?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Anomalous strange-face illusions (SFIs) are produced when mirror gazing under a low level of face illumination. In contrast to past studies in which an observer’s task was to pay attention to the reflected face and to perceive potential facial changes, the present research used a mirror gazing task (MGT) that instructed participants to fixate their gaze on a 4-mm hole in a glass mirror. The participants’ eye-blink rates were thus measured without priming any facial changes. Twenty-one healthy young individuals participated in the MGT and a control panel-fixation task (staring at a hole in a gray non-reflective panel). The Revised Strange-Face Questionnaire (SFQ-R) indexed derealization (deformations of facial features; FD), depersonalization (bodily face detachment; BD), and dissociative identity (new or unknown identities; DI) scales. Mirror-fixation increased FD, BD, and DI scores compared to panel-fixation. In mirror-fixation, FD scores revealed fading specific to facial features, distinct from “classical” Troxler- and Brewster-fading. In mirror-fixation, eye-blink rates correlated negatively with FD scores. Panel-fixation produced low BD scores, and, in a few participants, face pareidolias as detected on FD scores. Females were more prone to early derealization and males to compartmentalization of a dissociative identity. SFQ-R may be a valuable instrument for measuring face-specific dissociation (FD, BD, DI) produced by MGT. Use of MGT and panel-fixation task for differential diagnoses between schizophrenia and dissociative identity disorder is discussed.
Introduction
The mirror is a technological artifact that triggers, through its reflected image, unique anthropological behaviors. First, the participant can perceive their visual image as revealed by self-mirroring behaviors in front of the mirror. Second, the participant can receive proprioceptive feedback as shown by self-grimacing behaviors, for example, in front of the mirror. Third, the participant consciously awakes their self-identity, which is distinct from other-identities, as exhibited by self-talking, self-thinking, self-daydreaming, and self-imagining behaviors in front of the mirror (see, e.g., Brandl, Citation2018; Gallup et al., Citation2014). Since the inception of mirror usage, such as obsidian or metal mirrors, humans became aware of anomalous experiences that mirrors produced, which were interpreted as divine epiphanies, and the mirror was a doorway within the mere physical reality that led to an invisible world of spirits (Delatte, Citation1932; Healey, Citation2021; Kerenyi, Citation1976; Vollrath, Citation2018).
Recent psychological studies scientifically investigated these anomalous self-experiences with mirrors. Under a low-level face illumination, the mirror-gazing task (MGT) reliably produces strange-face illusions (SFIs). In the first study of SFIs (Caputo, Citation2010b), after 10 min of mirror-gazing, 50 healthy individuals from the general population perceived: (a) face deformations that still represented one’s own face (66% of the 50 participants); (b) a parent’s face with altered traits (18%); (c) an unknown person with an independent identity (28%); (d) an old man or woman, a child or an adolescent (28%); (e) an animal face (18%); and (f) non-human beings (48%). Similar results were obtained through interpersonal eye-gazing in dyads (Caputo, Citation2019). For a systematic review of previous studies, see Caputo et al. (Citation2021).
Subsequent experiments demonstrated the usefulness of MGT for investigating anomalous experiences for people diagnosed with schizophrenia (Caputo et al., Citation2012), schizotypal personality (Fonseca-Pedrero et al., Citation2015; Derome et al., Citation2018; Derome et al. Citation2022), psychogenic non-epileptics seizure and functional movement disorder (Nisticò et al., Citation2020; Pick et al., Citation2020), as well as anorexia (Demartini et al., Citation2021). Schizophrenia and anorexia findings are further examined in the Discussion section. Moreover, a previous eye-gazing study showed that interpersonal anomalous experiences (measured with the Strange-Face Questionnaire, SFQ) were increased in dyads of mixed sex compared to same-sex dyads, with males being more prone than females toward dissociation in mixed-sex dyads (Caputo, Citation2019). Therefore, interpersonal set-up may exacerbate the sex differences and enhances the man’s illusory “projection” in the woman’s bodily face. In addition, the bias of sex emerged from advanced statistical analyses through Rasch-scaling on few SFQ items (Lange et al., Citation2022). Sex differences for anomalous self-experiences thus need to be further investigated in participants who may be tested individually through MGT.
Mirror- and eye-gazing involve face perception and may produce anomalous experiences that are specific to faces. Face processing is mediated by multiple cooperating neural systems. Two circuits have been distinguished: the core face network and the extended face network (Haxby et al., Citation2000; Zhao et al., Citation2018). These circuits operate after (i) early-vision processing has initially analyzed the visual information (primary visual cortex V1 and V2) with no relation to face or facial features. At (ii) intermediate-vision face processing, the core face network is engaged in extracting facial features (eye, mouth, nose, etc.) from the visual image (occipital face area), and in binding these facial features into the whole face Gestalt. This occurs whenever these features are in the appropriate spatial arrangement (fusiform face area), and in establishing invariants with respect to variable aspects of the face, like eye direction, face direction, face expression and emotion, lip movement, etc. (superior temporal sulcus). At (iii) late-vision face processing, the extended face network is involved in retrieving the identity of the face (anterior temporal lobe), integrating visual and auditory speech information from lip movements (anterior superior temporal sulcus), and distinguishing self-other identity. In this way, face processing construes a theory of mind of the self and of the other while encompassing affective and cognitive intersubjectivity (orbital prefrontal cortex).
According to this processing scheme, anomalous experiences that are nonspecific to faces can occur at (i) early-vision processing stage where “classical” fading of colors (Troxler-fading of iso-luminous color patches that were presented away from the fixation point; Troxler, Citation1804) and fading of things (Brewster-fading of objects that were presented away from fixation point; Brewster, Citation1818) occur. In contrast, anomalous experiences that are specific to faces [i.e., deformations and/or disappearance of facial features (“face-feature-fading”) and holistic deformations of the whole face Gestalt] may likely occur at the (ii) intermediate-vision face processing stage (in core face network). Ultimately, “strange-face illusions,” that reflect an others’ identity may involve the (iii) late-vision face processing stage (in extended face network) for top-down mental construction of personalities and narratives of new and strange identities.
Mirror gazing involves at least three different processing stages related to the participant’s face reflected in the mirror. First, the visual face is the perception of the image reflected in the mirror. This image is bottom-up visual information, which requires exteroceptive (i.e., external world representations) visual processing.
Second, the bodily face is the proprioceptive feeling of the face (i.e., feeling of muscular states) that can eventually be integrated with the visual image of the reflected face. This proprioceptive feeling is bottom-up information based on interoceptive bodily signals (i.e, representations of internal body; Chen et al., Citation2021) and multisensory integration (Blanke et al., Citation2015; Park & Blanke, Citation2019; Tsakiris, Citation2017) of interoceptive bodily signals with exteroceptive signals, mainly visual, but also from other modalities. For example, mouth movement involves both proprioceptive feeling of muscular activity and seeing the moving mouth in the mirror. Moreover, two facets can be distinguished based on phenomenological studies (Gallagher, Citation2000) in bodily processing. One facet is body ownership, which is the participant’s ownership awareness of their body. The second facet is embodied agency, which is the participant’s awareness of “I” i.e., the author of the bodily action. Both of these facets naturally coincide, while they can be isolated on specific neurological diseases, and may be experimentally separated (Braun et al., Citation2018). The integration between interoception and exteroception likely occurs within the salience network, which involves, in particular, the anterior insular cortex (Uddin, Citation2015)
Third, in the processing stages of the participant’s image reflected in the mirror, the identity face is the recognition either as an image of own-self, or of another individual, or even as another being. This top-down recall involves memory of faces by self and by other beings, and active integration of an affective and cognitive theory-of-mind.
The Present Study
SFIs by mirror-gazing can be regarded as either anomalous self-experiences, visual, and bodily hallucinations, or phenomenal outcomes of dissociative states. In any case, SFIs are grounded in the mirror reflection of the participant’s face, which makes SFIs a truly unique study case aimed at the clinical purposes of distinguishing different neurological and functional pathologies, in addition to ascertaining the role of individual differences (Rogers et al., Citation2020).
The main purpose of the present article is to provide a comprehensive and exhaustive set of items, based on the Strange-Face Questionnaire, which may account for possible anomalous experiences in front of the mirror under low-level face illumination. The original SFQ displayed an uneven number of items across the three factors extracted through factor analysis in previous studies (Caputo, Citation2019; Lange et al., Citation2022), requiring a revised SFQ. Three types of anomalous experiences can be defined a priori on the basis of the current knowledge pertaining to cognitive processes that are involved in, respectively, visual face perception, body-ownership and embodied agency awareness, as well as identity recall. Moreover, previous findings and reports of anomalous experiences were used (Caputo, Citation2010a, Citation2019) together with advanced statistical data analyses (Lange et al., Citation2022). The three types of anomalous experiences are described in the following paragraphs.
Design of the Revised Strange-Face Questionnaire (SFQ-R)
First, visual face deformation (FD) consists of both facial-feature and whole-face Gestalt deformations, which the participant perceives in their reflected face (see ). Items 1, 16 and 32 assess facial feature deformations. Items 8 and 11 assess strong deformations of face skin (item 8, old person) or holistic face misperceptions (item 11, animal face). Items 31 and 34 assess aspects of derealization as a dream-like face (item 31) or face through a fog (item 34). In addition to this 7-item scale for visual face deformations, there are items 28 for extreme detachment of the actual mirror-reflected visual face toward a cartoon-like face and 29 for face transformation.
Table 1. Items of SFQ-R and endorsements among participants (N = 21).
Second, bodily face detachment (BD) consists of changes of two facets of body awareness (i.e., body-ownership and embodied agency), which the participant both feels interoceptively and perceives multimodally through their reflected face. Items 23, 30, and 33 assess loss of embodied agency. Items 15, 17, and 22 assess loss of body-ownership and out-of-body experiences. (Essentially, item 15 assesses both dissociated self-identity as it concerns perception of “another person” and BD, given that the person is staring at the participant from behind mirror.) Item 24 assesses time shrinking as previous statistical analyses found that it was specifically correlated to bodily detachment (see Table 5 in Caputo, Citation2019) and also indicated by diagnostic criteria of depersonalization (DSM-5: 300-6-A-1; American Psychiatric Association, Citation2013). In addition to this 7-item scale for detachment of bodily face, there are items 18 for dead-like face (i.e., an Out-of-Life or non-living face) and 20 for face-body detachment. It should be noted that bodily feelings (which are usually difficult to articulate verbally) can be tied to visual perception, as the participant’s feeling can often be seen in the mirror as a multisensory integration or detachment of the bodily face from the body image.
Third, dissociated identity (DI) or anomalous self-identity occurs when the participant does not recognize themself in the reflected face, so that the participant perceives another individual. Items 5, 21, 25, 26, and 27 assess different forms of other-identity either in a present (items 5, 21, 25), past (item 26), or future time (item 27). Items 10 and 14 assess other-identity with a different gender (item 10) or manifesting a special spirituality (item 14). In addition to this 7-item scale for DI, there are items 7 (child) and 9 (adolescent) for idealized identity.
There is a considerable juxtaposition between this classification of anomalous mirror-gazing experiences and descriptions of pathological dissociative traits as found in Diagnostic Statistical Manual (DSM-5; American Psychiatric Association, Citation2013). In fact, the three aforementioned types of anomalous experiences stem from perceptions of the external mirror-reflected visual reality, the internal (interoceptive) bodily feeling, and the participant’s identity, respectively. In contrast, we find dissociative traits of derealization, depersonalization, and dissociative identity in pathological patients. Derealization and depersonalization can be grouped into detachment of external and internal worlds, respectively (Allen, Citation2001; Holmes et al., Citation2005). Compartmentalization of alter identities characterizes identity disturbances, which are a deficit in the ability to deliberately control processes and actions, such as dissociative fugue and dissociative identity disorder (Allen, Citation2001; Holmes et al., Citation2005). Possible relations among these three dissociative conditions and the three types of SFIs are discussed in the following paragraphs.
First, FDs may be conceived as detachments of the exteroceptive visual world, which is grounded in the participant’s mirror-reflected visual face. On a more basic level, there is fading of visual facial features. As a consequence, the participant can experience the vision of the face as seen through a fog or through a wide-angle photographic lens (i.e., facial features can appear distorted or, eventually, disappear). Pathological patients could manifest derealization (DSM-5: 300-6-A-2) as persistent (i.e., relatively stable), while in healthy individuals derealization is a temporary and reversible state i.e., detachment of the external reality.
Second, BDs may be regarded as detachments of the interoceptive (proprioceptive) world, which is grounded in the participant’s bodily face. On a more basic level, there appears to be a disconnection of the participant’s face from their body that the participant can both feel proprioceptively in the body and perceive as visually detached from the body in the mirror-reflected image. There can be changes in feeling of the participant’s body and numbing of emotional expressions as a dead and immobile face, as well as a distorted subjective sense of time as in a flow of illusions of deformed faces. Pathological patients could manifest depersonalization (DSM-5: 300-6-A-1) as persistent (i.e., relatively stable), while in healthy individuals depersonalization is a temporary and reversible state.
Third, DIs may be envisaged as a compartmentalization of the participant’s self-identity into a participant’s other-identity, which is grounded in the participant’s mirror-reflected image. On a more basic level, another individual unknown to the participant appears, a stranger, or a child instead of themself. In other cases, there can be a non-human vision. In general, new faces can be considered “projections” of the participant’s potential or latent identities into and beyond the mirror. Pathological patients could manifest this experience of dissociative identity (DSM-5: 300–14-A) as persistent (i.e., relatively stable), while in healthy individuals this experience is a temporary and reversible state of dissociated self-identity.
The advantage of using mirror-gazing task as a tool for studying dissociation is that dissociative states are produced in a standardized set-up, which can be experimentally replicated, in contrast with widely used dissociation questionnaires that are based on self-reports of memory of past dissociative experiences [e.g., Dissociative Experiences Scale (DES), Carlson and Putnam (Citation1993); Clinician Administered Dissociative States Scale (CADSS), Bremner et al. (Citation1998); Cambridge Depersonalization Scale (CDS), Sierra and Berrios (Citation2000); Structured Clinical Interview for dissociative disorders (SCID-D), Steinberg and Schnall (Citation2001)].
The DES (Carlson & Putnam, Citation1993) has only one item (item 11 of DES) that concerns mirrors, which clusters within “alterations in the perception of the external world” (Schimmenti & Sar, Citation2019, p. 413). The CADSS (Bremner et al., Citation1998) and CDS (Sierra & Berrios, Citation2000) have no items concerning mirrors. The SCID-D (Steinberg & Schnall, Citation2001) has three scales, and only one item in the depersonalization scale (item 9 of SCID-Depersonalization) concerns mirrors. Therefore, the SFQ-R can be clearly distinguished from self-report measures and possesses the advantage of focusing on anomalous self-experiences, including dissociation, specifically produced by mirror gazing under a controlled set-up.
Hypotheses of the present study
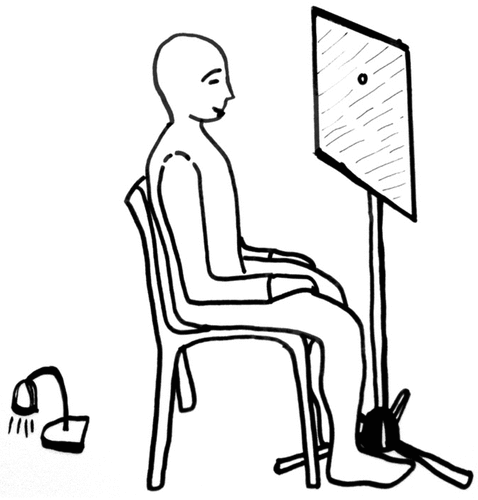
The participant’s task in previous studies was to stare at their eyes reflected in the mirror (Caputo, Citation2010a, Citation2010b) or to stare at the other’s eyes during eye-gazing in dyads (Caputo, Citation2019; Lange et al., Citation2022). In general, participants had no difficulty in executing this task, while some participants were easier to fixate on only one eye at a time, or the nasal septum in between the eyes. It was possible that these differences in task execution could influence perceptions of anomalous self-experiences, and explain, at least in part, differences between participants. Therefore, the present study implemented several procedural innovations. More specifically, we used a small hole (4-mm diameter) in the glass mirror that served as the fixation point (). The height of the mirror was regulated so that the hole was in-between the reflected image of the eyes. We also designed a control condition for assessing the effect of suggestions and subjective biases to report anomalous self-experiences and SFIs. The control condition was a gray non-reflective panel with the same size of the mirror and a 4-mm diameter hole that served as the fixation point. In the present experiment, the participant was instructed to stare at the hole, with no pre-information regarding face perception or potential facial changes. Given these set-up modifications with respect to previous studies, some changes in SFIs can be predicted and, concomitantly, hypotheses and research question can be advanced and evaluated through the experiment.
Figure 1. Set-up of mirror-fixation and panel-fixation. The 4-mm hole was used as fixation point for the participant’s task of staring at the hole. The square surface was either a glass mirror (in the mirror-fixation experimental condition) or a gray non-reflective panel (in the panel-fixation control condition). Behind the hole a micro-camera was mounted for monitoring the participant’s correct fixation and for measuring eye-blink rate (EBR).
Research Question: suggestions and biases introduced by task instructions. In previous studies of SFIs, the participant’s task involved noting experiences of changes of their own face reflected in the mirror (Caputo, Citation2010a, Citation2010b) or seen in the other participant’s face during eye-gazing (Caputo, Citation2019; Lange et al., Citation2022). Thus, it was possible that this instruction had primed the participant to detect face changes and biased the participant’s response to face changes. In the present study, this bias was excluded because the participant’s task was to stare at the hole, engaging, in turn, eye fixation as the primary task with no bias regarding face observation. If the aforementioned suggestion had an effect, then SFQ scores in the present experiment should be lower than previous studies that primed participants to detect of face changes (Caputo, Citation2019).
Hypothesis 1:
Mirror-fixation produces more dissociative phenomena than panel-fixation under low-level illumination. This finding may elucidate the differences between mirror-gazing and dot-staring, which some studies (e.g., Miller et al., Citation1994) posit as equivalent in producing dissociative states under normal illumination levels. Hence, our first hypothesis is that mirror-fixation will produce higher dissociation and anomalous experiences with respect to panel-fixation, as detected through SFQ-R scales of dissociation.
Hypothesis 2:
dissociative derealization. Hole-fixation increases colors, objects, facial features, and whole face Gestalt fading in mirror-fixation compared to panel-fixation. Fading can be of different types: (i) Troxler- and Brewster-fading at early-vision processing stage; (ii) fading of facial features (“face-feature-fading”) and/or whole face Gestalt (when the spatial arrangement of facial features is scrambled) at intermediate-vision face processing stage. No CADSS item is specific to face or to facial features, while most CADSS items index the fading of “things” in the visual field. As a result, the second hypothesis is that classical measures of derealization (CADSS-Derealization) will detect fading of “things” at early-vision stages, such as Troxler- and Brewster-fading, with no difference between mirror-fixation and panel-fixation. In contrast, we predict that face-feature-fading and whole face Gestalt deformations will be detected by the FD scale of SFQ-R in mirror-fixation, whereas, in contrast, they will not occur in panel-fixation. Therefore, FD scale of SFQ-R will discriminate between mirror-fixation and panel-fixation. Moreover, we expect no statistical correlation between CADSS-Derealization and FD scale of SFQ-R.
Hypothesis 3:
dissociative depersonalization. CADSS-Depersonalization and BD scale of SFQ-R are both grounded in the participant’s bodily interoception. Therefore, we expect that a positive correlation will be found between CADSS-Depersonalization and the BD scale of the SFQ-R.
Hypothesis 4:
dissociated self-identity of the participant. Strange faces of unknown individuals will be perceived during mirror-fixation, while none will be perceived during panel-fixation. Consequently, a large score difference will be present between mirror-fixation and panel-fixation in DI scale of SFQ-R.
Hypothesis 5:
the effect of eye movements and eye blinking during the mirror-fixation task, on both strength and frequency of anomalous self-experiences. It has long been assumed that lower eye-blink rates (EBRs) can result in more SFIs, even without direct evidence. Eye blinks are indeed associated with active visual suppression of processing information from the external world (Volkmann et al., Citation1980). Thus, eye blinks can intervene as a “defense mechanism” whenever a threat reaches a critical level for overwhelming illusions. In other words, the eye blink functions as an information “reset” for external world processing. EBRs show a negative correlation with attention loads during cognitive tasks, as EBRs decrease when cognitive loads increase (Maffei & Angrilli, Citation2018). Therefore, the fifth hypothesis is that lower EBRs will be associated with higher SFQ-R scores. As a result, a negative correlation will be found between SFQ-R scores and EBRs.
Methods
Participants
The experiment was run in accordance with the Helsinki declaration of human rights. Ethical approval for this study was obtained from a University based research ethics committee. Individuals were recruited through a public advertisement for “research aimed at investigating eye-movements.” Twenty-one naïve individuals from both students and the general population responded to the advertisement and they all met the selection criteria (males/females 6/15; mean age 21.4; SD 1.78; range 19–25 years). All participants were Caucasians.
Selection criteria were based on participants being between 18 and 25 years of age, with normal or corrected-to-normal vision. Exclusion criteria were: psychiatric or neurological deficits, and being under the effect of psycho-active substances (alcohol, illicit drugs, etc.). The sample size was chosen in advance by statistical power analysis (see Statistical Analyses section).
Each candidate arrived at the laboratory. For each candidate, an experienced clinical psychologist assessed his/her neurological health, psychiatric health, and dependency from alcohol or psychoactive substances via a brief interview and self-report questionnaire. When selected, the participant read the written instructions for the experiment (see below, Procedure) and signed a written informed consent form. All participants did not know the actual aim of the experiment and were naïve concerning strange-face illusions, with no previous involvement in psychological studies nor assessments. Participation was voluntary, with no monetary or credit rewards. Anonymity of participants was granted through assignment of a numeric code, and this code was used throughout endorsements of questionnaires and scales. The experiment was run in a double-blind condition (i.e., the experimenter who carried out the experiment did not know the experimental hypotheses).
Materials
Two conditions were tested: fixation to a hole in a glass mirror (mirror-fixation) and fixation to a hole in a gray non-reflective panel (panel-fixation). Both panel and mirror were square, measuring 400 mm × 400 mm, mounted on tripods, and with a hole that served as the fixation point. The hole was 4-mm in diameter, drilled at 150 mm from the top, and on the horizontal midline (see photos in Supplementary materials). A micro video-camera was mounted behind the hole and connected to a portable computer. The computer was placed in a laboratory location that was not visible to participants.
The mirror and the panel were mounted on tripods (). The tripod was placed at the center of the laboratory room that measured 3 m × 5 m. The room was obscured from external light and illuminated by a spotlight (20 W, halogen bulb) placed on the floor at an approximate distance of 0.5 m behind the chair, where the observer was seated, and 0.3 m from the nearest wall. The spotlight pointed toward the floor to provide indirect illumination of the participant’s face and to minimize shadows and other light artifacts. The level of illumination of the observer’s face (i.e., the light that is “incident” to the frontal plane where the face is located) is set around 0.8 lux, measured through a digital photometer (TES-1330A). This level of illumination is empirically set through regulating the distance between the spotlight and the floor so as to obtain the correct level of illumination. This distance is determined empirically because it depends on the reflectance of the floor and walls of the laboratory, as well as the size of the laboratory. As a reference, a full-moon on a dark night provides an illumination of about 0.3 lux.
Measures
Strange-Face Questionnaire Revised (SFQ-R)
The SFQ-R comprised 34 items (33 items that describe anomalous experiences and 1 control item, which is item-19). SFQ-R items are shown in and in Supplementary materials. The response to each SFQ-R item concerns the frequency of experiences described by the same item and anchored on a 5-point scale: 0 (No, never), 1 (rarely), 2 (sometimes), 3 (often), 4 (very often).
Total score of SFQ-R was calculated as the sum of all items, excluding item-19. The range of SFQ-R total score is 0–132. Three subscales were defined a priori: visual face deformation (FD) was calculated as the sum of SFQ-R items 1, 8, 11, 16, 31, 32, 34; bodily face detachment (BD) was calculated as the sum of SFQ-R items 15, 17, 22, 23, 24, 30, 33; dissociated self-identity (DI) was calculated as the sum of SFQ-R items 5, 10, 14, 21, 25, 26, 27. The range of subscales is 0–28.
Clinician-Administered Dissociative States Scale (CADSS)
The CADSS (Bremner et al., Citation1998) comprised 19 items. The original items (see ) were adapted to past-tense verbal time. The response to each item concerned the frequency of dissociative experiences described by the item rated on a 5-point scale: 0 (No, never), 1 (rarely), 2 (sometimes), 3 (often), 4 (very often). The CADSS score was calculated as the sum of the 19 items. The items were a priori subdivided into three subscales: CADSS-Amnesia (items 14, 15), CADSS-Depersonalization (items 3–7), and CADSS-Derealization (items 1, 2, 8–13, 16–19).
Table 2. Subcales of SFQ-R.
Self-evaluation of the difficulty of eye-fixation
The difficulty of maintaining eye-fixation on the hole was self-evaluated on a scale from 1 (minimum difficulty) to 100 (maximum difficulty). At the end of the session, the participant was asked to rate the difficulties of the two fixation tasks (hole-fixation of mirror vs. panel).
Procedure
A within-subject design was chosen for the experiment. The two conditions (mirror-fixation vs. panel-fixation) were counterbalanced among participants in random order. The experimental procedure involved written instructions of the tasks, which were described as follows: “There will be two tasks, one with a mirror and the other with a panel. Both mirror and panel have a hole. Your task is to stare at the hole. Keep staring at the hole. You should limit eye-blinks while staring at the hole. The task will last 10-minutes.” Then, the participant signed the written informed consent module.
The participant sat in the chair in front of the mirror or panel. For each individual, the height of the mirror or panel was regulated so that the hole was at the same height as the participant’s eyes. In the case of the mirror, the hole was perceived just in-between the image of the eyes reflected in the mirror. The room light was turned to the required low-level illumination. After a few minutes of light adaptation, the first fixation task started.
At the end of the first 10-min fixation-task, the light was turned on, and the SFQ-R and CADSS, which were printed on the two faces of the same sheet of paper, were administered in a random counterbalanced order. Then, the second 10-min fixation-task was given and measures were administered accordingly.
When both fixation-tasks were completed, the participant was asked to self-evaluate the difficulties in maintaining eye-fixation on the hole (mirror-fixation vs. panel-fixation). Finally, the participant was debriefed.
Statistical analyses
Through statistical power-analysis (Faul et al., Citation2009), we estimated that the sample size of 21 would allow 90% power to detect a large effect size (0.8) using a paired-sample t-test at one-tail significance level that was Bonferroni-corrected for 3-scales ( = 0.016).
Means and standard errors were calculated based on participants’ responses. Student t-test was used for statistical assessment of response differences to each item from zero value and for paired-samples comparison between the two experimental conditions (mirror-fixation vs. panel-fixation). The statistical significance-level was Bonferroni-corrected for number of items (34).
Means and standard errors were calculated on participants’ scores on FD, BD, and DI scales. Student t-test was used for statistical assessment of scale differences for each scale from zero value and for paired-samples comparison between the two experimental conditions (mirror-fixation vs. panel-fixation). The statistical significance-level was Bonferroni-corrected for number of scales (3). Reliability of scales was calculated through Cronbach’s alpha. Reliabilities were considered acceptable for alphas>0.7. Effect size was calculated through Cohen’s d.
N(yes) was calculated for each item as the number of participants who endorsed a response from 1 (rarely) to 4 (very often). This number gives an estimation of the number of participants who actually had the anomalous experience described by the item.
Sex differences were analyzed through mixed between-within (2 × 2 × 2 × 3) RM-ANOVA with sex (2-levels) as between-subjects factor, and task (2-levels: mirror- vs. panel-fixation), measure (2-levels: CADSS vs. SFQ-R) and subscale (3-levels) as within-subject factors. Afterward, item-by-item independent-samples t-tests with one factor (2-levels: sex) were carried out. The statistical significance level for item-by-item analysis was (uncorrected) p < .05. This choice is justified if more items would converge toward differential subscales of dissociation for either sex.
Eye-blink rates (EBRs) were counted for complete eye-blinks (when the eyelids completely closed; Volkmann et al., Citation1980) during the 10-min fixation task by watching video-recordings of participants. Recordings were acquired during fixation-tasks from the micro-camera mounted behind the hole.
Self-rated difficulty of hole fixation in the two fixation tasks (mirror-fixation vs. panel-fixation) was compared through paired-samples t-test. Statistical significance level was p < .05.
The effect of task order (mirror-fixation first- vs. second-task) was analyzed through mixed within-between ANOVAs on items, scales, and subscales. Statistical significance level was p < .05.
Correlations were calculated using statistical coefficients of Pearson. Statistical significance level was p < .05.
Results
Statistical analyses
SFQ-R scales
displays responses to SFQ-R items and the total score. We found that a large number of SFQ-R items obtained higher scores in mirror-fixation than panel-fixation. These increments were confirmed by paired comparisons in the mirror vs. panel column of . Thus, SFQ-R scores effectively discriminated between anomalous experiences due to mirror vs. panel, with much higher SFQ-R scores on mirror-fixation than panel-fixation for most items.
displays values of SFQ-R scales of FDs (derealization), BDs (depersonalization), and DIs (dissociative identity). Paired comparisons found highly significant differences between mirror-fixation and panel-fixation. Effect-sizes were quite high and beyond those expected in preliminary power-analysis. Reliabilities of SFQ-R scales of FDs, BDs, and DIs were above the acceptance threshold.
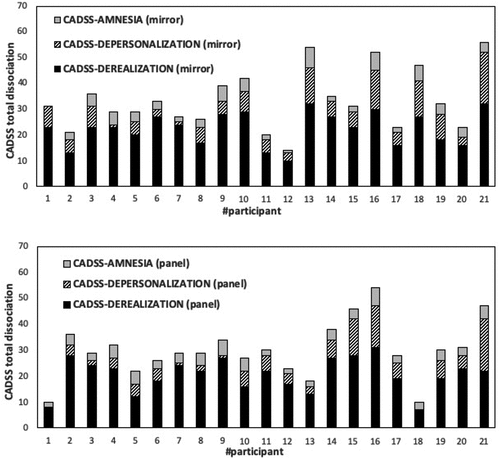
In , individual scores on SFQ-R scales are plotted in mirror-fixation and panel-fixation. In the horizontal axis, participants are sorted by the ordinal scale with respect to scores (sum of FD, BD, and DI scores, in the vertical axis) that they obtained in mirror-fixation.
Figure 2. Individual scores on SFQ-R subscales. The horizontal axis identifies participants sorted by increasing scores in mirror-fixation (sum of FD, BD, and DI), which are represented in the vertical axis. (Top chart) mirror-fixation. (Bottom chart) panel-fixation. Participants were identified by numbers in the horizontal axis.
CADSS scales
displays responses to CADSS items. Only two items showed statistically significant differences between mirror and panel. No statistically significant difference was found between CADSS total scores [mirror-fixation vs panel-fixation: mean (SEM) = 33.33 (2.51) vs. 29.95 (2.36); t = 1.08; p = .292]. Therefore, CADSS was not able to effectively distinguish between dissociative states produced by mirror-fixation vs. panel-fixation.
Table 3. Items of CADSS and endorsements among participants (N = 21).
shows CADSS scores on scales of derealization, depersonalization, and amnesia. Statistical comparisons between mirror-fixation and panel-fixation found no significant differences in the three scales of CADSS. Hence, CADSS dissociation scales of derealization, depersonalization, and amnesia were not able to distinguish between mirror-fixation and panel-fixation. This absence of differentiation between mirror-fixation and panel-fixation is not surprising as CADSS items are not specific to faces nor to mirror experiences.
Table 4. Scales of CADSS-Amnesia, CADSS-Depersonalization, and CADSS-Derealization. CADSS total score of dissociation is the algebraic sum of these three scales.
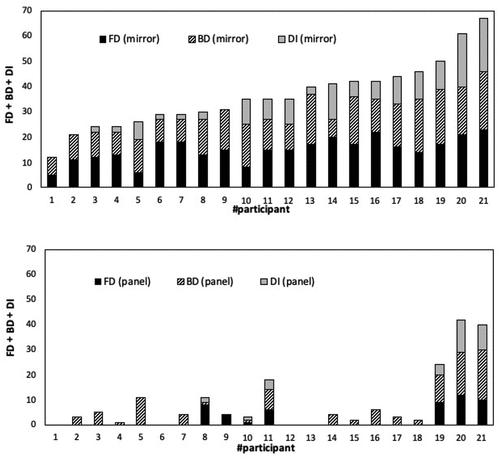
In , individual scores on CADSS scales of Amnesia, Depersonalization, and Derealization are plotted (participants were identified with the same numbers as in ). The high prevalence of CADSS-Derealization in both mirror-fixation and panel-fixation is noteworthy. This is brought about by staring at the hole (independently whether being mirror or panel), thus increasing both Troxler- and Brewster-fading, which are perceptual effects that are nonspecific to faces. Indeed, CADSS-Derealization items concern thing-fading, color-fading, sound-fading, and so forth.
Sex differences
RM-ANOVA showed a statistically significant interaction between subscale and sex of participants [F(1,19) = 11.11; p < .005]. SFQ-R item-by-item analyses showed the following (uncorrected) statistical effects (negative sign indicates females scoring higher than males): in mirror-fixation, item-9 (adolescent face, t = 2.71, p < .05), item-34 (face through a frog, t = −2.61, p < .05); in panel-fixation, item-15 (looked at, t = 2.66, p < .05). CADSS item-by-item analyses showed the following (uncorrected) statistical effects (negative sign indicates females scoring higher than males): in mirror-fixation, item-17 (special sense of clarity, t = 2.84, p < .05), item-18 (vision through a fog, t = −2.30, p < .05), item-19 (brighter colors, t = −2.21, p < .05); in panel-fixation, item-1 (things moving, t = −2.67, p < .05), item-12 (longer duration, t = −3.15, p < .01), and item-18 (vision through a fog, t = −2.10, p < .05). Summarizing these effects throughout tasks (mirror- or panel-fixation) and measures (CADSS or SFQ-R) allows the following explanations: (a) female participants were more prone to early derealization (correspondingly: vision/face through a fog, things moving, brighter colors) than male participants; (b) male participants were more prone to idealized/spiritualized/compartmentalized dissociative identities (respectively: adolescent face, special sense of clarity, looked at by another person) than female participants.
Eye-Blink rate and other minor variables
There was no EBR statistical difference between mirror-fixation and panel-fixation [mean (SEM) = 42.29 (4.51) vs. 43.86 (5.66); t = −0.270; p = .790]. No statistically significant effects of task order (mirror-fixation first- vs. second-task in the session) were found. The difference between self-rated difficulties of the fixation task showed a non-significant difference [mirror-fixation vs. panel-fixation: 55.0 (5.02) vs. 65.8 (5.49); t = −1.199; p = .244].
Comparisons with a previous study
Comparisons can be made between the results of the present experiment and data from previous studies, when CADSS and SFQ (the previous version had 18 items and one control item) were administered, since the first 18 items of SFQ-R remained the same. Comparing present data with data of eye-gazing (Caputo, Citation2019), total scores of both CADSS [mirror-fixation vs. eye-gazing: 33.33 (2.51) vs. 27.11 (1.07); t = 2.46; p = .02] and first 18 items of SFQ-R [mirror-fixation vs. eye-gazing: 22.52 (1.69) vs. 18.06 (0.92); t = 2.16; p = .03] showed a significant increase in the task of staring at the hole in the mirror with respect to the previous task that gave explicit instructions to observe experiences of face changes.
Correlations
SFQ-R scales
In mirror-fixation, the SFQ-R scales FD and BD showed a non-significant correlation (r = 0.321; p = .078); both FD and BD showed statistically significant correlations to DI (FD-DI: r = 0.508, p < .01; BD-DI: r = 0.531, p < .01). In panel-fixation, the number of participants who obtained scores on SFQ-R subscales that were different from zero was quite limited for FD and DI (7 and 6 out of 21 participants, respectively) and more consistent for BD (16 out of 21). Hence, in panel-fixation, correlations between SFQ-R scales are inconsistent from a statistical viewpoint.
CADSS scales
In mirror-fixation, the three CADSS subscales were reciprocally correlated (Derealization-Depersonalization: r = 0.572, p < .005; Derealization-Amnesia: r = 0.577, p < .005; Depersonalization-Amnesia: r = 0.491, p < .05). In panel-fixation, the three CADSS subscales were reciprocally correlated (Derealization-Depersonalization: r = 0.434, p < .05; Derealization-Amnesia: r = 0.507, p < .01; Depersonalization-Amnesia: r = 0.416, p < .05).
SFQ-R and CADSS
In mirror-fixation, SFQ-R and CADSS total scores were correlated (r = 0.432; p < .05). In particular, BD scale of SFQ-R and CADSS-Depersonalization were highly correlated (r = 0.587; p < .005). BD scale of SFQ-R and CADSS-Amnesia were correlated (r = 0.455; p < .05). The other scales were non-correlated (FD scale of SFQ-R and CADSS-Derealization: r = 0.235; p = .152).
In panel-fixation, SFQ-R and CADSS total scores were non-correlated (r = 0.267; p = .121). BD scale of SFQ-R and CADSS-Depersonalization were correlated (r = 0.536; p < .01).
Eye-Blink Rate
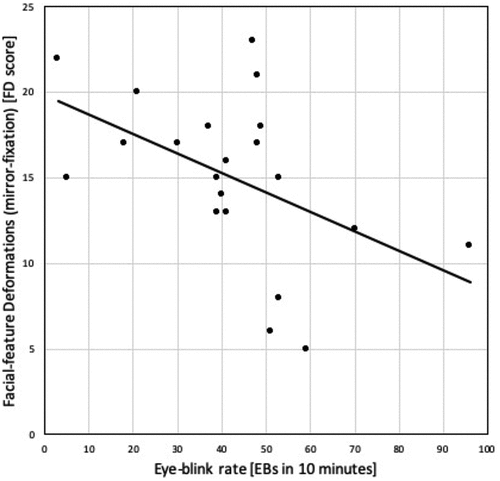
In mirror-fixation, EBRs showed a negative correlation to SFQ-R total score (r = −0.372; p < .05), and, specifically, to FD scale of SFQ-R (r = −0.487; p < .05). As shown in , lower rates of eye-blinking are associated to higher scores on FDs in mirror-fixation. In mirror-fixation, EBRs were non-correlated to BDs (r = −0.129; p = .288) and DIs (r = −0.247; p = .141). In mirror-fixation, no significant correlations were found between EBRs and the CADSS scale and subscales.
In panel-fixation, EBRs were not correlated to the SFQ-R scale and subscale scores (p > .40). However, this lack of correlation could originate from small SFQ-R item endorsements, as reported above. In fact, in panel-fixation, EBRs showed a negative correlation to CADSS scores (r = −0.452; p < .05) and, specifically, to CADSS-Derealization scores (r = −0.400; p < .05).
Self-rated task difficulty
Self-rated difficulty of hole fixation showed a positive correlation to EBRs in mirror-fixation (r = 0.571; p < .01). In contrast, in panel-fixation, a non-significant correlation was found (r = −0.262; p > .12).
Discussion
Our study was the first to not prime observers in advance of mirror gazing to focus on experiences of face changes. We found that even in the absence of priming/suggestions, participants still reported anomalous self-experiences. Accordingly, it is unlikely that the instructions to observe face changes in previous experiments accounted for reports of anomalous experiences or produced an appreciable bias toward reports of such experiences.
We found support for our first hypothesis: Participants’ SFQ-R scores were higher in the mirror-fixation than in the panel-fixation condition. However, CADSS scores did not differ between mirror and panel-fixation. Interestingly, CADSS scores were (a) higher than scores reported for PTSD patients comorbid for dissociative disorders [mean (SD) = 19.3 (18.7); Bremner et al. (Citation1998), p. 132] and (b) similar to scores of PTSD patients “after exposure to a traumatic memory group” [35.0 (21.9); Bremner et al. (Citation1998), p. 132].
A possible explanation of findings of high CADSS scores in our study is that measurements were completed (after mirror-fixation or panel-fixation) by healthy naïve individuals who performed the task apart from any clinical context (e.g., a hospital or a psychiatric center) and absent any doubt that participants’ identity would be recognized. Indeed, participants were anonymized, preventing disclosure of their identity to the experimenter, thereby not constraining their responses as might be the case in a hospital or other clinical setting where their responses might affect their diagnosis, for example. CADSS high scores for both mirror- and panel-fixation suggest, interestingly, a common cause, i.e., the restricted focus of attention on the fixation point. This result is exactly what is expected if CADSS scores are based on Troxler- or Brewster-fading.
Derealization
We also found support for the second hypothesis: The FD scale of SFQ-R discriminated between mirror-fixation and panel-fixation. Thus, FDs are not the effect of Troxler- or Brewster-fading (which occurs, most likely, at the early-vision processing stage in V1-V2), but, rather, of processing stages that are specific to facial features (“face-feature-fading” effect) that occurs, most likely, in the occipital face area and to distortions of whole face Gestalt that takes place in the fusiform face area. Conversely, CADSS-Derealization showed high scores in both mirror-fixation and panel-fixation. Indeed, CADSS-Derealization items concern thing-fading, color-fading, sound-fading, and so forth, classified as Troxler- and Brewster-fading; thus being nonspecific to faces. Moreover, a high score on CADSS-Derealization was associated with a high score also in CADSS-Depersonalization and CADSS-Amnesia, as these three CADSS subscales are highly correlated. In contrast, FD items of SFQ-R are specific to facial features and whole face Gestalt, and are relatively independent on the BD scale of depersonalization.
Depersonalization
We found support for the third hypothesis as well: BD scale of SFQ-R and CADSS-Depersonalization were statistically correlated, as both scales are grounded in the participant’s interoceptive bodily experiences. However, the BD scale of the SFQ-R was able to distinguish between mirror and panel-fixation. CADSS-Depersonalization was unable to do so. Thus, the BD scale of the SFQ-R was more successful in measuring and evaluating depersonalization in mirror-gazing task. In the panel-fixation task, minimal/low BD scores were found in 16 out of 21 participants, hence indicating a latent tendency to depersonalization in healthy individuals. Only 3 to 5 participants obtained medium BD scores in panel-fixation, and this will be discussed later.
Dissociative identity
We also found support for the fourth hypothesis: Mirror-fixation produced far higher scores on DI scale of SFQ-R than panel-fixation in 86% of participants (18 out of 21; -mirror). As summarized in the Introduction, this does not indicate, in itself, that DIs imply a persistent dissociative identity disorder (DID). In general, this is a common phenomenon in front of the mirror. For instance, in SFQ-R item 27, the perception of an unknown individual (i.e., a “projection” of the participant’s mind onto a deformed face in the mirror), whose inner urges lead to communicating something to the participant – the will of the former being independent – may indicate a latent dissociated aspect of the participant’s identity, which would become overwhelming in DID patients.
Relatedly, “mirror confrontation” (i.e., participants are confronted with their face in a mirror; Borgmann et al., Citation2014) was carried out under a normal level of illumination on patients suffering for partial dissociative identity disorder (pDID or DDNOS; Schäflein et al., Citation2020), who reported that their mirror reflected image looked unfamiliar or unrecognizable. Schäflein et al. found that pDID patients experienced more subjective stress, acute dissociation, and blunted autonomic reactivity than healthy controls when mirror confrontation was imposed for 2 min (Schäflein et al., Citation2018).
Dissociation is often present as a comorbid condition in patients with eating disorders. A recent meta-analysis reported that dissociation was present more in patients diagnosed with anorexia than in healthy controls and in individuals with other psychiatric disorders (Longo et al., Citation2021). A preliminary version of the SFQ-R has successfully been used with patients diagnosed with anorexia who scored particularly high on both BD and DI scales (Demartini et al., Citation2021) after MGT. Items that most significantly distinguished (p < .005) patients diagnosed with anorexia from healthy controls were item 25 (another unexpected personality) and item 27 (another communicating individual) of the DI scale of SFQ-R. Thus, in addition to an easily predictable increase of BDs, anorexia may also imply a strong or even extreme compartmentalization of an alter dissociative identity. The association of DI scores of the SFQ-R with anomalous experiences connected to multiple-selves or “multiple-personalities” could be investigated with DID patients in future studies, as DID “is a true disorder of self-perception in which individuals come to believe in and act based on narratives of distinct indwelling selves” (Lynn et al., Citation2022, p. 266).
Eye-blinking effects
The fifth and final hypothesis was also supported: Lower rates of eye-blinking correlated to higher SFQ-R scores. Indeed, a negative correlation between EBRs and FD scores of SFQ-R was found. This finding could be easily explained by lower EBRs as the cognitive load increases (Maffei & Angrilli, Citation2018). However, a more interesting explanation is suggested by the finding that EBRs showed a specific correlation to FDs, which is a measure of derealization, i.e., the detachment of the external world. Thus, it can be suggested that eye-blinking is similar to a brain processing “reset” of external reality when detachment of external reality produces excessive threat in the participant. There could be a sort of “rebound to reality” toward the “real face” from an overwhelming “distorted face” that has become analogous to frightening hallucinations.
Therefore, participants who are more prone to evading threatening situations and less resilient will increase their EBRs for reducing SFIs, whereas more courageous and resilient participants will reduce their EBRs thus enhancing SFIs. Differences between the participants in EBRs and SFIs () might be explained by differences of “absorption” (Tellegen & Atkinson, Citation1974) and absorbed attention.
Spontaneous eye blinks are time-locked to saccadic eye movements (Fogarty & Stern, Citation1989). When gazing at photos of faces, patients diagnosed with schizophrenia showed anomalous eye movements with reduced fixation scan-path lengths (Manor et al., Citation1999), fewer fixations as well as restricted scan-path style (Williams et al., Citation1999) with respect to healthy controls. These anomalies may partially explain the SFI increase and, in particular, the large FD increase that was found in patients diagnosed with schizophrenia compared to healthy controls (Caputo et al., Citation2012, p. 49).
Pareidolias and hallucinations
In panel-fixation, anomalous experiences were often identified as landscape-pareidolias or face-pareidolias. For example, the hole was perceived as the eye of a fish or a frog by two participants. These kinds of pareidolias have been famously described by Leonardo da Vinci in his Treatise on Painting (Leonardo da Vinci, Citation1651, Part 2, n. 63). Pareidolias are “visual illusions of meaningful objects which arise from ambiguous forms embedded in visual scenes” (Mamiya et al., Citation2016, p. 2; Zhou & Meng, Citation2020). Therefore, the tendency to perceive fleeting and fuzzy visions as faces could explain the responses by the three participants who obtained high scores (participants # 21, 20, and 19 in panel). Notice that these participants, who are highlighted by SFQ-R scores, are not detected by CADSS scores ().
It is noteworthy that the control item of SFQ-R (item-19), which was never specifically endorsed by the participants in mirror-fixation, was, instead, endorsed by some participants (5 out of 21) in panel-fixation, who recognized spiral vortices circling the hole and colored shadows on the panel as mountains or trees, resulting in typical landscape pareidolias. The hole in the panel largely facilitates pareidolias. Pareidolias of faces were relatively frequent in panel-fixation (7 out of 21 participants, 33%). When it is perceived as an eye, the lens of the micro-camera, which is mounted behind the hole, plays an important role since the lens may be perceived and/or hallucinated by some individuals as an “artificial” eye. In fact, the convex lens surface just behind the gray panel reflects an anamorphic miniature image of the participant within the laboratory set-up. This image is similar to Parmigianino’s painting Self-portrait in a convex mirror, dated 1524, and is reminiscent of Plato’s philosophical investigations on the eye as a mirror (Plato, Alcibiades I, 133, Citation2001) given that the participant sees the reflection of themself in the other’s eye pupil.
Furthermore, it is remarkable that the three individuals with highest SFQ-R scores in panel-fixation (i.e., participants # 19, 20, and 21 in panel were the same participants who obtained the highest scores in mirror-fixation mirror. Thus, it is possible to hypothesize that these individuals were susceptible to hallucinations during hole fixation. Three considerations can be helpful for accurately specifying the continuum from a veridical perception to an unconstrained hallucination (Rogers et al., Citation2020). First, in panel-fixation, FDs were higher for these three individuals compared to the other participants in panel. Second, in patients diagnosed with schizophrenia, facial feature distortions (FDs) were much more frequent than in healthy controls (Caputo et al., Citation2012, p. 49), as previously discussed. Third, in patients diagnosed with schizophrenia, mirror-gazing produced unusual strange-face illusions very similar to hallucinations. Therefore, a hypothesis regarding visual hallucinations of faces can be advanced (examples were adapted from Harrington et al., Citation1989, p. 379): (1) there is a latent tendency to depersonalization (which is likely based on the tendency to make “things” alive or to embody inanimate “things” as if they were living) for most participants as shown by low BD scores also in panel-fixation (e.g., the “artificial” lens becomes a “living” eye of an illusory mouse); (2) an increase of FDs can set off the change from anomalous self-experiences to hallucinations in both mirror-fixation and panel-fixation (e.g., Mickey Mouse makes its first apparition); (3) this FD increase pulls up both BD (providing illusions of “living” beings) and DI scores (that recollect known/unknown identities), and all three develop into hallucinations (e.g., Mickey Mouse owns his own body and autonomous agency); (4) DIs may be the source of delusions and identity possessions as an attempt by the participant to construct narrative explanations about their own FDs and BDs – as if both of these were veridical perceptions (e.g., Mickey Mouse wants to share secret knowledge with me about my past and future). This model of visual face hallucinations hypothesizes both bottom-up (FD, BD) and top-down (DI) processing stages and involves both posterior and anterior areas of the brain.
DID patients share some psychotic symptoms with patients diagnosed with schizophrenia, such as alter-related auditory and visual hallucinations. In general, both auditory and non-auditory hallucinations are dissociative in nature and related to childhood abuse (Nesbit et al., Citation2022). “Awareness of the alters may take other forms as well, including auditory hallucinations (e.g., hearing a baby cry) or visual hallucinations (e.g., seeing the alter in the corner of the room or when the patient looks in the mirror)” (Foote & Park, Citation2008, p. 217). Therefore, administering panel-fixation after mirror-fixation may be vital for revealing visual hallucinations of alter-faces in some DID patients. Our hypothesis is that patients diagnosed with schizophrenia will have more FDs than healthy controls, whereas patients diagnosed with DID will have more delusions and DIs than healthy controls. Mickey-Mouse like hallucinations that indicate an extreme derealization through perception of a cartoon-like face (see SFQ-R item 28) will most likely be perceived by patients diagnosed with schizophrenia. The three participants who were isolated through SFQ-R high scores on panel-fixation task # 19, 20, 21 of -panel may have a (latent) identity disorder. Conversely, they are certainly not affected by (latent) schizophrenia because their FD scores do not differ from the other participants in the mirror-fixation task (-mirror).
Concluding remarks
Sex difference showed that female participants may be more prone to early derealization (e.g., a vision through a fog), while male participants may be more prone to compartmentalization of dissociative identities (e.g., an idealized adolescent face). The former finding may be supported by superior performance of females in recognition, memory, and discrimination of human faces with respect to males (McBain et al., Citation2009; Rehnman & Herfitz, Citation2007; Sommer et al., Citation2013). In contrast, the propensity of males for face abstraction (idealization, spiritualization, and compartmentalization) is a new unreported finding – which, in any case, can be easily acknowledged in more “abstract” face portraits by male artists compared to more “figurative” face portraits by female artists (e.g. Francis Bacon vs. Frida Kahlo). Sex differences in face processing might be explained through major anterior brain activity of extended face network in males, and major posterior brain activity of core face network in females. However, studies that addressed this hypothesis have yet to be found.
Another advantage of using SFQ-R after MGT may be that FD scores and BD scores are reasonably independent (i.e., statistically non-correlated). Thus, in some patients, administering SFQ-R after MGT might be helpful for disentangling the two detachment states [FD (derealization) vs. BD (depersonalization)], which would be, instead, diagnosed along with Depersonalization/Derealization Disorder (DDD) of DSM-5 (American Psychiatric Association, Citation2013).
Statistically significant correlations were found between FD and DI, and between BD and DI, which contradicts the hypothesis of independence of compartmentalization from both derealization and depersonalization (Holmes et al., Citation2005). Nonetheless, these correlations agree with the idea of “progressive dissociation” that implies a successively triggered or “layered” involvement of dissociative states, from both visual distortion of facial features (FD) and bodily-face detachment (BD), to late-processing of new and strange identity (DI), as the intensity of dissociation increases (Lange et al., Citation2022). We posit that the change from derealization and depersonalization toward dissociative identity concerns the balance between posterior and anterior brain areas, which, in terms of face processing, corresponds to the balance between core face network and extended face network, i.e., the balance between distortions of facial features or whole face Gestalt, and transformations of self-identity into other potential facets of identity, so-called alter identity in the DID literature.
In conclusion, we demonstrated that mirror-gazing under a low illumination level is an effective tool for producing anomalous self-experiences, strange-face illusions, and face hallucinations that are stimulated by observing the participant’s face. Dissociative derealization specific to visual facial features and whole visual face Gestalt can be measured through FD scale of SFQ-R, while, instead, CADSS-Derealization scale seems more useful as a measure of early-vision distortions that are likely to be produced by “classical” Troxler- and Brewster-fading. The FD scale of SFQ-R detects a new class of fading (“face-feature-fading”) in mirror-fixation, which is entirely distinct from the “classical” fading effects of Troxler- and Brewster-fading. Dissociative depersonalization can be measured through the BD scale of the SFQ-R, because this scale successfully discriminates between mirror-fixation and panel-fixation. Finally, compartmentalization of strange dissociative identities may be measured through the DI scale of the SFQ-R. However, the latter requires a more definitive conclusion that could be drawn through correlations with scores on SPID-DID (Steinberg & Schnall, Citation2001) in healthy individuals, or though testing DID patients directly with MGT.
Limitations
In mirror-fixation, a clear advantage of the hole in the mirror is that it provides a fixation point for stable staring. Nevertheless, the hole modified the stimulus (i.e., the mirror) and introduces an element (the hole) within the image reflected. For example, in the present experiment, for some participants the hole produces the anomalous perception of a third eye in between the transformed eyes or in between double-face illusions. Therefore, the standard set-up of mirrors without a hole is a better choice if singular SFIs, like a child’s face, are investigated. However, the placement of a micro video-camera behind the hole was very useful for monitoring participants and to control their correct execution of the MGT. Future researches can choose placement of video-camera just above the mirror in order to monitor the observer’s correct execution of MGT. This placement would free the mirror surface from extraneous elements, such as the non-reflecting hole.
In panel-fixation, staring at the hole is rather important for producing pareidolias and visual hallucinations. In fact, the hole introduced an element that provides a pivot where fleeting and fuzzy sensations of shapeless, fluid colors can be anchored, and acts as a fixed point of perceptual stability. As previously described, the lens of the micro-camera, which was visible behind the hole, plays a relevant role in producing face pareidolias, given that it behaves as an “artificial” eye.
A limitation of the present study concerns statistical correlations. In fact, significance of correlations is dependent on the sample size, and the sample may be relatively small to detect minor significant correlations. Moreover, the sample employed in the present study, which comprises only healthy young individuals, limits the generalization to other populations (e.g., clinical samples, individuals with diagnosed dissociative conditions, older individuals).
Another limitation concerns the technical apparatus. Complete eye-blink rates were easily measured through video-camera recordings, while other aspects of eye movements, such as saccades, were not possible to detect and measure. However, tonic immobilization in eye movements, such as inhibition of saccades during SFIs, were already evident in some observers after a few minutes of mirror-fixation. In fact, their eye blink rate decreases, becoming a steady fixation and point of absorbed attention. Many observers described a feeling of “fascination” that they experienced during perception of SFIs in mirror-fixation. Future studies should perhaps consider measuring pupil dilation as indicators of attention, interest, and absorption on SFIs. These more subtle aspects of eye movements should be analyzed when video capture systems will be technologically more advanced.
Acknowledgement
The author thanks Esky Bolognese, James Houran, and Steven Jay Lynn for thoughtful comments on earlier versions of the article.
Supplemental Material
Download Zip (6.3 MB)Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
The data that support the findings of this study are openly available in figshare at (http://doi.org/10.6084/m9.figshare.22337242).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15299732.2023.2195394
Additional information
Funding
References
- Allen, J. G. (2001). Traumatic relationships and serious mental disorders. John Wiley and Sons.
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders. 5th. (DSM. American Psychiatric Publishing.
- Blanke, O., Slater, M., & Serino, A. (2015). Behavioral, neural, and computational principles of bodily self-consciousness. Neuron, 88(1), 145–166. https://doi.org/10.1016/j.neuron.2015.09.029
- Borgmann, E., Kleindienst, N., Vocks, S., & Dyer, A. S. (2014). Standardized mirror confrontation: Body-related emotions, cognitions and level of dissociation in patients with posttraumatic stress disorder after childhood sexual abuse. Borderline Personality Disorder and Emotion Dysregulation, 1(1), 1–10. https://doi.org/10.1186/2051-6673-1-10
- Brandl, J. L. (2018). The puzzle of mirror self-recognition. Phenomenology Cognitive Sciences, 17(2), 279–304. https://doi.org/10.1007/s11097-016-9486-7
- Braun, N., Debener, S., Spychala, N., Bongartz, E., Sörös, P., Muller, H. H. O., & Philipsen, A. (2018). The senses of agency and ownership: A review. Frontiers Psychology, 9, 1–17. https://doi.org/10.3389/fpsyg.2018.00535
- Bremner, J. D., Krystal, J. H., Putnam, F. W., Southwick, S. M., Marmar, C., Carney, D. S., & Mazure, C. M. (1998). Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). Journal Traumatic Stress, 11(1), 125–136. https://doi.org/10.1023/A:1024465317902
- Brewster, D. (1818). On a singular affection of the eye in the healthy state, in consequence of which it loses the power of seeing objects within the sphere of distinct vision. Annals of Philosophy, 11, 151.
- Caputo, G. B. (2010a). Apparitional experiences of new faces and dissociation of self-identity during mirror-gazing. Perceptual and Motor Skills, 110(3_suppl), 1125–1138. https://doi.org/10.2466/pms.110.C.1125-1138
- Caputo, G. B. (2010b). Strange-face-in-the-mirror illusion. Perception, 39(7), 1007–1008. https://doi.org/10.1068/p6466
- Caputo, G. B. (2019). Strange-face illusions during eye-to-eye gazing in dyads: Specific effects on derealization, depersonalization and dissociative identity. Journal of Trauma & Dissociation, 20(4), 420–444. https://doi.org/10.1080/15299732.2019.1597807
- Caputo, G. B., Ferrucci, R., Bortolomasi, M., Giacopuzzi, M., Priori, A., & Zago, S. (2012). Visual perception during mirror gazing at one’s own face in schizophrenia. Schizophrenia Research, 140(1–3), 46–50. https://doi.org/10.1016/j.schres.2012.06.029
- Caputo, G. B., Lynn, S. J., & Houran, J. (2021). Mirror-and eye-gazing: An integrative review of induced altered and anomalous experiences. Imagination Cognition Personality, 40(4), 418–457. https://doi.org/10.1177/0276236620969632
- Carlson, E. B., & Putnam, F. W. (1993). An update on the dissociative experiences scale. Dissociation: Progress in the Dissociative Disorders, 6, 16–27.
- Chen, W. G., Schloesser, D., Arensdorf, A. M., Simmons, J. M., Cui, C., Valentino, R., Gnadt, J. W., Nielsen, L., St. Hillaire-Clarke, C., Spruance, V., Horowitz, T. S., Vallejo, Y. F., & Langevin, H. M. (2021). The emerging science of interoception: Sensing, integrating, interpreting, and regulating signals within the self. Trends in Neurosciences, 44, 3–16. https://doi.org/10.1016/j.tins.2020.10.007
- Da Vinci, L. (1651). Trattato della pittura di Lionardo da Vinci. Parigi.
- Delatte, A. (1932). La catoptromancie greque et ses dérivés. Liège.
- Demartini, B., Nisticò, V., Tedesco, R., Marzorati, A., Ferrucci, R., Priori, A., Gambini, O., & Caputo, G. B. (2021). Visual perception and dissociation during Mirror Gazing Test in patients with anorexia nervosa: a preliminary study. Eat Weight Disord, 26(5), 1541–1551. https://doi.org/10.1007/s40519-020-00977-6
- Derome, M., Fonseca-Pedrero, E., Badoud, D., Morosan, L., Van De Ville, D., Lazeyras, F., Eliez, S., Chan, R., Rudrauf, D., Schwartz, S., & Debbane, M. (2018). Resting-state networks of adolescents experiencing depersonalization-like illusions: Cross-sectional and longitudinal findings. Schizophrenia Bulletin, 44(suppl_2), S501–S511. https://doi.org/10.1093/schbul/sby031
- Derome, M., Fonseca-Pedrero, E., Caputo, G. B., & Debbané, M. (2022). A developmental dtudy of mirror-gazing-induced anomalous self-experiences and self-reported schizotypy from 7 to 28 years of age. Psychopathology, 55(1), 49–61. https://doi.org/10.1159/000520984
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
- Fogarty, C., & Stern, J. A. (1989). Eye movements and blinks: Their relationship to higher cognitive processes. International Journal Psychophysiology, 8, 35–42. https://doi.org/10.1016/0167-8760(89)90017-2
- Fonseca-Pedrero, E., Badoud, D., Antico, L., Caputo, G. B., Eliez, S., Schwartz, S., & Debbane, M. (2015). Strange-face-in-the-mirror Illusion and schizotypy during adolescence. Schizophrenia Bulletin, 41(suppl 2), S475–S482. https://doi.org/10.1093/schbul/sbu196
- Foote, B., & Park, J. (2008). Dissociative identity disorder and schizophrenia: Differential diagnosis and theoretical issues. Current Psychiatry Reports, 10, 217–222. https://doi.org/10.1007/s11920-008-0036-z
- Gallagher, S. (2000). Philosophical conceptions of the self: Implications for cognitive science. Trends Cognitive Sciences, 4(1), 14–21. https://doi.org/10.1016/S1364-6613(99)01417-5
- Gallup, G. G., Platek, S. M., & Spaulding, K. L. (2014). The nature of visual self-recognition revisited. Trends Cognitive Sciences, 18(2), 57–58. https://doi.org/10.1016/j.tics.2013.10.012
- Harrington, A., Oepen, G., & Spitzer, M. (1989). Disordered recognition and perception of human faces in acute schizophrenia and experimental psychosis. Comparative Psychiatry, 30(5), 376–384. https://doi.org/10.1016/0010-440X(89)90003-5
- Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2000). The distributed human neural system for face perception. Trends Cognitive Sciences, 4(6), 223–233. https://doi.org/10.1016/S1364-6613(00)01482-0
- Healey, E. (2021). Not only a tool-stone: Other ways of using obsidian in the Near East. Journal of Lithic Studies, 8, 23–81. https://doi.org/10.2218/jls.5739
- Holmes, E. A., Brown, R. J., Mansell, W., Fearon, R. P., Hunter, E. C., Frasquilho, F., & Oakley, D. A. (2005). Are there two qualitatively distinct forms of dissociation? A review and some clinical implications. Clinical Psychology Review, 25, 1–23. https://doi.org/10.1016/j.cpr.2004.08.006
- Kerenyi, K. (1976). Dionysos. Langen-Müller.
- Lange, R., Caputo, G. B., Lynn, S. J., & Houran, J. (2022). Mirror- and eye-gazing perceptions in advanced psychometric perspective: Preliminary findings. Psychology of Consciousness: Theory, Research, and Practice, 9(3), 230–242. https://doi.org/10.1037/cns0000328
- Longo, P., Panero, M., Amodeo, L., Demarchi, M., Abbate Daga, G., & Marzola, E. (2021). Psychoform and somatoform dissociation in anorexia nervosa: A systematic review. Clinical Psychology Psychotherapy, 28(2), 295–312. https://doi.org/10.1002/cpp.2517
- Lynn, S. J., Polizzi, C., Merckelbach, H., Chiu, C., Maxwell, R., van Heugten, D., & Lilienfeld, S. O. (2022). Dissociation and dissociative disorders reconsidered: Beyond sociocognitive and trauma models toward a transtheoretical framework. Annual Review of Clinical Psychology, 18, 259–289. https://doi.org/10.1146/annurev-clinpsy-081219-102424
- Maffei, A., & Angrilli, A. (2018). Spontaneous eye blink rate: An index of dopaminergic component of sustained attention and fatigue. International Journal Psychophysiology, 123, 58–63. https://doi.org/10.1016/j.ijpsycho.2017.11.009
- Mamiya, Y., Nishio, Y., Watanabe, H., Yokoi, K., Uchiyama, M., Baba, T., Iizuka, O., Kanno, S., Kamimura, N., Kazui, H., Hashimoto, M., Ikeda, M., Takeshita, C., Shimomura, T., Mori, E., & Sleegers, K. (2016). The pareidolia test: A simple neuropsychological test measuring visual hallucination-like illusions. Plos One, 11(5), e0154713. https://doi.org/10.1371/journal.pone.0154713
- Manor, B. R., Gordon, E., Williams, L. M., Rennie, C. J., Bahramali, H., Latimer, C. R., Barry, R. J., & Meares, R. A. (1999). Eye movements reflect impaired face processing in patients with schizophrenia. Biological Psychiatry, 46(7), 963–969. https://doi.org/10.1016/S0006-3223(99)00038-4
- McBain, R., Norton, D., & Chen, Y. (2009). Females excel at basic face perception. Acta Psychologica, 130(2), 168–173. https://doi.org/10.1016/j.actpsy.2008.12.005
- Miller, P. P., Brown, T. A., DiNardo, P. A., & Barlow, D. H. (1994). The experimental induction of depersonalization and derealization in panic disorder and nonanxious subjects. Behaviour Research Therapy, 32(5), 511–519. https://doi.org/10.1016/0005-7967(94)90138-4
- Nesbit, A., Dorahy, M. J., Palmer, R., Middleton, W., Seager, L., & Hanna, D. (2022). Dissociation as a mediator between childhood abuse and hallucinations: An exploratory investigation using dissociative identity disorder and schizophrenia spectrum disorders. Journal of Trauma & Dissociation, 23(5), 521–538. https://doi.org/10.1080/15299732.2022.2064579
- Nisticò, V., Caputo, G. B., Tedesco, R., Marzorati, A., Ferrucci, R., Priori, A., Gambini, O., & Demartini, B. (2020). Dissociation during mirror gazing test in psychogenetic non-epileptic seizures and functional movement disorders. Epilepsy & Behavior, 112, 107368. https://doi.org/10.1016/j.yebeh.2020.107368
- Park, H. D., & Blanke, O. (2019). Coupling inner and outer body for self-consciousness. Trends Cognitive Sciences, 23(5), 377–388. https://doi.org/10.1016/j.tics.2019.02.002
- Pick, S., Rojas-Aguiluz, M., Butler, M., Mulrenan, H., Nicholson, T., & Goldstein, L. (2020). Dissociation and interoception in functional neurological disorder. Cognitive Neuropsychiatry, 25(4), 294–311. https://doi.org/10.1080/13546805.2020.1791061
- Plato, A. (2001). Cambridge Greek and Latin Classics. Alcibiades. Cambridge University Press. ISBN: 978-0-521-63414-4.
- Rehnman, J., & Herfitz, A. (2007). Women remember more faces than men do. Acta Psychologica, 124(3), 344–355. https://doi.org/10.1016/j.actpsy.2006.04.004
- Rogers, S., Keogh, R., & Pearson, J. (2020). Hallucinations on demand: The utility of experimentally induced phenomena in hallucination research. Philosophical Transactions Royal Society B: Biological Sciences, 376(1817), 20200233. https://doi.org/10.1098/rstb.2020.0233
- Schäflein, E., Sattel, H., & Sack, M. (2020). Masking and mirror – What can we learn from facial recognition in patients with (partial) Dissociative Identity Disorder? Journal of Trauma & Dissociation, 21(1), 14–15. https://doi.org/10.1080/15299732.2019.1675223
- Schäflein, E., Sattel, H., Schmidt, U., & Sack, M. (2018). The enemy in the mirror: Self-perception-induced stress results in dissociation of psychological and physiological responses in patients with dissociative disorder. European Journal of Psychotraumatology, 9(sup3), 1472991. https://doi.org/10.1080/20008198.2018.1472991
- Schimmenti, A., & Sar, V. (2019). A correlation network analysis of dissociative experiences. Journal of Trauma & Dissociation, 20(4), 402–419. https://doi.org/10.1080/15299732.2019.1572045
- Sierra, M., & Berrios, G. E. (2000). The cambridge depersonalisation scale: A new instrument for the measurement of depersonalisation. Psychiatry Research, 93(2), 153–164. https://doi.org/10.1016/S0165-1781(00)00100-1
- Sommer, W., Hildebrandt, A., Kunina-Habenicht, O., Schacht, A., & Wilhelm, O. (2013). Sex differences in face cognition. Acta Psychologica, 142(1), 62–73. https://doi.org/10.1016/j.actpsy.2012.11.001
- Steinberg, M., & Schnall, M. (2001). The stranger in the mirror: Dissociation: The hidden epidemic. Harper Collins.
- Tellegen, A., & Atkinson, G. (1974). Openness to absorbing and self-altering experiences absorption, a trait related to hypnotic susceptibility. Journal of Abnormal Psychology, 83(3), 268–277. https://doi.org/10.1037/h0036681
- Troxler, D. (1804). Ueber das Verschwinden gegebener Gegenstaende innerhalb unsers Gesichtskreises. In K. Himly & J. A. Schmidt (Eds.), Ophthalmologische Bibliothek (pp. 51–53). Fromman.
- Tsakiris, M. (2017). The multisensory basis of the self: From body to identity to others. The Quarterly Journal of Experimental Psychology, 70(4), 597–609. https://doi.org/10.1080/17470218.2016.1181768
- Uddin, L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature reviews Neuroscience, 16(1), 55–61. https://doi.org/10.1038/nrn3857
- Volkmann, F. C., Riggs, L., & Moore, R. K. (1980). Eyeblinks and visual suppression. Science, 207(4433), 900–902. https://doi.org/10.3758/BF03202953
- Vollrath, G. C. (2018). Cultural techniques of mirroring from lecanomancy to Lacan. Communication+1, 7, 1–31.
- Williams, L. M., Loughland, C. M., Gordon, E., & Davidson, D. (1999). Visual scanpaths in schizophrenia: Is there a deficit in face recognition? Schizophrenia Research, 40(3), 189–199. https://doi.org/10.1016/S0920-9964(99)00056-0
- Zhao, Y., Zhen, Z., Liu, X., Song, Y., & Liu, J. (2018). The neural network for face recognition: Insights from an fMRI study on developmental prosopagnosia. NeuroImage, 169, 151–161. https://doi.org/10.1016/j.neuroimage.2017.12.023
- Zhou, L. F., & Meng, M. (2020). Do you see the “face”? Individual differences in face pareidolia. Journal of Pacific Rim Psychology, 14, 1–8. https://doi.org/10.1017/prp.2019.27