Abstract
Hiccups are a rare but potentially debilitating side effect of opioid treatment, with only a handful of reported cases in the medical literature. The pathophysiological mechanism linking opioids and hiccups is unknown, and a lack of evidence exists concerning the optimal management of the condition. We report on a 64-year-old man diagnosed with advanced renal cancer and painful osteolytic metastases, presenting persistent hiccups while on opioid treatment. Hiccups recurred after multiple challenges with codeine, morphine and hydromorphone on separate occasions. Hiccups ceased only after opioid discontinuation, although various pharmacological treatments were tried to shorten the duration of hiccups. Eventually, fentanyl was introduced and was well tolerated by the patient, without any recurrence of hiccups. The chronological correlation between opioid initiation and the onset of hiccups, as well as opioid discontinuation and the termination of hiccups leads to the conclusion that a causal role of codeine, morphine and hydromorphone in this occurrence is likely. Individual susceptibility probably plays a central role in the development of opioid-related hiccups. Opioid rotation is a promising strategy in the management of opioid-related hiccups, particularly when the mere discontinuation of the opioid is not a viable option, such as in the oncology and palliative care field.
Introduction
Hiccups (singultus) are a common phenomenon in the general population, and can occur in up to 9% of patients with advanced cancer (Citation1,Citation2). When lasting more than 48 hours it is referred to as persistent whereas a duration of more than one month defines the hiccups as intractable (Citation3). Although apparently harmless, hiccups may become extremely debilitating when protracted, and be associated with other symptoms such as discomfort, pain, lack of sleep, weight loss, anxiety and depression (Citation4,Citation5). Hiccups may have a major impact on patients’ quality of life and is associated with increased healthcare resource use (Citation6). Recent systematic reviews showed that several treatment options have been used, but there is a lack of knowledge regarding optimal management of hiccups, and the evidence is insufficient to make strong recommendations (Citation3,Citation4). In this article, we briefly summarize the present knowledge about causes, pathophysiological mechanisms and management options of hiccups. We then report on the case of a 64-year-old man developing persistent hiccups after taking several different opioids for cancer pain. The patient agreed to the publication and gave written informed consent. A literature review of opioid-related hiccups was performed and is then discussed at the end of the article.
Etiology and pathophysiology
Causes of hiccup
More than 100 causes of acute, persistent or intractable hiccups have been identified, and divided into two main groups: central nervous system-related causes (ischemic, hemorrhagic, infectious, structural, inflammatory or degenerative), and peripheral nervous system-related causes. The latter are mostly associated with gastrointestinal conditions, but cardiovascular, pulmonary, and head and neck conditions are also involved. A third group of other miscellaneous causes includes toxic and metabolic conditions, surgical, anaesthesiologic and endoscopic procedures, psychosomatic disorders, and various drugs (Citation3,Citation5,Citation7). Hiccups were associated with antibiotics (e.g., beta-lactams, macrolides, fluoroquinolones), antineoplastic agents (e.g., fluorouracil, platin-based compounds, irinotecan (Citation8,Citation9)), dopamine agonists (e.g., rotigotine, pramipexole (Citation10)), benzodiazepines, steroids (in particular dexamethasone (Citation11)), and opioids (Citation3).
The hiccup reflex
Typically described as a reflex arc, hiccups are the result of involuntary repetitive contractions of the diaphragm and other inspiratory muscles, leading to rapid air intake, immediately followed by abrupt closure of the glottis giving its characteristic sound. Because of the great diversity of known causative stimuli, comprehensively defining the afferent routes of the hiccup reflex is a challenging task. The most important peripheral afferent pathways are considered to be the vagal nerve, the phrenic nerves, the pharyngeal plexus from C2 to C4, and the sympathetic chain from T6 to T12 (Citation4,Citation7).
The central mechanisms leading to hiccups are even less understood. They include structures in the brainstem (respiratory center, reticular activating system), and the proximal cervical cord (C3–C5). The neurotransmitters involved are mostly undefined, but seem to include gamma-aminobutyric acid (GABA), dopamine and serotonin (Citation5,Citation12–14).
The efferent response of the reflex arc is transmitted by the phrenic nerve to the diaphragm, the accessory nerves to the intercostal and scalene muscles, and the recurrent laryngeal branch of the vagal nerve to the glottis (Citation3,Citation4,Citation7).
Management of hiccups
A systematic review from 2015 (Citation3) aimed at outlining the optimal pharmacological management of hiccups. Persistent or intractable hiccups without an evident cause may find relief from an empirical treatment of gastroesophageal reflux disease. If this fails, the authors recommend baclofen (5–20 mg TID), a GABA-B agonist, as first line therapy, and gabapentin (300–600 mg TID), a voltage-gated calcium channel modulator, as an alternative, especially if a central nervous system condition is present or suspected (e.g., cerebrovascular disease, brain tumor or intracranial injury). The use of metoclopramide (10 mg TID), chlorpromazine (25–50 mg up to QID) and other anti-dopaminergic drugs may be considered for acute, but not long-term management of hiccups (Citation3).
Another systematic review from 2017 (Citation15) came to similar conclusions, defining baclofen, gabapentin and metoclopramide as good options to use when initially treating persistent and intractable hiccups. The authors of this review are even more cautious in their conclusions, stating that there is not enough evidence to be able to recommend any single pharmacological therapy over another for a patient with hiccups.
Non-pharmacological therapies such as acupuncture, hypnosis, positive pressure ventilation, and surgical or anesthetic procedures to disrupt or stimulate nerves involved in the reflex arc of hiccups have been tried, and may also be effective. Nevertheless, the evidence to support them is very limited (Citation3).
Case report
We report on the case of a 64-year-old man with a history of arterial hypertension, obstructive sleep apnea syndrome, hiatal hernia with gastroesophageal reflux disease and renal cell carcinoma with painful bone metastases, presenting recurrent persistent hiccups while being treated with different opioids on separate occasions.
Oncological history
The patient was diagnosed in 2015 with non-metastatic, Fuhrman IV clear cell renal cell carcinoma, for which he initially underwent right surgical nephrectomy. In 2016 an oligometastatic cancer progression to the right lung was identified and a surgical treatment was performed, followed by stereotactic radiotherapy. In 2017, due to multifocal pulmonary progression, a first line of systemic therapy by pazopanib was begun (6 cycles). In 2018 a metastatic progression in the lungs, mediastinal lymph nodes, and bones (right ilium wing, D12 vertebral body, and right femoral neck) was found at follow-up. A pathological bone fracture of the right femoral neck was treated by total hip replacement surgery and local radiotherapy (20 Gy, for consolidation and analgesia). Later in 2018, the patient also underwent a D12 vertebrae cementoplasty followed by local radiotherapy (20 Gy). A palliative immunotherapy of ipilimumab and nivolumab was initiated, but had to be stopped in 2019 due to pneumonitis. In 2020 osteolytic metastases were found in the right acetabulum and the left gluteal muscle and treated with local radiotherapy (20 Gy and 35 Gy respectively). In April 2021, a progression of the known right femoral neck lesion, as well as an infiltrating mass of the right iliotibial tract was found on the MRI. A local radiotherapy was performed for analgesic purposes (25 Gy). A treatment of cabozantinib, a tyrosine kinase inhibitor, was initiated in May 2021 (7 cycles, until December 2021).
The patient was directed to our outpatient palliative care consultation for the first time in May 2021 for the introduction of a pharmacological analgesic treatment. His oral medication at the time is detailed in . No systematic or as-needed pharmacological analgesic treatment was in place. During the first consultation, the patient reported a progressively increasing right trochanter pain that had started in 2018, with a clear exacerbation since he had begun radiotherapy the previous week. The pain was described as severe, continuous and dull. It had no neuropathic character, woke up the patient at night and was partially relieved by the sitting position.
Table 1. Characteristics of opioid-related hiccup in the present case, whilst the patient was followed at our outpatient clinic.
Opioids and hiccups history
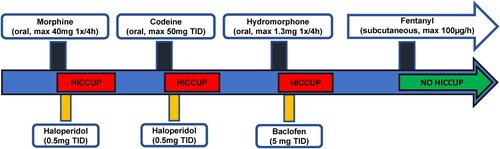
The patient had a history of previous oral morphine (formulation: 10 mg/ml oral solution; administered dose: up to 40 mg every 4 hours, plus 25 mg TID as needed) and oral codeine (formulation: 50 mg tablets; administered dose: 50 mg TID) treatments at separate times, both requiring interruption due to the occurrence of persistent hiccups. The first episode of hiccups while on morphine treatment occurred after the hip replacement surgery in 2018, and it persisted up to three weeks after morphine discontinuation, and was partially relieved by oral haloperidol (0.5 mg TID). The second episode of hiccups while on codeine treatment occurred in 2019, and it persisted up to ten days after codeine discontinuation and was resolved after four days of treatment with oral haloperidol (0.5 mg TID). On the latter occasion, an oral treatment of gabapentin followed by metoclopramide (unknown doses) was first tried to relieve hiccups, but was ineffective. Of note, a treatment with oral hydromorphone (formulation: 1 mg/ml oral solution; administered dose: up to 10 mg every 4 hours systematically, plus 5 mg TID as needed) short after hip replacement surgery, and with sublingual buprenorphine (formulation: 0.2 mg sublingual tablets; administered dose: up to 0.4 mg TID systematically, plus 0.2 mg BID as needed) after D12 cementoplasty later in 2018, had been well tolerated, without any occurrence of hiccups at the time.
Based on that information, an analgesic treatment with oral hydromorphone (formulation: 1.3 mg capsules; administered dose: 1.3 mg every 4 hours, plus 1.3 mg 6 times a day as needed) was prescribed at the end of our first consultation, in May 2021.
One day after hydromorphone initiation, the patient reported a slight improvement of his pain, but also the occurrence of hiccups, which began less than 4 hours after the first dose of hydromorphone. The hiccups became intolerable after the second dose of hydromorphone, keeping the patient awake at night and resulted in the interruption of the opioid treatment. Along with hiccups, he also described 5 to 10 episodes of what seems to be an involuntary spastic glottis closure, lasting no longer than 2 seconds and accompanied by a suffocating sensation. These episodes presented sporadically, only during daytime, and were generally precipitated by food intake and preceded by eructation. Due to the persistence of hiccups 3 days after hydromorphone discontinuation, an oral treatment of haloperidol 0.5 mg QID was initiated. A slight improvement, especially at night, was noted 3 days after haloperidol initiation and 6 days after hydromorphone cessation, but the resolution of the hiccups was incomplete. The haloperidol treatment was then replaced with oral baclofen 5 mg TID. Thirty-six hours after baclofen initiation the patient reported a complete resolution of the hiccups and glottis spasms. Baclofen treatment was stopped and the hiccups did not reoccur. The patient did not wish to discuss any further opioid treatment at this point. Oral paracetamol 1000 g TID was then prescribed, and was satisfactory alongside the analgesic effects of the recently completed radiotherapy.
In June 2021, the patient experienced a recurrence of persistent hiccups unrelated to opioid use. It started 24 hours after the initiation of an oral dexamethasone treatment (4 mg BID) for analgesic purposes, and right after a copious meal. Hiccups resolved after dexamethasone cessation, baclofen 10 mg TID and pantoprazole 40 mg daily treatment.
Six months later, the patient was admitted to the hospital because of a functional decline and right hip pain exacerbation. At that time, his pain treatment consisted of oral paracetamol 1000 mg TID and tramadol (formulation: 50 mg capsules; administered dose: 50 mg TID as needed). Tramadol had been started a month earlier by his oncologist and was well tolerated at the maximal prescribed dose, but was ineffective in reducing pain. It was therefore replaced with intravenous fentanyl (formulation: 0.02 mg/ml injectable solution; administered dose: 35 µg 6 times a day as needed) upon admission. Due to good tolerance and no hiccup recurrence, a subcutaneous continuous fentanyl pump was initiated after 5 days and cautiously increased over 3 weeks up to a rate of 100 µg per hour, plus 200 µg of sublingual fentanyl (formulation: 100 µg sublingual tablets) as needed. No hiccups were observed and a transition from subcutaneous to transdermal fentanyl administration was made. A timeline of opioid treatments, hiccup occurrence and management are represented graphically in .
Literature evidence for opioid-related hiccup
We conducted a literature search using two electronic databases, PubMed and Embase, on January 31, 2022. The following search strategy was adopted: (hiccup OR hiccups OR singultus OR hiccough) AND (opioids OR opioid OR tramadol OR codeine OR morphine OR fentanyl OR hydromorphone).
We found five case reports describing a total of six cases of opioid-related hiccups. Four of them were associated with oral opioid intake, whereas the remaining two were related to intrathecal opioid infusion. The six cases are detailed in . Additionally, we found two prospective studies describing an association between opioids and hiccups (Citation16,Citation17). In these studies, however, opioids were administered as part of a multi-drug protocol for the induction of general anesthesia and it is therefore impossible to make any inference on their role in the appearance of hiccups.
Table 2. Characteristics of opioid-related hiccup case reports in literature.
In a review of the French Pharmacovigilance Database, Bagheri et al. (Citation18) found that opioids accounted for 6% of the 53 cases of drug-induced hiccups reported in France between 1985 and 1997 (three cases in 12 years). In a case-noncase study conducted on the European Pharmacovigilance Database and published in 2021, García et al. (Citation19) showed a statistical association between tramadol and hiccups. Their data also suggest that tramadol is more often related to hiccups than other opioids.
On April 14, 2022 we also searched for reports of hiccups associated with different opioids in VigiBase®, the World Health Organization pharmacovigilance database. The results of the search are detailed in .
Table 3. Frequency of hiccup reports for different opioids in VigiBase as of 04.14.2022.
Discussion
Although the association between opioids and persistent hiccups has been reported to pharmacovigilance systems, it is rarely described in the medical literature. Our case report may help to improve the lack of data documenting a correlation between opioid initiation and the development of hiccups. Furthermore we could document several trials of various opioids – codeine, morphine, hydromorphone – in our patient’s history and show that a causal relationship between opioids and hiccups was likely (Citation20).
Interestingly, the patient did not develop hiccups when fentanyl was administered. In one of the six cases detailed in , the rotation to fentanyl was followed by hiccup cessation (Citation21). Fentanyl is a synthetic opioid with the chemical structure of a phenylpiperidine, whereas codeine, morphine and hydromorphone share a molecular structure based on the 4,5-epoxymorphinan ring (Citation22). These observations suggest that opioid-related hiccups could be mediated by a different pathway than µ-opioid receptor binding, and that the different molecular structure might partly explain fentanyl tolerance in our patient. The underlying mechanism is probably complex since tramadol, which is chemically related to codeine, was also well tolerated by our patient. Furthermore, hiccup reports with both fentanyl and tramadol were found in VigiBase, and the ratio of hiccup reports to total side effect reports for fentanyl is similar to that of many 4,5-epoxymorphinan ring-based opioids. Tramadol is the opioid with both the highest number of hiccup reports and the highest ratio of hiccup reports to total side effect reports. Nevertheless, it should be noted that these reports do not allow one to infer any causality between an adverse event and a drug, nor do they determine the likelihood of a side effect occurring. For instance, they do not take into account multiple other factors like the number of patients exposed to the drug, the duration and dose of exposure, and the bias of individual reporting practices from different national pharmacovigilance centers (Citation23).
Although our patient had risk factors for hiccups such as gastroesophageal reflux disease and hiatal hernia, he did not present any hiccups when not on opioids. Only one of the persistent hiccup episodes that he experienced was unrelated to opioid intake and began 24 hours after the initiation of an oral dexamethasone treatment. Hiccups are considered a potential side effect of dexamethasone (Citation11), and the causality in this patient’s case is possible (Citation20). Our patient developed hiccups when taking codeine 150 mg per day only, which corresponds to a morphine equivalent dose of 15 mg per day. Moreover, hiccups reoccurred after a first dose of hydromorphone, which is not typical of a dose-dependent adverse effect, but rather suggestive of an idiosyncratic reaction. These observations suggest that individual susceptibility plays a major role in the development of opioid-, and more generally drug-related hiccups. The delay between opioid discontinuation and the resolution of the hiccups was longer in our patient compared to previously published cases. Individual susceptibility may play a role, alone or in addition to contributing factors such as the irritation of the vagal or phrenic nerves found in gastroesophageal or head and neck conditions. A self-sustaining mechanism, with bursts of hiccups stimulating vagal and phrenic nerves, could also lead to prolonged symptoms after drug discontinuation.
A feature of our patient’s history that is common to all the other case reports of opioid-related hiccups is the fact that persistent hiccups could not be successfully treated with any pharmacological means other than opioid discontinuation or rotation. No symptomatic treatment was completely effective as long as the opioid responsible for hiccups was continued. In the oncology and palliative care field, opioids are often a necessity to effectively treat pain. Given the latter, a strategy based on the mere discontinuation of the opioid for treating an opioid-related hiccup is not a satisfying option, whereas opioid rotation is an interesting alternative. Our patient was effectively relieved from pain with fentanyl, without any recurrence of hiccups.
The mechanism by which opioids can cause hiccups is to this day unknown and it is difficult to make a strong hypothesis based on the available scientific literature.
Conclusions
This case report shows that, although rare, hiccups are a potentially debilitating side effect of opioids. The underlying pathophysiological mechanism is not known but individual susceptibility likely plays a central role. After multiple opioid trials associated with persistent hiccups, fentanyl was well tolerated in our patient, supporting the idea that opioid rotation is a promising strategy in the management of opioid-related hiccups. The use of a symptomatic treatment in addition to opioid rotation could help accelerate hiccup regression, although the available literature does not lead to any strong recommendation of a determined molecule over another. Further studies are needed to better understand the biochemical and pathophysiological basis of opioid-related hiccups as well as to define its optimal management.
| Acronyms and abbreviations | ||
| BID | = | twice a day |
| TID | = | three times a day |
| QID | = | four times a day |
Acknowledgements
Giacomo Xausa is the principal investigator. Each author meets criteria for authorship, has approved the content of this article, takes full responsibility for publication and has agreed to the journal’s submission policies.
Disclosure statement
None of the authors report any conflict of interest.
Additional information
Funding
References
- Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. Maggio 2000;8(3):175–9.
- Jeon YS, Kearney AM, Baker PG. Management of hiccups in palliative care patients. BMJ Support Palliat Care. 2018;8(1):1–6. doi:10.1136/bmjspcare-2016-001264.
- Steger M, Schneemann M, Fox M. Systemic review: the pathogenesis and pharmacological treatment of hiccups. Aliment Pharmacol Ther. 2015;42(9):1037–50. doi:10.1111/apt.13374.
- Adam E. A systematic review of the effectiveness of oral baclofen in the management of hiccups in adult palliative care patients. J Pain Palliat Care Pharmacother. 2020;34(1):43–54. doi:10.1080/15360288.2019.1705457.
- Becker DE. Nausea, vomiting, and hiccups: a review of mechanisms and treatment. Anesth Prog. 2010;57(4):150–6. doi:10.2344/0003-3006-57.4.150.
- Hendrix K, Wilson D, Kievman MJ, Jatoi A. Perspectives on the medical, quality of life, and economic consequences of hiccups. Curr Oncol Rep. 2019;21(12):113. doi:10.1007/s11912-019-0857-4.
- Kahrilas PJ, Shi G. Why do we hiccup? Gut. 1997;41(5):712–3. doi:10.1136/gut.41.5.712.
- Hosoya R, Uesawa Y, Ishii-Nozawa R, Kagaya H. Analysis of factors associated with hiccups based on the Japanese adverse drug event report database. PLoS One. 2017;12(2):e0172057. doi:10.1371/journal.pone.0172057.
- Hosoya R, Ishii-Nozawa R, Kurosaki K, Uesawa Y. Analysis of factors associated with hiccups using the FAERS database. Pharm Basel Switz. 2021;15(1):27. doi:10.3390/ph15010027.
- Lertxundi U, Marquínez AC, Domingo-Echaburu S, Solinís MÁ, Calvo B, Del Pozo-Rodríguez A, García M, Aguirre C, Isla A. Hiccups in Parkinson’s disease: an analysis of cases reported in the European pharmacovigilance database and a review of the literature. Eur J Clin Pharmacol. 2017;73(9):1159–64. doi:10.1007/s00228-017-2275-6.
- Go S-I, Koo D-H, Kim ST, Song H-N, Kim RB, Jang J-S, Oh SY, Lee KH, Lee SI, Kim S-G, et al. Antiemetic corticosteroid rotation from dexamethasone to methylprednisolone to prevent dexamethasone-induced hiccup in cancer patients treated with chemotherapy: a randomized, single-blind, crossover phase III trial. Oncologist. 2017;22(11):1354–61.
- Nausheen F, Mohsin H, Lakhan SE. Neurotransmitters in hiccups. Springerplus. 2016;5(1):1357. doi:10.1186/s40064-016-3034-3.
- Petroianu GA, Lorke DE. The role of serotonin in singultus: a review. Front Neurosci. 2020;14:629. doi:10.3389/fnins.2020.00629.
- Alefishat E, Aloum L, Baltatu OC, Petroianu GA. The action of aripiprazole and brexpiprazole at the receptor level in singultus. J Integr Neurosci. 2021;20(1):247–54.
- Polito NB, Fellows SE. Pharmacologic interventions for intractable and persistent hiccups: a systematic review. J Emerg Med. 2017;53(4):540–9. doi:10.1016/j.jemermed.2017.05.033.
- Payen JF, Riondel JP, Denis MJ, Doublier C, Badji R, [Arvieux C, Stieglitz P. Comparison of the clinical effects of propofol and methohexital in induced abortion. Ann Fr Anesth Reanim. 1987;6(4):293–6. doi:10.1016/S0750-7658(87)80043-6.
- Wang X, Bai H, Shu L. Application of intravenous and inhalational combined anesthesia with midazolam, fentanyl 1 and enflurane in cleft palate repair operation. Hua Xi Kou Qiang Yi Xue Za Zhi Huaxi Kouqiang Yixue Zazhi West China J Stomatol. 1999;17(3):239–41.
- Bagheri H, Cismondo S, Montastruc JL. Drug-induced hiccup: a review of the France pharmacologic vigilance database. Therapie. 1999;54(1):35–9.
- García M, Lertxundi U, Aguirre C. Tramadol-induced hiccups: a case-noncase study in the European pharmacovigilance database. Ther Adv Drug Saf. 2021;12:20420986211021230. doi:10.1177/20420986211021230.
- The use of the WHO-UMC system for standardised case causality assessment [Internet]. Uppsala Monitoring Centre; 2018. Disponibile su: https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf.
- Wilcox SK. Persistent hiccups after slow-release morphine. Palliat Med. 2005;19(7):568–9.
- Opioids. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. 2012.
- https://www.vigiaccess.org/.
- Lauterbach EC. Hiccup and apparent myoclonus after hydrocodone: review of the opiate-related hiccup and myoclonus literature. Clin Neuropharmacol. 1999;22(2):87–92. doi:10.1097/00002826-199903000-00004.
- Panchal R, Bhutt V, Anovadiya A, Purohit B, Dekhaiya F, Goswami N. Tramadol-induced hiccups: a report of two cases. Drug Saf Case Rep. 2018;5(1):3. doi:10.1007/s40800-017-0066-8.
- Ruan X, Couch JP, Shah R, Wang F, Liu HN. Persistent hiccup associated with intrathecal morphine infusion pump therapy. Am J Phys Med Rehabil. 2007;86(12):1019–22. doi:10.1097/PHM.0b013e31815206c8.
- Loomba V, Gupta M, Kim D. Persistent hiccups with continuous intrathecal morphine infusion. Clin J Pain. 2012;28(2):172–4. doi:10.1097/AJP.0b013e31822364a8.