Abstract
Di-(2-ethylhexyl)phthalate (DEHP) helps provide flexibility to plastic products including food containers and medical IV devices. Age-based sensitivity to DEHP-induced immunotoxicity was compared in female CD strain rats following administration for 16 consecutive days in utero vs. adult exposure. Pregnant and non-pregnant rats received 0, 37.5, 75, 150, or 300 mg/kg/day of DEHP in soybean oil daily by oral gavage. In pregnant animals, this corresponded to days 6–21 of gestation. In exposed offspring, anogenital distance (AGD) was measured at one and three weeks of age. For a subset of offspring, immune assessment was conducted at 5 weeks of age following KLH immunization at 3 and 4 weeks. KLH immunizations occurred in remaining offspring at 11 and 12 weeks with immune assessment at 13 weeks. Nonpregnant adults were immunized concurrently with adult offspring and underwent immune assessment 13 weeks post-DEHP exposure. Litter size, sex ratio, and 1-week body weights were unaffected by treatment. However, AGD was altered at both ages assessed. Three-week (but not 5-week) body weights were elevated in exposed offspring. Immune organ weights, thymus histology, antibody levels (IgG and IgE), DTH reaction to KLH, total and differential leukocyte counts, ex vivo cytokine production (IL-2, -4, -10, -12, interferon-gamma), as well as TNF-alpha and nitric oxide production by macrophages were not altered by treatment at any age. Flow cytometry analysis of splenocytes showed no differences during the juvenile assessment. In conclusion, in utero exposure of female rats to DEHP alters some developmental parameters (i.e., AGD) but has no persistent effect on the immune system based on the assessment parameters. Adult female rats also had no detectable immune alteration following similar exposures.
INTRODUCTION
Developmental immunotoxicology (DIT) assessment has garnered increasing attention as concerns have risen over the reliability of extrapolating adult exposure-outcome data to predict the immunological consequences of early exposures. To address the need for a more robust DIT database, 3 known adult immunotoxicants (lead, dexamethasone, and cyclosporin A) were previously compared for immunotoxicity following in utero vs. adult exposure of rats. Results of these studies suggested that the early life stages were more sensitive to these immunotoxicants than adults. Additionally, the adult exposure data were not effective in predicting the dose-response sensitivity of the fetus, the spectrum of fetal-induced immune alterations or the potential persistence of the immunotoxicity following early exposure. Hence, these prior studies indicated that direct DIT testing was helpful, if not required, in order to ascertain immunotoxicological risk to the fetus.
In the current study, the same protocol employed to compare age-related effects of known immunotoxicants was used to evaluate the developmental immunotoxicity of an environmental contaminant of potential concern particularly during early human life. This protocol is specifically designed to assess immune alterations that persist long after the exposure to an immunotoxicant has ended rather than immediate changes after exposure. The previous studies with lead (Bunn et al., Citation2001a, b), dexamethasone (Dietert et al., Citation2003), and cyclosporin (Hussain et al., Citation2005) demonstrated that exposure to immunotoxicants during fetal development resulted in measurable changes in the immune system lasting into the juvenile and adult stages of life. Even though acute changes were evident in females exposed as adults (Hussain et al., Citation2005), these immune alterations did not persist like those of offspring exposed to the immunotoxicant in utero.
Di-(2-ethylhexyl)phthalate (DEHP) is the plasticizer most commonly used in the preparation of flexible polyvinychoride (PVC) plastics, which can include food containers, wall coverings, and vinyl flooring. Because of leaching from the plastic, DEHP is a widespread environmental contaminant. Human exposure is thought to occur primarily through ingestion of foods contaminated while stored in plastic containers, as well as through IV exposure from plastic tubing used in certain medical procedures. However, some dermal and inhalation exposure may also occur. DEHP has been implicated as an endocrine disrupter (Lovekamp-Swan and Davis, Citation2003) and a reproductive toxicant (Moore et al., Citation2001; Kavlock et al., Citation2002). It is also a peroxisome proliferator (Klaunig et al., Citation2003). Exposure levels in pregnant women are significant, and this has led to a recent concern for possible in utero-induced alterations to the offspring (Adibi et al., Citation2003).
More recently, it has been suggested that early-life DEHP exposure may play a role in the risk of asthma in children (Bornehag et al., Citation2004). The authors found a significant association of DEHP in house dust and childhood asthma among a Swedish population. Furthermore, DEHP has been reported by some investigators to shift immune parameters toward elevated T-helper-2 responses (IL-4 and IgE) in adult mice (Yang et al., Citation2000; Lee et al., Citation2004). However, other investigators have reported no immunotoxicity following DEHP exposure of adult mice (Butala et al., Citation2004). Because these immunotoxicology studies produced conflicting results and were restricted to adult DEHP exposures, investigation of the DIT risk associated with early life exposure to DEHP is warranted.
This report compares the immunotoxicity of DEHP in CD strain female rats following in utero vs. adult exposure. The findings are discussed in light of the other developmental immunotoxicity studies using known immunotoxicants as well as previous studies with DEHP.
MATERIALS AND METHODS
Reagents
Di-(2-ethylhexyl)phthalate (DEHP, 99% pure, CAS No:117-81-7) was purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI). Sigma Chemical Company (St. Louis, MO) supplied Escherichia coli lipopolysaccharide (LPS), concanavalin A (Con A), o-phenylenediamine dihydrochloride (OPD), bovine serum albumin (BSA), Tween 20, sodium azide, ingredients for PBS, and carbonate coating buffer. Monoclonal antibodies for flow cytometry were obtained from BD Biosciences Pharmingen (San Diego, CA). Horseradish peroxidase conjugated antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). RPMI-1640 was from Gibco (Invitrogen Corp., Grand Island, NY). Keyhole limpet hemocyanin (KLH) was purchased from Calbiochem (San Diego, CA). Monoclonal anti-rat IgE heavy chain and rat IgE-6 were purchased from Serotec USA (Washington, DC). Rat cytokine immunoassay kits were obtained from Biosource International (Camarillo, CA).
Animals
Protocols were approved by the Cornell University Institutional Animal Care and Use Committee and complied with NIH guidelines. A total of 64 2-day timed pregnant and another 60 nonpregnant female adult (CD strain Sprague–Dawley-type) rats were received from Charles River (Wilmington, MA). Rats were individually housed in polycarbonate cages and were provided with a certified rodent diet, and water ad libitum. Rats were randomly divided into five treatment groups of 13 pregnant and 12 nonpregnant rats per group (except the control group that contained 12 pregnant and 12 nonpregnant rats). A 12-hour light/dark cycle was maintained during the entire experiment. Temperature was maintained between 20°C and 24°C and humidity was kept between 40–60%. The animals were weighed weekly during the period of the experiment.
Di-(2-Ethylhexyl)phthalate (DEHP) Exposure
Following 4 days of acclimation, treatment was initiated. Treatments consisted of administering soybean oil or 1 of 4 concentrations of DEHP (37.5, 75, 150, or 300 mg/kg) diluted in soybean oil by gavage, daily from gestational day 6 to 21. The amount dosed by gavage was determined by the previously recorded body weights and was adjusted each week. Animals receiving the control treatment were administered a volume of soybean oil equivalent to the volume of DEHP mixture given to the animals on other treatments based on weight. Male pups were culled at 7 days of age. Female offspring were weaned at 21 days and housed 4 per dam per cage until analyses.
Blood Collection and Differential Leukocyte Count
On day of sample collection, the female rats were anesthetized with a combination of ketamine and xylazine (The Butler Company, Columbus, OH). Blood was collected in syringes by cardiac puncture after opening the chest cavity. Smears were made from blood samples containing sodium EDTA as an anti-coagulant and stained with Diff-Quick stain set (Dade Behring Inc., Newark, DE) for differential leukocyte count. Blood without anti-coagulant was allowed to clot at room temperature and serum was harvested following overnight incubation of clotted blood in the refrigerator. Sera were stored at –20°C until further use.
Delayed-Type Hypersensitivity (DTH) Response
Immunization with KLH was carried out as described by Exon et al. (Citation1990). Briefly, KLH (5 mg/ml) was injected into the intracaudal tail fold in a 200 μ l volume of sterile water. The primary and secondary sensitizations were done at 21 days (D) (3 weeks) and 28 D (4 weeks) of age for the juvenile offspring assessment groups (usually 2 of the 4 pups born to each dam) and at 77 D (11 weeks) and 84 D (12 weeks) of age for the adult offspring assessment groups. Non-pregnant adult-exposed females were immunized concurrently with the in utero-exposed adult offspring groups. At 5 weeks post exposure, the juvenile rats previously sensitized to KLH received a challenge with heat aggregated (80°C for 1 hour) 40 mg/ml KLH in 0.05 ml of saline. At 13 weeks post-exposure, both the remaining female offspring and adult nonpregnant females previously sensitized to KLH were challenged with heat aggregated (80°C for 1 hour) 20 mg/ml KLH in 0.1 ml of saline. The challenge antigen was injected into one footpad with the other footpad receiving sterile saline. The DTH response was measured 24 hours later using a digital caliper (Dyer, Model 304).
Spleen Cell Preparation
Spleens were removed from euthanized rats, weighed, and placed in cold Hank's balanced salt solution. Each spleen was crushed with the back of a syringe plunger, forced and washed through fine nylon mesh (400 μ m) using 10 ml ice-cold RPMI-1640 medium that contained 5% fetal bovine serum. Each sample was then centrifuged at 300 × g for 5 minutes at 4°C, and the pellets were resuspended in 2 ml fresh RPMI-1640 medium. Splenocytes were isolated by centrifugation at 400 × g for 25 minutes at room temperature after layering onto 2 ml Histopaque (density 1.077 g/ml) columns. The resulting layer of cells was washed and resuspended in fresh RPMI-1640 and then plated 5 × 106 cells/ml/well. After 2 hours of incubation, wells were gently flushed with RPMI-1640 to remove nonadherent cells and fresh medium was added containing 0 or 10 ng/ml LPS to stimulate cells. Unseparated cells (3 × 106 cells/ml/well) were cultured for 72 hours in 0 or 5 μg/ml Con A in RPMI-1640 medium, and supernatants were collected and frozen at –20°C for cytokine analysis.
Nitric Oxide and Cytokine Measurements
Supernatants of LPS stimulated cells were collected after 24 hours of incubation. Nitric oxide production was evaluated by measuring the accumulation of the more stable endproduct, nitrite, by the Griess reaction (Green et al., Citation1982). Using an ELISA kit (Biosource International), frozen aliquots of these supernatants were also tested for TNF-α production. Similarly, supernatants of Con A-stimulated splenocytes were collected after 72 hours of incubation and frozen at –20°C until analyzed for IFN-γ, IL-2, IL-4, IL-10, and IL-12 levels using commercially-available cytokine ELISA kits (Biosource International).
Flow Cytometry
Splenocytes as prepared above were examined for leukocyte subpopulation distribution by 3-color flow cytometry. Cell surface immunotyping was achieved by staining cells with direct (FITC-conjugated anti-CD8a; FITC-conjugated anti-natural killer (NK), NKR-P1A; PE-conjugated anti-CD3; PE-conjugated anti-macrophage, ED2-like antigen; Cy-5-conjugated anti-CD4; Cy-5-conjugated anti-B-cell, CD45RA) staining technique (Morris and Komoscar, Citation1997). Briefly, 1 × 106 cells were washed twice in RPMI-1640 complete medium and blocked for nonspecific IgG binding. Samples were then incubated with primary conjugated-antibodies for 30 minutes on ice. The cells were washed twice with wash buffer (PBS, 0.1% NaN3, 1% FBS). Each sample was resuspended in 0.5 ml of a fixative solution (PBS containing 2% formaldehyde and 0.05% sodium azide) and analyzed immediately or the following day with FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA). A minimum of 10,000 cells per sample was counted.
Antigen-Specific Antibody ELISA
Anti-KLH IgG and anti-KLH IgE titers were measured by using a noncompetitive, solid phase enzyme immunosorbant assay (ELISA) similar to the procedure described by Exon and Talcott (Citation1995). KLH (2 g/ml in carbonate coating buffer) was bound to 96-well high-protein-binding plates (Corning, NY) overnight at 4°C. After blocking the plates, serum samples from rats were serially diluted and added to the wells, and the plates incubated overnight at 4°C. Peroxidase conjugated anti-rat IgG (H+L) (Zymed Laboratories Inc, San Francisco, CA) or biotinylated anti-rat IgE-6 (Pharmingin, San Diego, CA) were added to the plates followed by chromogen (OPD) alone or peroxidase labeled streptavidin followed by OPD for the color to develop. The absorbance was read at 450 nm. Concentrations of IgE in serum were determined by comparing sample values against a standard curve. Using a pooled sample, it was determined that IgG concentration was not linearly associated with optical density. By using 1:2500 dilution of the pooled sample and then serially diluting, it was determined that the range of IgG concentration measured in this study would be best represented by the following equation:
To formulate this equation, the 1:2500 dilution was given an arbitrary value of 2500. The correlation of “X” to the concentration of pooled sample in the serial dilution (AU) was 0.996.
Histopathology
Thymuses from euthanized rats were removed, weighed, and stored in 10% buffered formalin at room temperature for 2 weeks. After fixation, tissues were embedded in paraffin and sectioned at 6 μm thickness. Hematoxylin and eosin were used for staining. Stained sections were scanned for cortico-medullary area measurements on a microscope equipped with a video camera. Image Pro Plus Software (Media Cybernetics, Silver Spring, MD) was used for analysis of thymus sections.
Statistical Analyses
Data were analyzed using a one-way analysis in Minitab for Windows (Release 11.21, Minitab, Inc., State College, PA). Data were subjected to Fisher's LSD test and treatment differences were considered to be significant at p < 0.05. Each age group was analyzed separately. Due to variation in staining in flow cytometry analyses, these data were subjected to the general linear model so that day-to-day variability in staining could be accounted for in the analysis. All data are reported as the mean ± SE. In several instances, where a variable is not statistically different based on treatment, only the mean and SE for control is reported in the text rather than summarized in a table or figure.
RESULTS
Pregnancy Outcome
One rat given 150 mg/kg/day DEHP was not pregnant, so only 12 litters were available for evaluation of that treatment. All pups were born within a 24 hour period between days 22 and 23. Stillbirths and early deaths were very low (). The ratio of female to male pups, as well as the total number of female pups per litter, was not effected by treatment. One of the rats in the control group was not lactating well. After removing the male pups, the remaining females died within 14 days of birth. Any collected results from these animals were removed from the data analysis.
TABLE 1 Pregnancy outcome following fetal exposure to di-(2-ethylhexyl)phthalate for the last 16 days of gestationFootnote1
Physical Characteristics of Pups
At 7 days of age, body weight was similar between the offspring in the 5 treatment groups (). Anogenital distance (AGD) was greater for pups that were exposed to150 or 300 mg/kg/day DEHP in utero than the ones exposed to 0 or 37.5 mg/kg/day DEHP. When expressed on a body weight basis, only pups exposed to 300 mg/kg/day had an AGD greater than controls. However expressing AGD on a metabolic body weight (body weight1/3) basis followed a similar pattern to AGD. At day 21 of age, all groups of rat pups exposed to DEHP were heavier than those pups in the control group (p < 0.05). Increasing DEHP dose also caused an increase in the AGD regardless of how it was expressed.
TABLE 2 Body weights and ano-genital distances (AGD) measured in offspring of Sprague-Dawley CD rats exposed to di- (2-ethylhexyl)phthalate for the last 16 days of gestation
Body Weights, Delayed-Type Hypersensitivity, Spleen, and Thymus
At 5 weeks of age (first immune assessment period), the differences in body weight observed at 21 days were no longer evident (131.2 ± 5.0 g). Delayed-type hypersensitivity was similar for all treatment groups (0.95 ± 0.06 mm). Spleen (0.56 ± 0.03 g) and thymus (0.47 ± 0.02 g) weight did not appear to be effected. Similarly, the ratio of medulla to cortex in the thymus was not different from control (0.30 ± 0.01) for any treatment.
All offspring at 13 weeks of age had similar body weights (314.3 ± 6.0 g). Likewise, DTH was not effected by treatment (1.58 ± 0.05 mm). Spleen (0.72 ± 0.04 g) and thymus (0.46 ± 0.03 g) weights, as well as corticomedullary ratios of thymus (0.35 ± 0.01), were unchanged by treatment.
The nonpregnant adults that were dosed with DEHP concurrently with pregnant rats for 16 days had similar body weights (342.4 ± 8.9 g) following 13 weeks of recovery after the last dose of DEHP was given. Likewise, DTH (1.25 ± 0.09 mm), spleen (0.69 ± 0.03 g) and thymus (0.37 ± 0.02 g) weights, and thymus corticomedullary ratios (0.32 ± 0.02) were not different from the control group.
Nitric Oxide and Tumor Necrosis Factor Alpha Production
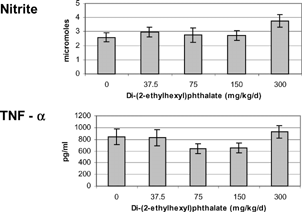
Adherent splenocytes were utilized to measure nitric oxide and TNF-α production after a 24 hour incubation with 10 ng/ml LPS. The Griess reaction was employed to measure the more stable metabolite, nitrite, to estimate nitric oxide production. Because of low cell numbers for 13-week-old offspring and nonpregnant adults, only data from 5-week-old offspring is presented (). Both nitrite (2.59 ± 0.33 μM) and TNF-α (843 ± 135 pg/ml) were not different from controls for all exposure levels of DEHP.
FIG. 1 Nitrite (top) levels and tumor necrosis factor alpha (TNFα, bottom) produced by adherent splenocytes isolated from 5-week-old female offspring exposed to di-(2-ethylhexyl) phthalate for the last 16 days of gestation. Cells were stimulated with 10 ng/ml LPS and supernatants were collected after 24 hours of incubation at 37°C. The minimum detection limit for TNFα was 0.7 pg/ml. There were no statistical differences (p > 0.05).
Total Serum Immunoglobulin E and G
The concentration of immunoglobulin (Ig) E was not effected by DEHP in 5-week-old offspring (2755 ± 534 ng/ml), 13-week-old offspring (8647 ± 3911 ng/ml), and nonpregnant adults (12469 ± 8366 ng/ml). The concentrations of IgG in serum were also similar between rats in the control and DEHP groups (297 ± 80 AU, 5-week-old offspring; 2,094 ± 397 AU, 13-week-old offspring; 1361 ± 166 AU, nonpregnant adults).
Cytokine Production
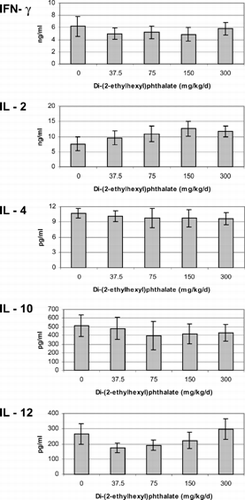
Cytokines were measured from mixed-splenocyte cultures after incubation with 5 μg/ml Con A for 72 hours. If there were any acute effects caused by DEHP, animals recovered by the time of assessment. Animals exposed to DEHP in utero or concurrently with pregnant rats produced similar concentrations of IFN-γ, IL-2, -4, -10, and –12 as the control group animals regardless if they were assessed at 5 () or 13 () weeks of age or 13 weeks post-adult exposure ().
FIG. 2 Cytokines produced by unseparated splenocytes isolated from 5-week-old offspring exposed to di-(2-ethylhexyl)phthalate in utero for the last 16 days of gestation. Cells were incubated with 5 μg/ml Con A for 72 hours. The minimum detection limit for each of the cytokines was as follows: IFN-γ < 13 pg/ml, IL-2 < 5 pg/ml IL-4 < 1.3 pg/ml, IL-10 < 5 pg/ml, IL-12 < 3 pg/ml. There were no significant differences between treatments (p > 0.05).
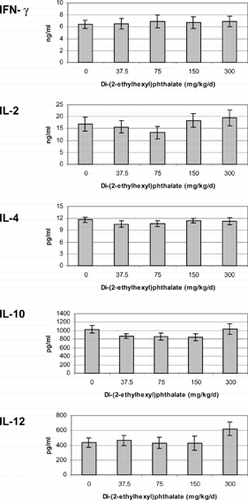
FIG. 3 Cytokines produced by unseparated splenocytes isolated from 13-week-old offspring exposed to di-(2-ethylhexyl)phthalate in utero for the last 16 days of gestation. Cells were incubated with 5 μg/ml Con A for 72 hours. The minimum detection limit for each of the cytokines was IFN-γ < 13 pg/ml, IL-2 < 5 pg/ml, IL-4 < 1.3 pg/ml, IL-10 < 5 pg/ml, IL-12 < 3 pg/ml. There were no significant differences between treatments (p > 0.05).
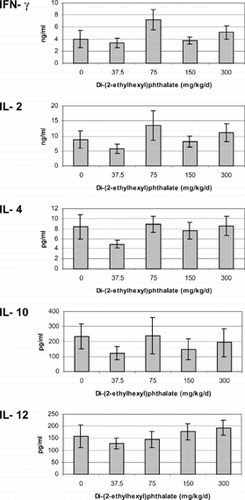
FIG. 4 Cytokines produced by unseparated splenocytes isolated from non-pregnant adults exposed to di-(2-ethylhexyl)phthalate for 16 days concurrently with pregnant dams. Cells were incubated with 5 μg/ml Con A for 72 hours. The minimum detection limit for each of the cytokines was as follows: Interferon gamma < 13 pg/ml, IL-2 < 5 pg/ml IL-4 < 1.3 pg/ml, IL-10 < 5 pg/ml, IL-12 < 3 pg/ml. There were no significant differences between treatments (p > 0.05).
Total and Differential Leukocyte Counts
Total leukocyte counts in blood smears of rats exposed to DEHP were not different from control rats. This was true for 5-[5.95 × (106 cells) ± 0.79] and 13-week-old offspring [9.64 × (106 cells) ± 1.30], as well as nonpregnant adults [9.77 × (106 cells) ± 1.15]. Similarly, counts of differential leukocytes revealed similar percentages of lymphocytes, neutrophils, monocytes, eosinophils, and basophils between treatment groups within assessment period (data not shown).
Flow Cytometry
In the 5-week-old offspring, the three-color flow cytometry did not show significant differences in CD4+CD8+, CD4+CD8−, CD4−CD8+, natural killer, macrophage, or B-cell populations in spleens (). Unfortunately, several samples from 13-week-old offspring and nonpregnant adults had inadequate cell numbers or poor CD3 staining. Therefore, these data are not presented.
TABLE 3 Flow cytometry profile of cell populations in 5-week-old offspring exposed to di-(2-ethylhexyl)phthalate in utero for the last 16 days of gestation
DISCUSSION
Because of widespread use in making flexible plastics and a diverse number of other products, phthalate ester consumption is relatively high at 3.5 million metric tons per year with close to half of that being DEHP (Cadogan and Howick, 1996). The chance of having repeated or even continuous exposure is probable given its prevalence in the environment. Those born premature, needing blood transfusions or hemodialysis have a higher degree of exposure due to the plastics used in tubing and medical equipment. The detection of DEHP and its metabolite mono (2-ethylhexyl) phthalate in cord blood (Latini et al., Citation2003) indicates that exposure starts even in utero.
Concern is based on DEHP's potential carcinogenicity and teratogenicity (reviewed in Wams, Citation1987) role as an endocrine disrupter (Lovekamp-Swan and Davis, Citation2003) and reproductive toxicant (Moore et al., Citation2001; Kavlock et al., Citation2002), and peroxisome proliferator activity (Klaunig et al., Citation2003). Furthermore, the possible association of DEHP in childhood asthma (Bornehag et al., Citation2004) and altered immune function (Yang et al., Citation2000, Lee et al., Citation2004) was suggested in some studies.
DEHP has been reported to impact some growth parameters. Body weight is reduced in adult male mice after exposure to DEHP as 0.2% of the diet when compared to controls (Yang et al., Citation2000). Likewise, rat pup weight on the day of birth was reduced by in utero exposure to DEHP compared to control but was similar at weaning for male pups (Gray et al., Citation2000). In the present study, the female offspring were heavier at 21 days of age after in utero exposure to DEHP () indicating a possible gender difference previously unseen. However, it should be noted that female offspring at 5 weeks of age had similar body weights showing that the earlier weight difference was short in duration.
The observed differences in AGD () do suggest an effect of in utero DEHP exposure on development. Male offspring tend to be feminized after in utero exposure to DEHP (Gray et al., Citation2000) showing similar AGD to females and retained nipples. However, incorrect identification can be discounted as an error in the current study because there was no difference in the female to male ratio or number of female offspring between treatment groups. Additionally, no anatomical reproductive abnormalities were seen in the offspring used in postnatal analysis ().
The available literature on the effects of DEHP on the immune system is limited and variable in findings. Because prior studies have emphasized in vitro or adult animal exposures with assessment during or immediately following exposure, these shed little light on life-stage-associated sensitivities to DEHP or on the potential for persistent effects. This present study marks the first time DEHP has been examined as a potential developmental immunotoxicant with direct in utero vs. adult exposure comparisons.
A series of adult and/or in vitro studies suggested that DEHP has immuntoxic potential. Yang et al. (Citation2000) found that feeding male C57B1/6 mice a diet containing 0.2% DEHP reduced the weight and DNA content of thymus and spleen as compared to controls indicating a potential role of DEHP in immunosuppression. Furthermore, Lee et al. (Citation2004) showed that mouse CD4+ T-cells increased production of IL-4 and serum IgE levels were also elevated after in vivo DEHP exposure. In studying a DEHP metabolite co-administered with antigen, Larsen et al. (Citation2001) found either enhanced or suppressed IgE production depending upon the metabolite dose. BALB/cJ mice were administered a 100 μl mixture containing ovalbumin (antigen) and either 1000, 100, 10, or 1 μg/ml mono-(2-ethylhexyl)phthalate, a DEHP metabolite. At the highest dose, IgE and IgG1 were reduced compared to controls. However, at 10 μg/ml, IgE was increased (Larsen et al., Citation2001).
Other investigators have compared in vitro and in vivo immune alterations. Murine spleen cells incubated with DEHP had increased Con A- and LPS-stimulated proliferation as well as enhanced IL-4, IFNγ, and IgG production compared to controls (Yamashita et al., Citation2002). Spleen macrophages also increased the production of TNF and IL-1 in response to DEHP. Similarly, DEHP increased murine thymocyte proliferative response especially after incubating thymocytes with antigen-presenting spleen cells (Yamashita et al., Citation2003b). Thymocytes also had higher production of IL-3, IL-4, and IFNγ with DEHP exposure. Following a 6-week exposure of mice to 10− 5 M DEHP in the drinking water, some of the responses seen in vitro were not duplicated in this in vivo exposure model (Yamashita et al., Citation2003a).
Because of these observations, it was believed that exposure to DEHP might contribute to the prevalence of asthma. Bornehag et al. (Citation2004) was able to correlate the diagnosis of asthma with higher amounts of DEHP in bedroom dust of children. Route of exposure may be important. Oie et al. (Citation1997) suggested that DEHP exposure as aerosols adsorbed to particulate matter may be an important factor in asthma, because the hydrolysis product of DEHP [mono-(2-ethylhexyl)phthalate] mimics prostaglandins and thromboxanes in the lung, causing inflammation in the airways.
In contrast with the studies suggesting DEHP to be a significant immunotoxicant, Butala et al. (Citation2004) found little immune impact following DEHP exposure of young adult B6C3F1 female mice. In a study designed to test the allergic asthma potential, DEHP, as well as other phthalates examined, did not produce any change in IgE, IL-4, or IL-13 following exposure. The authors concluded that phthalates probably have low or no potential to produce antibody-mediated respiratory allergy.
The present results would agree with the findings of Butala et al. (Citation2004) as extended to DIT exposure assessment of female rats. While DEHP exposure in utero did produce potential developmental alterations as suggested by AGD changes, little impact on the immune system was found based on the assessment measures employed. This included assessment following both adult and in utero exposure to DEHP.
One difference between the current study and most prior adult studies is the inclusion of a significant post-exposure recovery period (5 and/or 13 weeks) in our protocol. This protocol was used for the purpose of examining potential persistent immunotoxicity caused by DEHP, as well as permitting a comparison between these results and prior results with lead, dexamethasone and cyclosporin A using the same testing protocol.
The present result with DEHP is in stark contrast with prior age-based comparisons involving lead (Bunn et al., Citation2001a); dexamethasone (Dietert et al., Citation2003) and cyclosporin A (Hussain et al., Citation2005). For those xenobiotics, the fetus was relatively hyper-susceptible to immunotoxic alterations compared with the adult female rat. Depending upon the chemical, this increased sensitivity of the fetus vs. the adult could take several forms including: a greater dose sensitivity than the adult; a broader range of immune changes following exposure; and, persistence of the alterations while adult-exposed animals exhibited only transitory changes. However, some gender differences were observed following early exposures (Bunn et al., Citation2001a, Citation2001b) and this should not be discounted in the case of DEHP.
Previous work in our laboratory has shown the impact of sex hormone and toxicant on the developing immune system (Hussain, in press). This may explain some of the differences observed between studies regarding the immunotoxicity of DEHP. Because DEHP can act as an anti-androgen (Parks et al., Citation2000), it may produce different effects among the genders that can extend to the immune system. The current study employed only females, as this was necessary to permit the direct age-based immunotoxicity comparisons with sufficient numbers of animals. However, future studies should consider direct gender comparisons whenever possible in light of gender differences reported in various DIT studies (Blyler et al., Citation1994; Chapin et al., Citation1997; Bunn et al., Citation2001a, Citation2001b).
In conclusion, administering DEHP by gavage to pregnant rats for the last 16 days of gestation resulted in increases in AGD of female offspring when measured at both 7 and 21 days of age. Despite this change in a hormonally sensitive developmental measure, no changes were apparent for the various immunological measures assessed in this study. Therefore, both the developing immune system of female offspring as well as non-pregnant exposed adults appears to be resistant to persistent effects of DEHP.
ACKNOWLEDGMENTS
The American Chemistry Council graciously provided the funding for this project. We would like to thank Forrest Sanders, Fae Tompkins, Kevin Fitch, and Clarence Thompson for their assistance during gavage and tissue collection procedures. For daily animal care, we thank Roy Barriere and the staff of the Cornell Laboratory Animal Services.
REFERENCES
- Adibi J. J., Perera F. P., Jedrychowski W., Camann D. E., Barr D., Jacek R., Whyatt R. M. Prenatal exposure to phthalates among women in New York City and Krakow, Poland. Environ. Health Perspect. 2003; 111: 1719–1722, [PUBMED], [INFOTRIEVE], [CSA]
- Blyler G., Landreth K. S., Barnett J. B. Gender-specific effects of prenatal chlordane exposure on myeloid cell development. Fundam. Appl. Toxicol. 1994; 23: 188–193, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Bornehag C. G., Sundell J., Weschler C. J., Sigsgaard T., Lundgren B., Hassesgren M., Hägerhed-Engman L. L. The association between asthma and allergic symptoms in children and phthalates in house dust: A nested case-control study. Environ. Health Perspect. 2004; 112: 1393–1397, [PUBMED], [INFOTRIEVE], [CSA]
- Bunn T. L., Ladics G. S., Holsapple M. P., Dietert R. R. Developmental immunotoxicology assessment in the rat: Age, gender and strain comparison after exposure to Pb. Toxicol. Methods 2001a; 11: 41–58, [CSA], [CROSSREF]
- Bunn T. L., Parsons P. J., Kao E., Dietert R. R. Exposure to lead during critical windows of embryonic development: Differential immunotoxic outcome based on stage of exposure and gender. Toxicol. Sci. 2001b; 64: 57–66, [PUBMED], [INFOTRIEVE], [CSA]
- Butala J. H., David R. M., Gans G., McKee R. H., Guo T. L., Peachee V. L., White K. L., Jr. Phthalate treatment does not influence levels of IgE or TH2 cytokines in B6C3F1 mice. Toxicology 2004; 201: 77–85, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Cadogan D. F., Howick C. J. Plasticizers. Kirk-Othmer Encyclopedia of Chemical Technology, M. Howe-Grant. John Wiley and Sons, New York 1999; vol. 19
- Chapin R. E., Harris M. W., Davis B. J., Ward S. M., Wilson R. E., Mauney M. A., Lockhart A. C., Smialowicz R. J., Moser V. C., Burka L. T., Collins B. J. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam. Appl. Toxicol. 1997; 40: 138–157, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Dietert R. R., Lee J. E., Olsen J., Fitch K., Marsh J. A. Developmental immunotoxicity of dexamethasone: Comparison of fetal versus adult exposures. Toxicology 2003; 194: 163–176, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Exon J. H., Bussiere J. L., Mather G. G. Immunotoxicity testing in the rat: An improved multiple assay model. Int. J. Immunopharmacol 1990; 12: 699–701, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Exon J. H., Talcott P. A. Enzyme-linked immunosorbent assay (ELISA) for detection of specific IgG antibody in rats. Methods in Immunology, G. Burleson, J. Dean, A. Munson. Wiley-Liss, Inc., New York 1995; Vol 1: pp. 109–124
- Gray L. E., Ostby J., Furr J., Price M., Beeramachaneni D. N. R., Parks L. Perinatal exposure to the phthalates, DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000; 58: 350–365, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tanneubaum S. R. Analysis of nitrate, nitrite and [15N] nitrite in biological fluids. Anal. Biochem. 1982; 126: 131–138, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Hussain I., Piepenbrink M. S., Fitch K. J., Marsh J. A., Dietert R. R. Developmental immunotoxicity of cyclosporin A in rats: Age-associated differential effects. Toxicology 2005; 206: 273–284, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Hussain I., Piepenbrink M. S., Dietert R. R. Impact of in ovo-administered lead and testosterone on developing female thymocytes. J. Toxicology Environ. Health. Part A.
- Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., Seed J., Shea K., Tabacova S., Tyl R., Williams P., Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl)phthalate. Reprod. Toxicol. 2002; 16: 529–653, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Klaunig J. E., Babich M. A., Baetcke K. P., Cook J. C., Corton J. C., David R. M., DeLuca J. G., Lai D. Y., McKee R. H., Peters J. M., Roberts R. A., Fenner-Crisp P. A. PPARalpha agonist-induced rodent tumors: Modes of action and human relevance. CRC. Crit. Rev. Toxicol. 2003; 33: 655–780, [PUBMED], [INFOTRIEVE], [CSA]
- Larsen S. T., Hansen J. S., Thygesen P., Begtrup M., Poulsen O. M., Nielsen G. D. Adjuvant and immunosuppressive effect of six monophthalates in a subcutaneous injection model with BALB/c mice. Toxicology 2001; 169: 37–51, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 2003; 111: 1783–1785, [PUBMED], [INFOTRIEVE], [CSA]
- Lee M. H., Park J., Chung S. W., Kang B. Y., Kim S. H., Kim T. S. Enhancement of interleukin-4 production in activated CD4+ T-cells by diphthalate plasticizers via increased NF-AT binding activity. Int. Arch. Allergy Innunol. 2004; 134: 213–222, [CSA], [CROSSREF]
- Lovekamp-Swan T., Davis B. J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003; 111: 139–145, [PUBMED], [INFOTRIEVE], [CSA]
- Moore R. W., Rudy T. A., Lin T. M., Ko K., Peterson R. E. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di(2-ethylhexyl)phthalate. Environ. Health Perspect. 2001; 109: 229–237, [PUBMED], [INFOTRIEVE], [CSA]
- Morris D. L., Komoscar W. J. Immunophenotyping analysis of peripheral blood, splenic, and thymic lymphocytes in male and female rats. J. Pharmacol. Toxicol. Methods 1997; 37: 37–46, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Oie L., Hersoug L., Madsen J. O. Residential exposure to plasticizers and its possible role in pathogenesis of asthma. Environ. Health Perspect. 1997; 105: 972–978, [PUBMED], [INFOTRIEVE], [CSA]
- Parks L. G., Ostby J. S., Lambright C. R., Abbott B. D., Klinefelter G. R., Barlow N. J., Gray L. E. The plasticizer diethylhexylphthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000; 58: 339–349, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Wams T. J. Diethylhexylphthalate as an environmental contaminant—A review. Sci. Total Environ. 1987; 66: 1–16, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Yamashita U., Kuroda E., Yoshida Y., Sugiura T. Effect of endocrine disrupters on immune responses in vivo. J. UOEH. 2003a; 25: 365–374, [PUBMED], [INFOTRIEVE], [CSA]
- Yamashita U., Sugiura T., Kuroda E. Effect of endocrine disrupters on immune responses in vitro. J. UOEH. 2002; 24: 1–10, [PUBMED], [INFOTRIEVE], [CSA]
- Yamashita U., Sugiura T., Yoshida Y., Kuroda E. Effect of endocrine disrupters on thymocytes in vitro. J. UOEH. 2003b; 25: 161–170, [PUBMED], [INFOTRIEVE], [CSA]
- Yang Q., Xie Y., DePierre J. W. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol. 2000; 122: 219–226, [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]