ABSTRACT
Mitochondrial dysfunction is a central defect in cells creating the Warburg and reverse Warburg effect cancers. However, the link between mitochondrial dysfunction and cancer has not yet been clearly explained. Decrease of mitochondrial oxidative energy production to about 50 % in comparison with healthy cells may be caused by inhibition of pyruvate transfer into mitochondrial matrix and/or disturbed H+ ion transfer across inner mitochondrial membrane into cytosol. Lowering of the inner membrane potential and shifting of the working point of mitochondria to high values of pH above an intermediate point causes reorganization of the ordered water layer at the mitochondrial membrane. The reorganized ordered water layers at high pH values release electrons which are transferred to the cytosol rim of the layer. The electrons damp electromagnetic activity of Warburg effect cancer cells or fibroblasts associated with reverse Warburg effect cancer cells leading to lowered electromagnetic activity, disturbed coherence, increased frequency of oscillations and decreased level of biological functions. In reverse Warburg effect cancers, associated fibroblasts supply energy-rich metabolites to the cancer cell resulting in increased power of electromagnetic field, fluctuations due to shift of oscillations to an unstable nonlinear region, decreased frequency and loss of coherence.
Introduction
Biological systems are in a state far from thermodynamic equilibrium excited and maintained by continuous energy supply. The coherent electromagnetic field is an essential condition of life (Pokorný et al., Citation2013b). Energy transport, processing, parceling out into small bits and storing into adenosine triphosphate (ATP) and guanosine triphosphate (GTP) form a complex fermentative and oxidative pathway. Energy provides ordering of the living system. O. Warburg compared growth of disordered tumors and ordered embryos and wanted to find a reason for disordered growth of tumors. He assumed that there are essential physical–chemical differences between the two cases. Measurements of energy processes disclosed that cancers may obtain even more than 50 % of their ATP by metabolizing glucose directly to lactic acid even in the presence of oxygen (Warburg et al., Citation1924). This phenotype of cancers is known as the Warburg effect. O. Warburg believed that this discovery would enable a cure. He assumed that “The adenosine triphosphate synthesized by respiration therefore involves more structure than the adenosine triphosphate synthesized by fermentation.” (Warburg, Citation1956). But the structure and mechanism causing the difference in organization remained unknown—the effect of dysfunctional mitochondria on cellular processes causing disorganization was not revealed. The scientific world considered the disturbed mechanism of energy production a minor, unimportant side effect in cancer development.
Nearly 40 years after Warburg’s death Pavlides et al. (Citation2009) disclosed another phenotype of cancer—mitochondrial dysfunction in fibroblasts associated with a cancer cell with functional mitochondria and called it the reverse Warburg effect. Tumor growth and metastases are fueled by the supply of energy-rich metabolites such as lactate, pyruvate, glutamine and ketone BHB (beta-hydroxybutyrate) from the associated fibroblasts. In the reverse Warburg effect cancer, cells are overfed by energy supply. Their common property is aggressiveness, early invasion and metastases. The Warburg and reverse Warburg effect cancers correspond to the medical classification of differentiated and undifferentiated cancers, respectively. Analysis of the defects of chemical reactions related to mitochondrial dysfunction forms an enormous complex of published data. A few references are given as published in a special journal issue devoted to the Wartburg effect (Bartons & Caro, Citation2007; Chen et al., Citation2007; Cuezva et al., Citation2007; Gillies & Gatenby, Citation2007; Godinot et al., Citation2007; Herrmann & Herrmann, Citation2007; Ma-Ho et al., Citation2007; Pedersen, Citation2007; Semenza, Citation2007).
The Warburg effect is caused by inhibition of enzymatic activity of mammalian pyruvate dehydrogenase enzymes and pyruvate transfer into mitochondrial matrix. The enzymatic activity of mammalian pyruvate dehydrogenase is regulated through three phosphorylation sites—serine residues Ser–264, Ser–271 and Ser–203 (Kolobova et al., Citation2001). Activation is performed by pyruvate dehydrogenase phosphatases PDP1–2 and inhibition by pyruvate dehydrogenase kinases PDK1–4. However, mitochondrial dysfunction may result from a high parasitic energy consumption inhibiting transfer of pyruvate to mitochondrial matrix (Pokorný et al., Citation2016). Infection with a lactate dehydrogenase-elevating (LDH) virus increases the level of the lactate dehydrogenase enzymes (now classified as NAD 1.1.1.27 Oxidoreductase) in plasma and production of lactate from pyruvate. A very interesting analysis of mitochondrial properties was performed by Dakubo (Citation2010) who described the chain of oxidative energy production and its defects. However, the properties of the hydrogen ion layer outside the inner membrane and its potential were not analyzed.
Biochemical methods have not disclosed the defective forces responsible for disturbed organization of cancer cells—their type, origin and defects. The forces should be macroscopic, with a range comparable to cellular dimensions. Biophysical measurements of the mitochondrial inner membrane potential have been performed, but the analysis of the results remains incomplete. Measured values of the electric potential at the mitochondrial membrane do not correspond to the mitochondrial oxidation activity. Metabolic activity of mitochondria includes transport of H+ ions from the matrix to the intermembrane space and cytosol and formation of an electrochemical proton gradient with a negative electric potential across the inner membrane dependent on a distribution of positive and negative charges (Klingenberg & Rottenberg, Citation1977). The electrochemical proton gradient is an important part of the energy transformation pathway outside mitochondria and may be affected by processes in the cytosol. A long-term systematic study has overwhelmingly indicated that the value of the mitochondrial membrane potential measured by uptake and retention of positively charged fluorescent dyes is, respectively, lower and higher in the fully active functional mitochondria and in the dysfunctional mitochondria (Modica-Napolitano & Aprille, Citation1987), strongly indicating an external influence. The actual potential across the mitochondrial membrane depends on H+ transfer. Accordingly, a high absolute value of the actual mitochondrial membrane potential should correspond to high oxidative activity. It is obvious that the measurement of the mitochondrial membrane potential must be affected by another physical process. The process must uptake and bind positively charged fluorescent molecules at or near the mitochondrial membrane. We ask whether this process plays a role in cancer initiation and development.
Our analysis of the contradictory difference between the inner membrane potential of the functional and dysfunctional mitochondria in living cells and the possible effect of the cytosol processes is based on published experimental data on the electric potential of the proton electrochemical gradient, mitochondrial membrane potential in cells, water ordering and its dependence on pH, release of free electrons from ordered water layers, and the electromagnetic field generated in living cells. The experimental methods and resulting data are important for assessment of the acting physical mechanisms.
Experimental background of the hypothesis
Electric potential of electrochemical proton gradient
High-energy electrons are transported down the respiratory chain in the mitochondrial inner membrane, and the released energy is used to pump protons across the inner membrane into the intermembrane space and cytosol. The electrochemical gradient around mitochondria is formed. In functional mitochondria, the actual electric inner membrane potential of the electrochemical gradient is about −140 mV and the pH gradient of about −1 pH unit (Alberts et al., Citation1994). Cancer cells can obtain approximately the same amount of energy from fermentation as from respiration (Warburg, Citation1956). Assuming a similar state for all mitochondria in the cell, each dysfunctional mitochondria produces on average about 50 % of energy in comparison with fully functional ones. In dysfunctional mitochondria, the actual electric inner membrane potential is of about −70 mV.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential in living cells is measured by uptake and retention of positively charged fluorescent dyes. Specific dyes are Rhodamine (Rh) 6G and Rh 123 (Chen, Citation1988; Modica-Napolitano & Aprille, Citation1987; O’Connor et al., Citation1988; Wiseman et al., Citation1985). Beside Rh 6G and Rh123, other positively charged fluorescent dyes have been used, for example, carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and tetramethyl Rhodamine methyl ester (TMRM) (Bonnet et al., Citation2007). The potential measured by fluorescent dyes differs from the actual membrane potential corresponding to the electrochemical gradient, and, therefore, it is termed the apparent potential in this text.
Healthy cells exhibit a low uptake and retention of positively charged fluorescent dyes, and hence, low absolute value of the negative apparent mitochondrial potential. Bonnet et al. (Citation2007) proved that mitochondria with a suppressed oxidative metabolism are hyperpolarized—that is, the absolute value of the apparent potential is increased. In the large majority of cancer cells, mitochondria have a high absolute value of the negative apparent potential (Chen, Citation1988; Lampidis et al., Citation1983; Summerhayes et al., Citation1982). Normal monkey kidney epithelial cells CV-1 exhibit the apparent mitochondrial membrane potential of −104 ± 9 mV. Human colon carcinoma cells CX-1 and MIP101 exhibit the apparent mitochondrial membrane potential of −163 ± 7 mV and −158 ± 8 mV, respectively (Modica-Napolitano & Aprille, Citation1987). The difference between the apparent potential of healthy and cancer cells is about 60 mV. However, some cancer cells have an apparent potential similar to healthy cells or even lower. For instance, v-fes oncogene transformed mink fibroblasts change their normal state to a state of an abnormally low apparent mitochondrial membrane potential accompanied by a relatively high pH gradient of about three pH units (Chen, Citation1988; Johnson et al., Citation1982).
Dependence of water ordering on pH factor
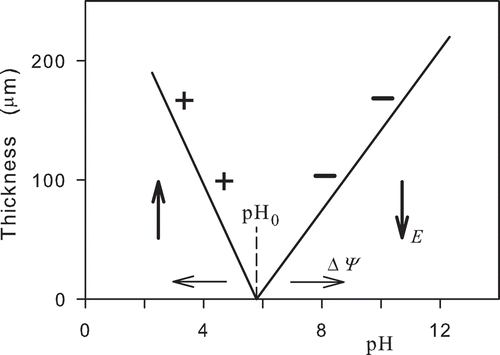
The electric field can produce ordering of water (Zheng et al., Citation2006; Zheng & Pollack, Citation2003). At charged surfaces, water forms ordered layers with macroscopic thicknesses up to about 500 µm. The layers are called exclusion zones, as charged particles are excluded from them. shows a schematic picture of the layer thickness as a function of pH. The plotted lines are evaluated from experimental data (Zheng & Pollack, Citation2003). The charged surface with a constant charge density was formed by polyvinyl alcohol (PAV) gel. For pH < pH0 and pH > pH0, particles with positive and negative charges are excluded, respectively. The excluded particles were positively charged carboxylate microspheres with a diameter 2 µm and negatively charged amidine microspheres with a diameter 1.5 µm (Zheng & Pollack, Citation2003).
Figure 1. Thickness of the ordered water layer (exclusion zone) above a plane charged surface as a function of pH. Positively and negatively charged particles denoted by plus and minus signs are excluded from the ordered water layer for pH < pH0 and pH > pH0, respectively. Vertical arrows denote orientations of the intensity of the electric field in the ordered water layer excluding charged particles. The intermediate value pH0 denotes a point on the pH axis where ordered water layer is not formed. The change of the charge in the surface layer should result in a change of the potential barrier ΔΨ in the water and shift of the pH0 along the pH axis. Horizontal arrows denote possible shifts of the pH0 point.
Release of electrons from ordered water layers
Formation of an ordered state is related to a transformation mechanism of water molecules. The process is driven by collection of low-grade energy and its transformation into high-grade energy which is able to produce electronic excitations. Energy of thermal fluctuations is transformed into energy of the ordered state. The energy of a molecule in the ordered state is lower than its energy in the disordered state. All constituents in the ordered state oscillate in a common rhythm within a space termed a coherence domain with a linear dimension about 100 nm. Theoretical description is based on quantum electrodynamics (Arani et al., Citation1995; Del Giudice & Preparata, Citation1998; Del Giudice et al., Citation1988; Del Giudice & Tedeschi, Citation2009; Preparata, Citation1995). External electric field organizes the coherence domains into layers. In each molecule, coherent oscillations take place between a fundamental state and an excited state with strongly and weakly bound electrons whose ionization energy corresponds to 12.6 and 0.54 eV, respectively (Del Giudice et al., Citation2009). The coherent state of water has a much higher tendency to release electrons than the non-coherent state which rather forms H2O− ions. Ordered water is able to give rise to a significant electron transfer which is beneficial to supply redox reactions in biological systems (Del Giudice et al., Citation2009).
Generation of electromagnetic field in living cells
Electromagnetic field generated in living cells and conditioning biological activity is a nature of life (Pokorný et al., Citation2013b). In eukaryotic cells, the electromagnetic field is generated by microtubules composed of tubulin heterodimers with a strong electric dipole (Pokorný et al., Citation1997). Long-range order, large distance cooperation, and the whole body control are significant properties of biological systems. Chemical bonds (including covalent, ionic, hydrogen and van der Waals types) have been commonly assumed to be dominating for biological organization and activity. However, these bonds represent forces acting at short distances in the nm region. Biological systems maintain order at every dimension scale (Pokorný et al., Citation2013b).
Fröhlich’s hypothesis of coherent electric polar vibrations (Fröhlich, Citation1969) in living cells was gradually proved by measurements performed on living cells. Dielectrophoretic attraction of dielectric particles to living cells was observed, and a corresponding frequency of oscillations was assessed in the range below 10 MHz (Pohl et al., Citation1981). Oscillations in the frequency range 1.5–52 MHz were measured (Hölzel, Citation2001). Increased electromagnetic activity in the M phase (Pohl et al., Citation1981) was confirmed on synchronized Saccharomyces cervisiae cells in the frequency range 8–9 MHz. (Pokorný et al., Citation2001). Interaction between living cells in the red and near-infrared range depends on electromagnetic field generated by them (Albrecht-Buehler, Citation1992). Elongated BHK cells on opposite faces of thin and thick glass films were oriented in transverse and random directions, respectively. Interaction between cells is mediated by cellular electromagnetic field in the near-infrared range (Albrecht-Buehler, Citation2005). Coherent mechanical vibrations of living cells were measured by atomic force microscopy in the acoustic frequency range (Pelling et al., Citation2004, Citation2005). Frequencies of the mechanical vibrations and of the electromagnetic field generated by a cell are equal (Jelínek et al., Citation2009). Vibrations of cells are considered a signature of life (Kasas et al., Citation2014).
Resonant frequencies of microtubules are important parameters for assessment of the cellular functions and interactions with other cells in the tissue. Microtubules are capable to generate electromagnetic field in a wide spectrum in classical frequency bands up to 20 GHz, at 20 THz, and in the UV range (Sahu et al., Citation2013, Citation2014) as follows from measured absorption and emission spectra excited by external irradiation. External electromagnetic signals are selectively damped by some cancer tissues at a frequency about 465 MHz and its first harmonic (Pokorný et al., Citation2011; Vedruccio & Meessen, Citation2004). Therefore, the fundamental biological role of the generated electromagnetic field in living cells is strongly supported by experimental data.
Hypothesis: Damping of electromagnetic activity
Water exhibits extraordinary properties which can explain the observed contradictions of the measured uptake and retention of positively charged fluorescent dyes and the actual mitochondrial membrane potential. Development of the ordered water layers around a mitochondrion is similar to that at a charged surface but the potential barrier and the intensity of the electric field depend on transferred H+ ions. Most of the mitochondrial membrane potential is generated by electrogenic pumping of H+ ions from the inner membrane. Intensity of the electric field is about −3.5 MV/m at the membrane and about −0.6 MV/m at a distance 2 µm from mitochondria (Tyner et al., Citation2007). Formation of ordered water layers around mitochondria is analyzed in (Pokorný, Citation2012).
shows a model of ordered water layers around functional and dysfunctional mitochondria. The dashed lines denote a thickness of the ordered water layers in functional mitochondria. Their slopes correspond to the values in . The working point is at pH = 6, depending on the inner membrane potential. The mitochondrial matrix has a pH value of about 8 (Alberts et al., Citation1994), which is created by pumping H+ ions out of the mitochondrion into the intermembrane space and cytosol with a pH value of about 7. The pH gradient resulting from pumping is ΔpH = − 1 pH unit (Alberts et al., Citation1994). The molecules of the positively charged fluorescent dyes used for measurement of the mitochondrial membrane potential remain at the outer rim of the ordered water layer (denoted by + signs). The potential of the ordered water layer is subtracted from the actual potential of −140 mV (Alberts et al., Citation1994), and the apparent potential of −100 mV (Modica-Napolitano & Aprille, Citation1987) is formed (). Dysfunctional mitochondrion producing only about one half of ATP by respiration should have a pH gradient ΔpH = − 0.7 pH unit. The decrease of the absolute value of the actual membrane potential results in a shift of the intermediate pH0 point to higher values of pH. The apparent potential is of about −160 mV (Modica-Napolitano & Aprille, Citation1987). The potential of the ordered water layer is added to the actual potential to form the apparent potential (). The actual potential of the dysfunctional mitochondria can be about −70 mV. In this case, the absolute value of the apparent membrane potential is about 90 mV higher.
Figure 2. Model assessment of the thickness of the ordered water layer as a function of pH at the membrane of a healthy functional (H) and dysfunctional (Ca) mitochondrion. The dashed lines and the dotted lines represent the thickness of the ordered water layer of the functional and dysfunctional mitochondrion, respectively. ΔpH denotes decrease of the number of transferred hydrogen ions from the matrix space to cytoplasm in a dysfunctional mitochondrion. The thicknesses of the ordered water layers are related to the absolute values of electric potentials.
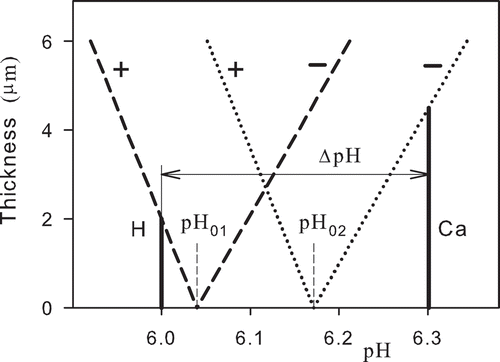
Figure 3. Potential at the inner mitochondrial membrane for functional (Healthy) and dysfunctional (Cancer) mitochondria. A—apparent potential, R—actual potential corresponding to the transfer of hydrogen ions across the inner membrane, T—combination of the actual potential and the potential across the ordered water layer (dark grey and black columns). The arrows denote orientations of the intensity of the electric field.
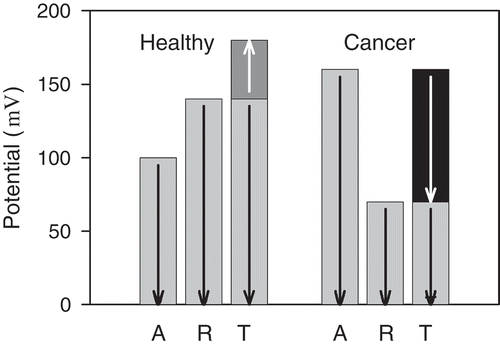
visualizes a relation between the actual potential corresponding to transfer of hydrogen ions across the inner membrane, the apparent potential measured by uptake and retention of positively charged fluorescent dyes, and the potential of the ordered water layer. The reorganized ordered water layer around dysfunctional mitochondria has a reversed orientation of the electric field which enables transport of electrons released from ordered water layer into cytosol. As water occupies 70 % of the cell volume, ordered water is capable of releasing a huge amount of electrons into the cytosol. Free electrons increase conductivity which causes damping of electromagnetic field. The mechanism of damping electromagnetic oscillations generated by microtubules may explain the disturbed organization in cells with dysfunctional mitochondria.
Discussion
The presented model of the membrane potential of a mitochondrion with ordered water layers can explain the discrepancy between the measured apparent values of its membrane potential and the actual values resulting from the mitochondrial function. The model is based on the experimental data on ordered water layers measured in the vicinity of charged surfaces. The value of the shift of the intermediate point pH01 to pH02 caused by mitochondrial dysfunction is estimated; its real position should correspond to the change of the apparent membrane potential to about −160 mV due to the increased negative potential of the ordered water layer. Available experimental data prove that there is an ordered water layer with exclusion of electrons around dysfunctional mitochondria, supporting the assumption of damping of the cellular electromagnetic field.
The model is based on the apparent potential measured by the uptake and retention of positively charged fluorescent dyes and measurement of mitochondrial activity transferring H+ ions from the matrix to the intermembrane space and cytosol. However, the real positions of the working regions and of the intermediate pH01 and pH02 points in might differ due to individual arrangements and combination with other processes. A decreased transfer of H+ ions into cytosol decreases the actual mitochondrial inner membrane potential and shifts the working point of mitochondria to higher values of pH. If this shift runs across the intermediate pH0 point, then it leads to reorganization of the ordered water layer, release of electrons and their transfer to the outer rim of the zone. The electrons form a conductive region around mitochondria and neighboring microtubules which can efficiently damp generated electromagnetic field. Therefore, the shift has fatal consequences for the living system regardless of the mechanism of its origin. Any inhibition of pyruvate transfer to the matrix, defects in electron transfer chain or transfer of hydrogen ions across the inner membrane may lead to a similar result. Production of energy in the form of ATP and GTP cannot overcome the effects of damping by released electrons.
The formulated hypothesis of damping of electromagnetic activity in afflicted cells is sufficiently supported by theoretical analysis and experimental data and can be experimentally proved. The absolute value of the actual electric potential of a layer of hydrogen ions transported from functional and dysfunctional mitochondria across the mitochondrial inner membrane (Alberts et al., Citation1994), respectively, is higher and lower than the apparent potential measured by the uptake and retention of positively charged fluorescent dyes (Modica-Napolitano and Aprille Citation1987). The assumed additional electric potential layer can be subtracted from or added to the actual potential of the mitochondrial membrane to form the apparent potential. The assumed layer is formed by ordered water. Ordered water layers were measured around microtubules (Amos, Citation1979) and at surfaces of charged macromolecules and structures (Marchettini et al., Citation2010; Voeikov Citation2007). Experimental proofs of water ordering are based on measured differences of attraction or exclusion of positively and negatively charged particles (Zheng & Pollack, Citation2003) and measured spectral radiance from the layer which corresponds to a very low temperature noise equivalent (Zheng et al., Citation2006).
Explanation of the Warburg effect by reorganization of the ordered water layer is supported by the analysis of formation of ordered water layers around mitochondria (Pokorný, Citation2012) and dependence of their properties on pH. Ordering of water in regions under a high electric field is its general property (Zheng et al., Citation2006; Zheng & Pollack, Citation2003). Ordered water can form layers up to about 0.5 mm thick. Using electric field of about 500–700 kV/m, a floating water bridge 1–3 cm long can be formed (Fuchs et al., Citation2007, Citation2008, Citation2009). Water ordering results from transformation of energy of random thermal fluctuations into energy of coherent motion of electrons in water molecules. At “biological” temperatures around 37 °C, water contains coherence domains and bulk water. In coherence domains, electrons of water molecules coherently oscillate between a fundamental state with strongly bound electrons and an excited state with weakly bound electrons. Weakly bound electrons can be released, and therefore, the ordered water layer can act as a donor of electrons. We assume that the damping mechanism causing disturbed organization forces in a cancer cell is similar for cancer initiation both by mitochondrial dysfunction and by short-circuiting by nanofibers like asbestos (Pokorný et al., Citation2016).
Living cells can gradually pass from healthy to cancer state by decreasing transfer of H+ ions from the matrix to cytosol due to various defects including decreased membrane permeabilization, adaptive processes, etc. Measurement of apparent and actual potential points out that ordered water layers at functional and dysfunctional mitochondria has an opposite direction of the intensity of the electric field. There should be an intermediate point pH0 where the ordered water layer is not formed (Zheng & Pollack, Citation2003). The thickness of the ordered water layer increases with increasing shift from pH0. For high transfer of H+ ions when pH < pH0 at the outer mitochondrial membrane, the electromagnetic activity is not damped and mitochondrial function corresponds to a healthy state. However, if the transferred amount of H+ ions across the mitochondrial membrane decreases to the intermediate point, the layer of the ordered water is not formed. For low transfer when pH > pH0, damping of electromagnetic activity corresponds to the cancer state. Any defect of the high-energy electrons passage along the respiratory chain and/or transfer of H+ ions across the inner or outer membrane may shift mitochondrial function to the cancer region. Hexokinase 2 bound by the voltage-dependent anion channel (VDAC) to the outer mitochondrial membrane is assumed to regulate transfer of H+ ions from the mitochondrial matrix (Pedersen, Citation2007) very likely by controlling membrane permeabilization.
The model can explain a gradual development of a cancer cell. Electrons released from the ordered water layer increase conductivity of cytosol in the cell. Increased cytosol conductivity is necessarily connected with damping of the electromagnetic field generated by vibrating microtubules. In the Warburg effect cancer cells, the power of the electromagnetic field is lowered, coherence disturbed and frequency of oscillations increased. In the reverse Warburg effect cancer cells, loss of Caveolin 1 expression in the tissue and transfer of reactive oxygen species from a cancer cell affects the function of the associated fibroblasts. The fibroblasts have dysfunctional mitochondria and limited biological activity due to damping of the electromagnetic field. They transport energy-rich metabolites such as lactate, pyruvate, glutamine and ketone beta-hydroxybutyrate (BHB) to the cancer cell. The reverse Warburg effect cancer cell has a high energy supply, and electromagnetic oscillations are shifted to a highly nonlinear but unstable region. Coherence is disturbed, frequency of oscillations lowered, and organization forces are strong but unstable.
The present knowledge about electromagnetic activity in living cells and effects of ordered water on living processes is considerably limited. The pathway of cancer transformation involves disturbance of physical processes (Pokorný, Citation2012; Pokorný et al., Citation2013a; Pokorný & Pokorný, Citation2013). The missing link between mitochondrial dysfunction and the disturbed mechanism of organization is discussed in terms of water ordering and damping of electromagnetic oscillations in (Pokorný et al., Citation2015). Breakdown of coherent states in living cells is described in (Pokorný et al., Citation2014). Energy parasitism may also result in limited pyruvate transfer into mitochondria (Pokorný et al., Citation2016). Cell-mediated immunity of a human body includes a response to energy parasites of the type of an LDH virus. Some diseases such as cancer, acute myocardial infarction, schizophrenia and recurrent spontaneous abortions without any gynaecologically apparent reason within the first three months of gravidity reveal a connection with defects of energy structures and disturbed coherence (Jandová et al., Citation2015). Experimental data on precancerous cervical lesions suggest that transformation from the healthy to the cancer state is triggered in this stage of development (Jandová et al., Citation2009). Disturbances of the coherence of electromagnetic activity are therefore processes endangering the living state.
Conclusion
Disorder in cancer cells and tissues in comparison with healthy cells and tissues suggests that the organization mechanism is disturbed, the acting forces changed and their coherence weakened. The coherent electromagnetic field is a fundamental component of the action forces, and its disturbance can condition cancer development. We present a physical model of transformation of the ordered water layer connected with mitochondrial function and dysfunction, based on published experimental data.
Reduced oxidative ATP production and transfer of hydrogen ions from the matrix of dysfunctional mitochondria to the cytosol and a diminished membrane potential result in transfer of the working point to a higher pH region and reorganization of the ordered water layer connected with a potential barrier formed by a charge layer on the inner membrane. The reorganized ordered water layer releases electrons which are transferred to its outer rim at the cytosol. The electrons form a conductive region which damps electric oscillations of microtubules. Lowered power and disturbed coherence of electromagnetic oscillations result in a disorder of cellular organization—the final effect of mitochondrial dysfunction. Warburg’s reference to “more structure” is provided by functional mitochondria in healthy cells with the actual inner membrane potential higher than certain critical value.
In a healthy cell, the absolute value of the apparent potential of the mitochondrial inner membrane is lower than that in a Warburg effect cancer cell. The differences between the apparent and actual potential are caused by the potential of the ordered water layers. In a Warburg effect cancer cell, the ordered water layer is reorganized and the water layer releases electrons, efficiently damping electromagnetic activity. The electromagnetic organization forces are weak, and coherence is disturbed. In the reverse Warburg effect cancers, the mitochondrial dysfunction and damped electromagnetic field occur in associated fibroblasts transporting energy-rich metabolites to the cancer cell whose electromagnetic field exhibits high energy with fluctuating power level and low coherence.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This work was supported by the Czech Science Foundation, project 16–12757S.
Additional information
Funding
References
- Alberts, B., Bray, D., Lewis, J., et al. (1994). Molecular Biology of the Cell, 3rd. ed. New York, NY: Garland Publishing Inc.
- Albrecht-Buehler, G. (1992). Rudimentary form of cellular ‘vision’. PNAS. 89:8288–8293.
- Albrecht-Buehler, G. (2005). A long-range attraction between aggregating 3T3 cells mediated by near-infrared light scattering. PNAS. 102:5050–5055.
- Amos, L. A. (1979). Structure of Microtubules. In: Roberts, K., and Hyam, J. S. ed. Microtubules. London, New York:Academic Press. pp. 1–64.
- Arani, R., Bono, I., Del Giudice, E., and Preparata, G. (1995). QED coherence and the thermodynamics of water. Int. J. Mod. Phys. B. 5:1813–1841.
- Bartons, R., and Caro, J. (2007). Hypoxia, glucose metabolism and the Warburg’s effect. J. Bioenerg. Biomembr. 39:223–229.
- Bonnet, S., Archer, S. L., Allalunis-Turner, J., et al. (2007). A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 11:37–51.
- Chen, L. B. (1988). Mitochondrial membrane potential in living cells. Ann. Rev. Cell. Biol. 4:155–181.
- Chen, Z., Lu, W., Garcia-Prieto, C., and Huang, P. (2007). The Warburg effect and its cancer therapeutic implications. J. Bioenerg. Biomembr. 39:267–274.
- Cuezva, J. M., Sánchez-Aragó, M., Sala, S., et al. (2007). A message emerging from development: The repression of mitochondrial β-F1-ATPase in cancer. J. Bioenerg. Biomembr. 39:259–265.
- Dakubo, G. D. (2010). Mitochondrial Genetics and Cancers. New York, NY: Springer-Verlag.
- Del Giudice, E., Elia, V., and Tedeschi, A. (2009). The role of water in the living organisms. Neural. Netw. World. 19:355–360.
- Del Giudice, E., and Preparata, G. (1998). A new QED picture of water: Understanding a few fascinating phenomena. In: Sassaroli, E., Srivastava, Y., Swain, J., and Widom, A. ed. Proceedings of the International Conference on Macroscopic Quantum Coherence. Singapore, Singapore: World Scientific. pp. 108–129.
- Del Giudice, E., Preparata, G., and Vitiello, G. (1988). Water as a free electric dipole laser. Phys. Rev. Lett. 61:1085–1088.
- Del Giudice, E., and Tedeschi, A. (2009). Water and autocatalysis in living matter. Electromagn. Biol. Med. 28:46–52.
- Fröhlich, H. (1969). Quantum mechanical concepts in biology. In: Marois, M. ed. Theoretical Physics and Biology. Amsterdam, The Netherlands: North Holland. Proc. 1st Int. Conf. Theor. Phys. Biol., Versailles, 1967. pp. 13–22.
- Fuchs, E. C., Bitschnau, B., Woisetschläger, J., et al. (2009). Neutron scattering of a floating heavy water bridge. J. Phys. D Appl. Phys. 42:065502. 1–4.
- Fuchs, E. C., Gatterer, K., Holler, G., and Woisetschläger, J. (2008). Dynamics of the floating water bridge. J. Phys. D Appl. Phys. 41:185502. 1–5.
- Fuchs, E. C., Woisetschläger, J., Gatterer, K., et al. (2007). The floating water bridge. J. Phys. D Appl. Phys. 40:6112–6114.
- Gillies, R. J., and Gatenby, R. A. (2007). Adaptive landscapes and emergent phenotypes: Why do cancers have high glycolysis? J. Bioenerg. Biomembr. 39:251–257.
- Godinot, C., De Laplanche, E., Hervouet, E., and Simonnet, H. (2007). Actuality of Warburg’s views in our understanding of renal cancer metabolism. J. Bioenerg. Biomembr. 39:235–241.
- Herrmann, P. C., and Herrmann, E. C. (2007). Oxygen metabolism and a potential role for cytochrome c oxidase in the Warburg effect. J. Bioenerg. Biomembr. 39:247–250.
- Hölzel, R. (2001). Electric activity of non-excitable biological cells radio frequencies. Electro. Magnetobiol. 20:1–13.
- Jandová, A., Pokorný, J., Kobilková, J., et al. (2009). Cell-mediated immunity in cervical cancer evolution. Electromagn. Biol. Med. 28:1–14.
- Jandová, A., Pokorný, J., Pokorný, J., et al. (2015). Diseases caused by defects of energy level and loss of coherence in living cells. Electromagn. Biol. Med. 34:151–155.
- Jelínek, F., Cifra, M., Pokorný, J., et al. (2009). Measurement of electrical oscillations and mechanical vibrations of yeast cells membrane around 1 kHz. Electromangn. Biol. Med. 28:223–232.
- Johnson, L. V., Summerhayes, I. C., and Chen, L. B. (1982). Decreased uptake and retention of Rhodamine 123 by mitochondria in feline sarcoma virus-transformed mink cells. Cell. 28:7–14.
- Kasas, S., Ruggeri, F. S., Benadiba, C., et al. (2014). Detecting nanoscale vibrations as signature of life. PNAS. 112:378–381.
- Klingenberg, M., and Rottenberg, H. (1977). Relation between the gradient of the ATP/ADP ratio and the membrane potential across the mitochondrial membrane. Eur. J. Biochem. 73:125–130.
- Kolobova, E., Tuganova, A., Boulatnikov, I., and Popov, K. M. (2001). Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites. Biochem. J. 358:69–77.
- Lampidis, T. J., Bernal, S. D., Summerhayes, I. C., and Chen, L. B. (1983). Selective toxicity of Rhodamine 123 in carcinoma cells in Vitro. Cancer Res. 43:716–720.
- Ma-Ho, W., Sung, H. J., Park, J. Y., et al. (2007). A pivotal role for p53: Balancing aerobic respiration and glycolysis. J. Bioenerg. Biomembr. 39:243–246.
- Marchettini, N., Del Giudice, E., Voeikov, V., and Tiezzi, E. (2010). Water: A medium where dissipative structures are produced by a coherent dynamics. J. Theor. Biol. 265:511–516.
- Modica-Napolitano, J. S., and Aprille, J. R. (1987). Basis for selective cytotoxicity of Rhodamine 123. Cancer Res. 47:4361–4365.
- O’Connor, J. E., Vargas, J. L., Kimler, B. F., et al. (1988). Use of Rhodamine 123 to investigate alterations in mitochondrial activity in isolated mouse liver mitochondria. Biochem. Biophys. Res. Commun. 151:568–573.
- Pavlides, S., Whitaker–Menezes, D., Castello-Cros, R., et al. (2009). Reverse Warburg effect. Aerobic glycolysis and cancer associated fibroblasts and their tumor stroma. Cell Cycle. 8:3984–4001.
- Pedersen, P. L. (2007). Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e. elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr. 39:211–222.
- Pelling, A. E., Sehati, S., Gralla, E. B., et al. (2004). Local nano–mechanical motion of the cell wall of Saccharomyces cerevisiae. Science. 305:1147–1150.
- Pelling, A. E., Sehati, S., Gralla, E. B., and Gimzewski, J. K. (2005). Time dependence of the frequency and amplitude of the local nanomechanical motion of yeast. Nanomed: Nanotechnol. Biol. Med. 1:178–183.
- Pohl, H. A., Braden, T., Robinson, S., et al. (1981). Life cycle alterations of the micro–dielectrophoretic effects of cells. J. Biol. Phys. 9:133–154.
- Pokorný, J. (2012). Physical aspects of biological activity and cancer. AIP Adv. 2:0112071.11.
- Pokorný, J., Foletti, A., Kobilková, J., et al. (2013a). Biophysical insight into cancer transformation and treatment. Sci. World J. 2013. 195028:1–11.
- Pokorný, J., Hašek, J., Jelínek, F., et al. (2001). Electromagnetic activity of yeast cells in the M phase. Electro- Magnetobiol. 20:371–396.
- Pokorný, J., Jelínek, F., Trkal, V., et al. (1997). Vibrations in microtubules. J. Biol. Phys. 23:171—179.
- Pokorný, J., and Pokorný, J. (2013). Biophysical pathology in cancer transformation. J. Clinic. Exper. Oncology. S1–003. 10.4172/2324-9110.S1-003
- Pokorný, J., Pokorný, J., Jr, Foletti, A., et al. (2015). Mitochondrial dysfunction and disturbed coherence: Gate to cancer. Pharmaceuticals. 8:675–695.
- Pokorný, J., Pokorný, J., Jr, Jandová, A., et al. (2016). Energy parasites trigger oncogene mutation. Int. J. Rad. Biol. 10:577–582.
- Pokorný, J., Pokorný, J., and Kobilková, J. (2013b). Postulates on electromagnetic activity in biological systems and cancer. Integrat. Biol. 5:1439–1446.
- Pokorný, J., Pokorný, J., Kobilková, J., et al. (2014). Cancer – pathological breakdown of coherent energy states. Biophys. Rev. Lett. 9:115–133.
- Pokorný, J., Vedruccio, C., Cifra, M., and Kučera, O. (2011). Cancer physics: Diagnostics based on damped cellular elastoelectrical vibrations in microtubules. Eur. Biophys. J. 40:747–759.
- Preparata, G. (1995). QED Coherence in Matter. New Jersey, London, Hong Kong, China: World Scientific.
- Sahu, S., Ghosh, S., Fujita, D., and Bandyopadhyay, A. (2014). Live visualizations of single isolated tubulin protein self-assembly via tunneling current: Effect of electromagnetic pumping during spontaneous growth of microtubule. Sci. Rep. 4:7303. 1–9.
- Sahu, S., Ghosh, S., Ghosh, B., et al. (2013). Atomic water channel controlling remarkable properties of a single brain microtubule: Correlating single protein to its supramolecular assembly. Biosens. Bioelectron. 47:141–148.
- Semenza, G. L. (2007). HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 39:231–234.
- Summerhayes, I. C., Lampidis, T. J., Bernal, S. D., et al. (1982). Unusual retention of Rhodamine 123 by mitochondria in muscle and carcinoma cells. PNAS. 79:5292–5296.
- Tyner, K. M., Kopelman, R., and Philbert, M. A. (2007). “Nanosized voltmeter” enables cellular–wide mapping. Biophys. J. 93:1163–1174.
- Vedruccio, C., and Meessen, A. (2004). EM cancer detection by means of non linear resonance interaction. In Proceedings PIERS progress in electromagnetic research symposium (pp. 909–912). Pisa, March 28–31.
- Voeikov, V. (2007). Fundamental Role of Water in Bioenergetics. In Beloussov, L. V., Voeikov, V. L., Martynyuk, V. S., ed. Biophotonics and Coherent Systems in Biology. New York: Springer Science + Business Media, LLC. pp. 89–104.
- Warburg, O. (1956). On the origin of cancer cells. Science. 123:309–314.
- Warburg, O., Posener, K., and Negelein, E. (1924). Über den Stoffwechsel der Carcinomzelle. [Metabolism of a cancer cell]. Biochem. Z. 152:309–344. (in German).
- Wiseman, A., Fields, T. K., and Chen, L. B. (1985). Human cell variants resistant to Rhodamine 6G. Somatic Cell. Mol. Gen. II:541–556.
- Zheng, J., Chin, W. C., Khijniak, E., et al. (2006). Surfaces and interfacial water: Evidence that hydrophilic surfaces have long–range impact. Advanc. Colloid Interface Sci. 127:19–27.
- Zheng, J., and Pollack, G. H. (2003). Long–range forces extending from polymer–gel surfaces. Phys. Rev. E. 68:031408. 1–7.
