
Safety was evaluated by monitoring adverse events (AEs), with relatedness to study drug determined by investigators. Further safety analyses are reported for subgroups based on demographic characteristics; these included renal laboratory parameters (estimated glomerular filtration rate [calculated using serum cystatin C; eGFRcystC], urine β-2 microglobulin:creatinine ratio, urine albumin:creatinine ratio, urine protein:creatinine ratio, and urine retinol-binding protein:creatinine ratio), lipid laboratory parameters, and for patients in the bone investigation substudy, changes over time in bone mineral density (BMD) at the hip, lumbar spine, and femoral neck.
Pre-specified subgroup analyses were performed on all randomized patients who received ≥1 dose of study drug. Demographic subgroups were based on age (≤50 vs >50 years), gender, and race (non-black/African American vs black/African American). Subgroups for clinical characteristics at baseline were based on VL (HIV-1 RNA ≤100,000 vs >100,000 copies/mL), CD4+ cell count (<200 vs ≥200 cells/µL), and World Health Organization (WHO) clinical stage of HIV infection (1 [asymptomatic] vs 2 [mild symptoms]).
In addition, age subgroups (≤50 vs >50 years) were further divided based on the presence or absence of polypharmacy (defined as use of ≥5 [non-ARV] concomitant medications at baseline), as this may be of particular concern among older patients.
Results
Study population
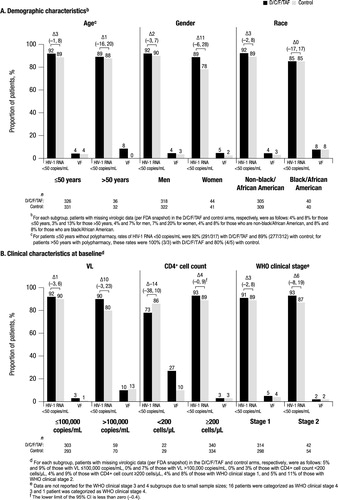
Overall, 725 patients were randomized and treated in AMBER (362 in the D/C/F/TAF arm and 363 in the control arm). Baseline demographic and clinical characteristics were generally balanced between both treatment arms (). Overall, 9% of patients were >50 years of age, 12% were women, 12% were black/African American, and 5% had polypharmacy. Few patients had advanced HIV-1 disease; overall, 18% of patients had HIV-1 RNA ≥100,000 copies/mL, 7% had a CD4+ cell count <200 cells/µL, and 2% had WHO clinical stage 3 or 4 infection. Due to the small numbers of patients with WHO clinical stage 3 or 4 infection, these patients were not included in the subgroup analyses, and in general the sample size for each subgroup should be taken into account when interpreting results.
Table 1 Baseline demographic and clinical characteristicsTable Footnotea
Efficacy
Overall, the virologic response rate at week 48 was high and similar in the D/C/F/TAF (91%; 331/362) and control (88%; 321/363) arms; as previously reported, D/C/F/TAF was found to be non-inferior to control.Citation14 Correspondingly, VF rates were low with both D/C/F/TAF (4%; 16/362) and control (3%; 12/363) arms. Similar virologic response and VF rates were observed with D/C/F/TAF and control across all subgroups (), indicating that the efficacy of D/C/F/TAF in treatment-naïve patients is not impacted by demographic or clinical characteristics at the time of treatment initiation.
Figure 1. Virologic response and VF rates at week 48 by subgroup.aVF: virologic failure; D/C/F/TAF: darunavir/cobicistat/emtricitabine/tenofovir alafenamide; HIV-1: human immunodeficiency virus–1; VL: viral load; WHO: World Health Organization; FDA: Food and Drug Administration.athe differences (95% CI) in virologic response rate between treatment arms are reported above the brackets.
Resistance
Of 9 patients with protocol-defined VF and available resistance data, none developed post-baseline darunavir, primary PI, or tenofovir RAMs.Citation14 Only one patient (D/C/F/TAF arm) developed M184I/V, an emtricitabine and lamivudine RAM; ad hoc deep sequencing showed that M184V was present as a minority species at baseline. This patient also had K103N at screening, indicating transmitted nonnucleoside reverse transcriptase inhibitor (efavirenz/nevirapine) resistance.
Safety
No clinically relevant differences were observed in the incidence of AEs with D/C/F/TAF versus control across subgroups based on demographic characteristics () or clinical characteristics at baseline (), consistent with the overall study population.Citation14 There was a low incidence of AEs leading to discontinuation with D/C/F/TAF and control both overall (2% and 4% [regardless of relatedness], respectivelyCitation14) and across subgroups. The incidences of serious AEs and grades 3–4 AEs were also low in both treatment arms across subgroups. The most common study drug–related AEs (≥5% in either arm) overall were diarrhea (D/C/F/TAF, 9%; control, 11%), rash (D/C/F/TAF, 6%; control, 4%), and nausea (D/C/F/TAF, 6%; control, 10%).Citation14
Table 2 Summary of AEs through week 48 by demographic characteristics
Table 3 Summary of AEs through week 48 by clinical characteristics at baseline
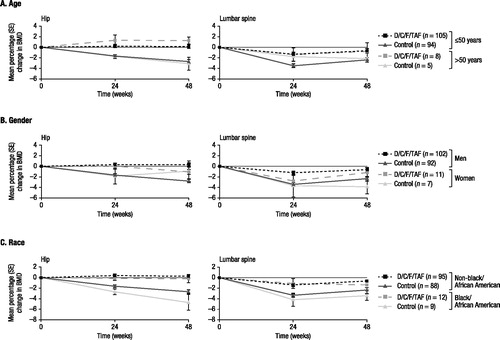
Renal function, as assessed by changes in eGFRcystC over time, improved from baseline to week 48 with D/C/F/TAF overall,Citation14 and results were generally consistent across subgroups by age, gender, and race (). Similarly, mean changes in markers of proteinuria were markedly improved from baseline to week 48 with D/C/F/TAF versus control overall, and results were generally consistent across demographic subgroups (Supplementary Figure S1). Median lipid parameter values measured at baseline and week 48 were similar across demographic subgroups in the D/C/F/TAF and control arms, and tended to increase with D/C/F/TAF (Supplementary Figure S2). Among patients in the bone investigation substudy, hip, lumbar spine, and femoral neck BMD remained stable from baseline to week 48 with D/C/F/TAF across demographic subgroups ( and Supplementary Figure S3).
Figure 2. Change in eGFRcystC from baseline to week 48 by demographic characteristics.aeGFRcystC: estimated glomerular filtration rate (calculated using serum cystatin C); SE: standard error; D/C/F/TAF: darunavir/cobicistat/emtricitabine/tenofovir alafenamide.aeGFRcystC by Chronic Kidney Disease Epidemiology Collaboration, and measured at weeks 0, 2, 4, 8, 12, 24, 36, and 48.
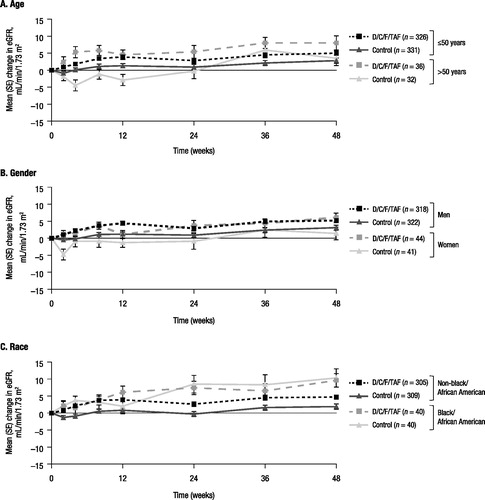
Figure 3 Changes from baseline in BMD over time (hip and lumbar spine) by demographic characteristics.aBMD: bone mineral density; SE: standard error; D/C/F/TAF: darunavir/cobicistat/emtricitabine/tenofovir alafenamide.adata are from the bone investigation substudy, which included 113 patients in the D/C/F/TAF arm and 99 patients in the control arm. The total number of substudy patients in each treatment arm for each subgroup is reported in the legends.
Discussion
For treatment-naïve individuals with HIV-1 infection, choosing an effective initial treatment strategy is dependent on numerous factors that vary across this heterogeneous population.Citation1,Citation2 In this analysis of AMBER, the efficacy and safety of D/C/F/TAF and darunavir/cobicistat + emtricitabine/TDF were consistent across subgroups based on demographic and clinical characteristics. Overall, patients in the D/C/F/TAF arm achieved a high virologic response rate of 91% at week 48, which was noninferior to control (88%).Citation14 The virologic response rate with D/C/F/TAF is among the highest achieved by an STR in phase 3 studies of treatment-naïve patients (range: 80%–93%).Citation15–22 VF rates in AMBER were low, and no emerging RAMs to darunavir or any other component of the regimen were observed.
Overall and across subgroups, the AE profile of D/C/F/TAF was generally similar to the control regimen.Citation14 Notably, the incidence of AEs tended to be numerically higher in patients >50 versus ≤50 years, and in women versus men, regardless of treatment arm; however, sample sizes were very small for patients >50 years and women versus their respective comparator subgroups. The potential impact of age and gender on the safety of ARVs has been examined in previous studies; no clinically meaningful impact has been observed.Citation23–26 Moreover, among the few patients >50 years with polypharmacy in the D/C/F/TAF arm in the current analysis, none discontinued due to a study drug–related AE. Favorable renal and bone outcomes were observed in AMBER with D/C/F/TAF relative to control,Citation14 which is consistent with the use of TAF versus TDF. These positive renal and bone safety results were also observed across age, gender, and race subgroups, with only a few exceptions. Lipid parameters tended to increase with D/C/F/TAF from baseline to week 48; importantly, low and similar proportions of patients in the D/C/F/TAF and control arms initiated lipid-lowering therapy during the treatment period (2% and 1%, respectivelyCitation14).
A limitation of this study was the small numbers of patients in some subgroups (e.g., >50 years, women, black/African American, and CD4+ cell count <200 cells/µL). In the case of the FDA snapshot analysis of virologic response, these small sample sizes were sometimes associated with large 95% CIs for the difference between the D/C/F/TAF and control arms in virologic response rate. These small subgroup sample sizes also limited the interpretation of changes in lipid parameters from baseline. Despite this, the current analysis of AMBER demonstrated that a diverse patient population could consider initiating ARV therapy with D/C/F/TAF. While statistical significance was not assessed, the results may, nevertheless, have clinical significance. As stated in US DHHS treatment guidelines,Citation2 a darunavir-based regimen is recommended in situations that may be encountered in newly diagnosed patients due to its high barrier to resistance. Rapid initiation of treatment following diagnosis, as recommended in recent WHO and International Antiviral Society–USA guidelines,Citation6,Citation27 would present such a scenario and, in the first known phase 3 trial of an STR in a rapid initiation model of care, D/C/F/TAF is being evaluated in the DIAMOND study (ClinicalTrials.gov Identifier: NCT03227861).Citation28 The high barrier to resistance of darunavir would be beneficial in cases of transmitted resistance or a propensity to be suboptimally adherent, and would help to preserve future treatment options. For these reasons, D/C/F/TAF may be a preferred treatment for rapid initiation.
In conclusion, results of this subgroup analysis of AMBER suggest that initiating ARV therapy with D/C/F/TAF is an effective and well-tolerated option, regardless of age, gender, race, VL, or CD4+ cell count.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Supplemental Material
Download Zip (4.1 MB)Acknowledgment
The authors would like to acknowledge Karam Mounzer and Romana Petrovic for their input on the study analyses. Medical writing support was provided by Courtney St. Amour, PhD, of MedErgy, and was funded by Janssen Scientific Affairs, LLC.
Disclosure statement
This study was funded by Janssen Scientific Affairs, LLC. B. Rashbaum, C.D. Spinner, C. McDonald, and C. Mussini contributed to the conduct of the study as investigators and to the interpretation of the data. J. Jezorwski and D. Luo contributed to statistical analysis and interpretation of the data. E. Van Landuyt, K. Brown, and E.Y. Wong contributed to the design of the analysis and interpretation of the data. All authors contributed to drafting the manuscript and approved the final version.
B. Rashbaum has participated in speakers bureaus for Janssen and Gilead, and is a Gilead stockholder. C.D. Spinner received research grants from Gilead, Janssen, and ViiV; has participated in speakers bureaus for Gilead and Janssen; and has received honoraria from AbbVie, Bristol Myers-Squibb, Gilead, Hexal/Teva, Merck, Janssen, and ViiV. C. McDonald reports personal fees from Gilead, Merck, ViiV, and Janssen. C. Mussini has received honoraria from AbbVie, Bristol Myers-Squibb, Gilead, Merck, Janssen, Angelini, and Viiv. J. Jezorwski, D. Luo, E. Van Landuyt, K. Brown, and E.Y. Wong are full-time employees of Janssen.
Additional information
Notes on contributors
Bruce Rashbaum
Bruce Rashbaum has been in private practice for the past 33 years and is an assistant professor of medicine at George Washington University School of Medicine. He has been a primary investigator for a majority of the drugs that are used today for people living with HIV. He is a Fellow of the American College of Physicians and a specialist with the American Association of HIV Medicine.
Christoph D. Spinner
Christoph D. Spinner is an associate professor in medicine at Technical University Munich, Germany. He serves as a consultant in infectious diseases at the University Hospital Klinikum rechts der Isar, Munich, Germany. His research focuses on aspects of the efficacy, safety, and tolerability of antiretroviral therapy, as well as on the prevention of HIV.
Cheryl McDonald
Dr. Cheryl McDonald is an infectious disease specialist in Fort Worth, Texas and is affiliated with multiple hospitals in the area, including Texas Health Harris Methodist Fort Worth, Baylor All Saints Medical Center at Fort Worth, and Medical City Fort Worth Hospital. She received her medical degree from University of Texas Southwestern Medical School and has been in practice for more than 20 years.
Cristina Mussini
Cristina Mussini, MD, is Professor of Infectious Diseases at the University of Modena and Reggio Emilia in Italy. She has published >200 papers in peer-reviewed journals. Prof. Mussini is a member of the Governing Council of the International AIDS Society, the Governing Board of EACS, and of the Scientific Committee of both European Conferences on HIV infections (European AIDS Clinical Society and International Congress on HIV and Drug Therapy in HIV Infection).
John Jezorwski
John Jezorwski has a master's degree in applied statistics and has over 12 years of experience as a statistician. For the past 2 years he has been employed by Janssen, Research & Development, Pharmaceutical Companies of Johnson & Johnson in the infectious disease and vaccine therapeutic area.
Donghan Luo
Donghan Luo is an experienced statistician with over 20 years of clinical research in pharmaceutical industry. He authored and published over 30 clinical research manuscripts in the areas of infectious disease, diabetes, oncology, and cardiovascular disease. He had academic training in mathematics and statistics with a Ph.D. in mathematics and M.S. in biometrics and statistics.
Erika Van Landuyt
Erika Van Landuyt earned her Medical Degree at the University of Ghent, Belgium, and a degree in Tropical Medicine at the Prince Leopold Institute of Tropical Medicine in Antwerp, Belgium. After a Fellowship in Surgery/Emergency Room and a mission with Médécins sans Frontières in Nicaragua, she joined the industry at Janssen Pharmaceutica (Beerse, Belgium) in 1997 and has built up over 20 years of experience in pharmacovigilance and clinical R&D activities, primarily in Infectious Diseases (HIV and HCV). Currently she is working as Director-Clinical Trial Physician on the new D/C/F/TAF single-tablet regimen development program. Recently she has been co-author on several papers published in peer-reviewed journals and on abstracts presented at international conferences.
Kimberley Brown
Kimberley Brown, PharmD, is Medical Director, HIV for Janssen Therapeutics. She has served other roles at Janssen across the Infectious Diseases franchise, including as part of the HCV and HIV clinical teams to lead efforts on research trials, launch activities, and business partner collaborations.
Eric Y. Wong
Eric Y. Wong is the medical communications lead for infectious diseases at Janssen Scientific Affairs, with primary responsibilities in the planning and development of publications for clinical studies and associated secondary analyses. He began his career as a researcher and reviewer at the Food and Drug Administration Center for Biologics Evaluation and Research. Eric earned his PhD in Chemical and Biomolecular Engineering from the University of Pennsylvania and MBA from Northeastern University, and is a certified medical publications professional (ISMPP CMPPTM).
References
- European AIDS Clinical Society. EACS Guidelines Version 9.1, October 2018. http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf. Accessed February 11, 2019.
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed February 11, 2019.
- Coffey S, Bacchetti P, Sachdev D, et al. RAPID ART: High virologic suppression rates with immediate ART initiation in a vulnerable urban clinic population. AIDS. 2018;33(5):825–832.
- Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med. 2017;14(7):e1002357.
- Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: The RapIT Randomized Controlled Trial. PLoS Med. 2016;13(5):e1002015.
- World Health Organization. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Geneva: World Health Organization; 2017.
- Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59.
- Lathouwers E, Wong EY, Luo D, Seyedkazemi S, De Meyer S, Brown K. HIV-1 resistance rarely observed in patients using darunavir once-daily regimens across clinical studies. HIV Clinical Trials. 2017;18(5–6):196–204.
- Ruane PJ, DeJesus E, Berger D, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455.
- Mills A, Crofoot G, Jr., McDonald C, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr. 2015;69(4):439–445.
- Post FA, Yazdanpanah Y, Schembri G, et al. Efficacy and safety of emtricitabine/tenofovir alafenamide (FTC/TAF) vs. emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) as a backbone for treatment of HIV-1 infection in virologically suppressed adults: subgroup analysis by third agent of a randomized, double-blind, active-controlled phase 3 trial. HIV Clin Trials. 2017;18(3):135–140.
- Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;58(9):1297–1307.
- Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther. 2018;15(1):17.
- Eron J, Orkin C, Gallant J, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naïve HIV-1 patients. AIDS. 2018;32(11):1431–1442.
- Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–2448.
- Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818.
- Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231.
- Cohen C, Wohl D, Arribas JR, et al. Week 48 results from a randomized clinical trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate vs. efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected adults. AIDS. 2014;28(7):989–997.
- Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–2615.
- Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–2082.
- Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–2072.
- Squires KE, Molina JM, Sax PE, et al. Fixed dose combination of doravirine/lamivudine/TDF is non-inferior to efavirenz/emtricitabine/TDF in treatment-naïve adults with HIV-1 infection: week 48 results of the Phase 3 DRIVE-AHEAD study. Paper presented at: 9th IAS Conference on HIV Science July 23–26, 2017.
- Currier J, Averitt Bridge D, Hagins D, et al. Sex-based outcomes of darunavir-ritonavir therapy: a single-group trial. Ann Intern Med. 2010;153(6):349–357.
- Crawford KW, Spritzler J, Kalayjian RC, et al. Age-related changes in plasma concentrations of the HIV protease inhibitor lopinavir. AIDS Res Hum Retroviruses. 2010;26(6):635–643.
- Brown K, Patterson-Browning B, Liu C, Patel M, Stewart L, Nettles RE. Treating older HIV-1-infected subjects with cobicistat-boosted darunavir in a 48-week phase 3 trial. Rev Recent Clin Trials. 2017;12(3):174–181.
- Schoen JC, Erlandson KM, Anderson PL. Clinical pharmacokinetics of antiretroviral drugs in older persons. Expert Opin Drug Metab Toxicol. 2013;9(5):573–588.
- Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA Panel. JAMA. 2018;320(4):379–396.
- Huhn GD, Crofoot G, Ramgopal M, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) rapid initiation for HIV-1 infection: primary analysis of the DIAMOND study. Paper presented at: 13th Annual American Conference for the Treatment of HIV (ACTHIV); April 11-13, 2019; Miami, FL, USA. Poster ACTHIV-5.
