ABSTRACT
Skin aging is primarily associated with the alterations in dermal extracellular matrix, in particular a decrease in collagen type-1 content. Recent studies have shown that collagen-degrading matrix metalloproteinase (MMP-1) is produced by fibroblasts in response to chronoaging, which in human dermal fibroblasts leads to the release of proinflammatory cytokines. Past studies showed that anti-inflammatory capabilities could be induced via non-chemical means. One of these methods makes use of ultra-weak fractal electromagnetic (uwf-EM) signals. Such ultra-/very-low frequency (U/VLF) signals (few nT in intensity and within 0.5–30 kHz) interact with aqueous solutions in living systems. The fractal nature of such EM-signals relates to the self-similar property by which a “cut-out” and magnified piece of this signal reveals again the original. Thus, the aim of this study is twofold, to i) investigate the extent of this modulating effect using Human Dermal Fibroblasts (HDF)-cells, and ii) analyse molecular rejuvenation markers therein. We could demonstrate that a 10 min uwf-EM exposure (prior to incubation) increases type-1 collagen and modulates elastin in human fibroblasts cultured up to 96 h, while at the same time reduces IL-6, TNF-α and MMP-1 (the later three being statistically significant). Such up- respectively down-regulation of corresponding genes are strong indicators of an EM-induced hormetic effect that influences the epigenomic landscape of HDFs. In the Appendix, we present, in the framework of Quantum Field Theory (QFT), water as a biphasic liquid and how its coherent fraction can be affected by uwf-EM signals while at the same time resolving the “kT paradox”.
Introduction
We propose an extension of classical physico-chemical investigations (inanimate world) by referring to living matter (animated world) as the latter is a much better probe to measure subtle perturbations of aqueous solutions. Biotic structures not only react to such stimuli but also do so by magnifying effects otherwise not measurable in abiotic entities. We present here the first of a series of investigations designed to follow this direction.
Skin aging is a natural process caused by both intrinsic changes and extrinsic damage. Much of the changes occur in the dermis, which is mostly composed of a dense, collagen-rich extracellular matrix (ECM) that provides structure and support for the skin cells and confers tensile strength and firmness to the skin. Collagen, the most prominent protein within the human body, is therefore the major structural protein in the skin. Fibroblasts synthesize roughly 80–85% collagen type-1. It accounts for skin strength, durability and is responsible for the smooth, compact appearance of young, healthy skin. However, skin collagen content decreases with age and is thought to be the main reason for loss in tonicity. This decrease is associated with a lowered procollagen gene expression on one side, and an increased expression of matrix metalloproteinases (MMPs) on the other – hence a two-fold effect often associated with photo–induced aging (Fisher et al. Citation2001). Photo-stress interferes with metalloproteinase, a zinc-dependent endopeptidase, which degrades the proteins of the ECM inducing collagen and elastin fiber degradation, responsible for wrinkled and aging skin. Elastic fibers, a key secondary components of the dermis, account for the elasticity of the skin and consist of cross-linked elastin cores with fibrillin-based microfibrils. In addition, elastin is an essential constituent of various human tissues – including the lung and arteries – by assuring that these tissues stretch and recoil, therefore attain a critical role in supporting and maintaining health (Daamen et al. Citation2007; Debelle and Alix, Citation1999). Together, the loss of both collagen and elastin is responsible for the poor ability of fibroblast to replace damaged fibers, particularly when subject to photo-induced skin aging.
Skin is a physical barrier, which safeguards the body from water loss, environmental insults such as pathogens, chemicals and physical agents, and from UV radiation. Furthermore, it provides essential physiological functions, such as the important antioxidant defense capacity, including photo-induced activation of certain vital substances, such as vitamin D.
Cytokines and growth factors are mobilized in tissue damage repair via several environmental stressors and include both chemical and physical stressors – with photo-induced aging falling into the latter class. In this regard, Del Giudice et al. (Citation2015) have demonstrated that a non-chemically driven method such as ultra-weak electromagnetic (uwf-EMF) exposure can be used to study the modulating effect of cytokines in plasma of patients afflicted by certain pathologies such as psoriasis. The uwf-EM approach is rooted in the intrinsic self-organization capacities and coherent states as are observed in photosynthesis, which emphasize electromagnetic susceptibility of living tissues (Del Giudice et al. Citation2013; Tedeschi Citation2010; Voeikov and Del Giudice Citation2009). The uwf-EM procedure was shown to work even in abiotic matter, yielding some kind of “informed” materials, in particular, glass-slates onto which the emissions have been imprinted (Del Giudice et al. Citation2011; Zheng et al. Citation2006). An outline of the theoretical framework and its practical relevance to this investigation is given in the Appendix.
Since decades, light and laser sources have been used for skin rejuvenation applications, mitigate adverse effects of tattoo-removal along with other skin treatments. Collagen synthesis via photodynamic therapy (Orringer et al. Citation2008) has therefore been suggested as one non-invasive option for epithelium renewal and keratinocyte proliferation.
Given the positive feedback obtained from a preliminary trial done at the University of Bologna (Ventura Citation2013) who obtained extremely interesting and worthy results from in vitro tests when using the analogue version of the fractal signals obtained from the Materia prima (Tedeschi Citation2010) – further elaborated in the appendix. In particular, the Bologna group observed significant results on human fibroblast cell cultures, such as a reduction in i) cell growth particularly evident after 72 h, with a peak at 144 h; ii) IL6 and IL8 levels at 120 h in the medium of cells exposed to treatment, compared to the medium of unexposed cells; furthermore iii) the accumulation of the ILs always evaluated at 120 h in the cell lysate; iv) an increase in cell vitality found in the context of an evaluation of the effectiveness of plating under unfavorable conditions (low seeding dilution). Similar effects could be observed when using human mesenchymal stem cells, via i) an increase in the transcription of “stemness genes” of such as Nanog, Oct4 and Isl1, i.e. genes capable of optimizing the expression of “multipotency” and therefore the ability to differentiate; ii) a very significant increase in the expression of the GATA4 gene of same cells, the directing gene of the cardiogenesis process; iii) a significant increase of the KDR gene, encoding for a receptor with autocrine/paracrine role for the Vascular Endothelial Growth Factor (VEGF), essential in the processes of vasculogenesis and vascular homeostasis; iv) the ability to inhibit cellular “gero-conversion” and gene expression associated with the modulation of cellular aging processes, such as p16INK4, ARF, p53, and p21CIP1; v) a significant increase in the migratory capacity derived from adipose tissue during in vitro “wound healing” assays; vii) a significant modulating effect of “Reactive Oxygen Species” in exposed cell cultures.
Thus, the aim of this study is, therefore, to demonstrate that an observable effect can also be induced when exposing human dermal fibroblasts with the updated, digitalized prototype a) to frequencies not in the visible range and b) at very low intensities yet with an underlying self-similarity pattern as is the case with uwf-EM signals. This is done by analyzing the modulating effect of rejuvenation molecular markers and of proinflammatory cytokines.
Materials and Methods
Cell culture and uwf-EM treatment
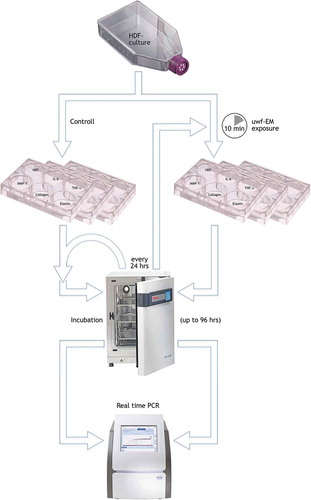
Human Dermal Fibroblasts (HDF) were grown in Medium 199 and Dulbecco’s Minimal Essential Medium (1:4), 0.01 mg/mL hygromycin B (Sigma), fetal bovine serum (FBS, ATCC n.30–2020), at 37°C in a 5% CO2 humidified atmosphere. HDF were used between the 5th and 20th passages. Around 5 × 105 HDF were plated in multiwells (6 wells, flat bottom, 9.60 cm2, 35 mm) and treated with the uwf-EM generating device (). The current prototype generating these emissions is an updated version of the WHITE Holographic Bioresonance method (Tedeschi Citation2010). It consists of a logarithmic coil printed on a circuit board, which emits a low-frequency digitized fractal audio signal from an electronic playback device. The entire setup – consisting of the Raspberry® processor board, high-end digital-audio converter and touch-screen interface constitutes a unit that we termed FRACTOS (for further details see Appendix). The overall duration of uwf-EM signal exposure in all trials was limited to 10 mins while subsequent incubation of the cells lasted for up to 96 h. Higher amplitudes or longer exposure time (>30 mins) do not modify these results (De Ninno and Congiu-Castellano Citation2014). The uwf-EM treatment was repeated every 24 hours without refreshing the culture medium. After stimulation, samples were taken at 24, 48, 72 and 96 h to determine mRNA levels of MMP-1, IL-6, IL-8, elastin, and collagen type-1. A batch of non-irradiated HDF has been used as negative controls.
Figure 1. Flow chart. Simplified sketch of the performed protocol. HDF-cells are cultivated in 25 cm2 flask; upon confluence transfer into six 6-multiwell plates (working volume: 1.90–2.90 mL, surface/well: 9.6 cm2); exposure of three plates to the uwf-EM signal (for 10 min, distance: approx. 5 mm from the log-antenna); incubation of all six plates in different compartments of the incubator for up to 96 hours; execution of PCR (determination of HDF-viability and genetic expression of IL-6, TNFa, MMP-1, collagen and elastin) followed by a repetition of the 10 min uwf-EM treatment cycle on exposed cells every 24 hours. Note: the HDF-well in this plate was used as for monitoring purposes only. The HDF-well in this plate was used as for monitoring purposes only.
MTT proliferation assay
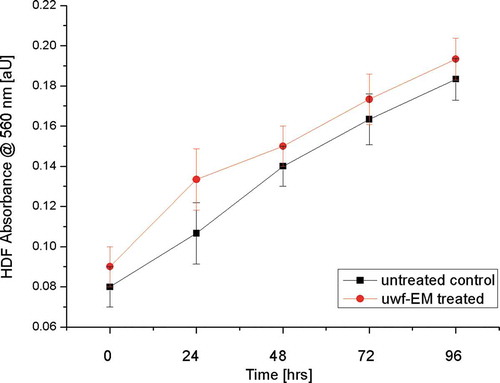
Cell viability of HDF after irradiation with uwf-EM signals was measured using i) an MTT assay (Alpha Kit, Biochrom, Berlin, Germany), ii) a colorimetric assay for cellular growth and iii) survival using 3-(4,5-dimethyl-thiazol-2-ly)-2,5-diphenyl-tetrazolium-bromide. 10 μL of MTT labelling reagent (Sigma-Aldrich, Milan, Italy) was added to each well (96 multiwells, flat bottom, 0.33 cm2, 6.4 mm; final concentration 0.5 mg/mL). After 4 hours, 100 μL of the solubilization solution was added to each well and plates were incubated overnight. Spectrophotometric absorbance was measured using a microplate (ELISA) reader at wavelength of 570 nm (Mosman Citation1983). The viability has been expressed as a percentage of increase in absorbance of samples vs negative control.
Real-time PCR analysis
Total RNA was isolated from HDF cells using the High Pure RNA Isolation Kit (Roche; Milan, Italy). A 200 ng sample of total cellular RNA were reverse-transcribed (Expand Reverse Transcriptase, Roche; Milan, Italy) into complementary DNA (cDNA) using random hexamer primers (Random hexamers, Roche; Milan, Italy) at 42°C for 45 min, according to the manufacturer’s instructions. Real-time PCR was carried out with the LC Fast Start DNA Master SYBR Green kit (Light Cycler 2.0 Instrument, Roche; Milan, Italy) using 2 mL of cDNA, corresponding to 10 ng of total RNA in a 20 mL final volume, 3 mM MgCl2 and 0.5 mM sense and antisense primers (). A melting curve was made at the end of each amplification to ensure the absence of non-specific reaction products. The accuracy of mRNA quantification depends on the linearity and efficiency of the PCR amplification. Both parameters were assessed using standard curves generated by increasing amounts of cDNA. Quantification is based on the threshold-cycle values, which are measured in the early stage of the exponential phase of the reaction, and on normalization to the internal standard curve obtained with the housekeeping b-actin gene to avoid discrepancies in input RNA or in the reverse transcription efficiency. The PCR products were examined on 1.4% agarose gel.
Table 1. Sense and antisense primers as obtained by real-time PCR.
Table 2. Two sided t- and p-values of t-test statistics. In accordance with the figures below, the diverging trends of controls and uwf-EMF-exposed cultures with a significance level of p < α (0.05) yields a non-significant difference in HDF data. However, H0 is rejected in the cases of IL-6, TNF-α and MM-1 (shaded areas); this is in line with tcrit being ≥2.777 among all data sets; this is further supported by tstat > tcrit leading also to the rejection of H0.
Statistical analysis
Both experiments as well as the measurements have been performed in triplicates, unless otherwise indicated. The results are expressed as means ± standard deviations (±SD. In order to determine the statistical significance of the results and to exclude that these have occurred by chance, a two-sample t-test for means was chosen. In particular, the two-sample option for unequal variances was selected, even though the various tests have been executed on cultures that originated from the same source batch but cultivation and incubation occurred in separate compartments of the incubator to avoid optical cross-talk, therefore, falsifying the results (Rossi et al., Citation2011). The Hypothesized Mean Difference (H0 states that there is no difference between the means) was set to zero. A standard alpha level (α) 0.05, or 5% was chosen. In the case of the HDF-morphology was no significant difference was detectable. In the cases of IL-6, TNF- α and MMP-1 however, the t-test unequivocally assigns those trends a significant difference between exposed and unexposed over time.
Results
Cell viability assay
As demonstrated by MTT assay after 24, 48, 72 and 96 h after exposure to uwf-EM signals did neither significantly influence HDF morphology nor viability ().
Pro-inflammatory cytokines expression in irradiated HDF cells
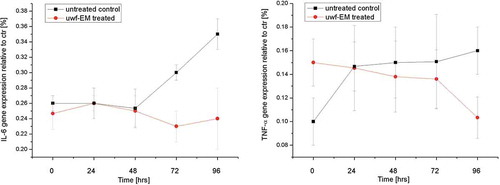
After 96 h of culture incubation, pro-inflammatory cytokines gene expression (IL-6 and TNF-α) in HDF was found to be increased. In particular, we have observed an enhancement of IL-6 gene expression in the order of 46% and an increase of TNF-α gene expression by 39% (). In samples that have been uwf-EM treated we observed a reduction of IL-6 and TNF-α gene expression by 28% and 32%, respectively.
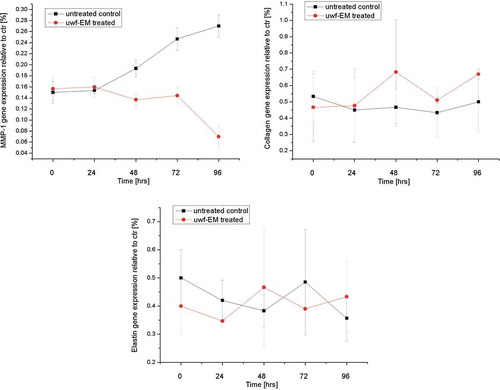
MMP-1, collagen type-1 and elastin gene expression from Human Dermal Fibroblast (HDF) stimulated with uwf-EM signals
HDF cultured for 96 h showed and increasing of MMP-1 until 35% and a reduction of collagen type-1 and elastin gene expression until 38% and 30%, respectively (). In HDF stimulated with the uwf-EM signal, a modulating effect of these genes became evident. MMP-1 gene expression was reduced by 28%, while collagen and elastin showed an increase of 35% and 33%, respectively. Major gene expression modulation was observed even beyond 96 h of culture.
Figure 4. Real-time PCR analysis using specific primers for MMP-1, collagen type-I and elastin. Relative MMP-1 expression from HDF (top left), relative collagen type-1 gene expression from HDF (top right) and relative elastin gene expression from HDF (bottom), treated with and without uwf-EM signals.
Discussion
Skin aging is a complex biological process influenced by combination of endogenous (genetics, cellular metabolism, hormone and metabolic processes) and exogenous (chronic light exposure, pollution, ionizing radiation, chemicals, toxins) factors. These factors lead together to cumulative structural and physiological alterations and progressive changes in each skin layer as well as changes in skin appearance. Keratinocytes and fibroblast play an important role to determine or prevent skin aging in response to intrinsic or extrinsic stimuli, modulating cytokines and several factors that are able to maintain the skin homeostasis.
In our experimental model, confluent HDFs have been treated with uwf-EM signals for 96 hrs. Photoaging and chronological aging are mainly characterized by the reduction of collagen, glucosaminoglycan and elastin. In addition, in photoaging leads to an overexpression of metalloproteinases that inhibit the synthesis of hyaluronic acid in fibroblasts. In this regard it is quite remarkable that our study could show, for the first time, that an increase (up to a maximum of 38%) in the expression of collagen type-1 and elastin following the treatment with an uwf-EM signal is possible, thus potentially reversing the effects of photo-induced aging. So far, skin rejuvenation growth factors are regular additives in most topical cosmetic products aiming to induce collagen synthesis; yet, due to their rather large molecular weight, they hardly penetrate the Stratum corneum, yielding rather poor results (Mehta and Fitzpatrick Citation2007).
Inflammatory processes that lead to coding for the mediators of inflammation, which are the cytokines, drive the process of aging; this process leads to the deconstruction of the three-dimensional architecture of the dermis resulting in the gradual appearance of wrinkles. While laser treatment results in increased expression of IL-6 and TNF-α in HDFs thus inducing a di-stress momenta in the exposed cells, in uwf-EM signal treated HDFs we observed a reduction of proinflammatory cytokines. This result confirms the role anti-inflammatory of uwf-EM exposed samples as obtained previously by Del Giudice et al. (Citation2015). These deviations are strong indicators that up- respectively down-regulation of the corresponding genes are of epigenetic origin as outlined by Beak et al. (Citation2019). In fact, a couple of years ago it was likewise shown that U/VLF-EMF exposure trials induced epigenetic regulation in the gene expression of neural stem cells; it did so by activating the histone acetyltransferase CBP recruitment, the Cav1-dependent binding of pCREB and histone H3K9 acetylation on the regulatory sequence of pro-proliferative and neuronal determination genes (Leone et al. Citation2014). This observation is in line with another study (Sage and Burgio Citation2017) who highlighted that EMF-exposures are indeed involved in epigenetic changes of DNA expression. Such epigenetic modulatory effects are not just limited to the U/VLF-spectrum but are a wider phenomenon as it has also been documented with mobile communication devices whereby microRNA modulation was observed to occur in rat brains at 900 and 2450 MHz at very low-intensity levels (Dasdag et al. Citation2015a, Citation2015b).
Given the low-intensities of the uwf-EM signal, we are dealing here with an EM-induced hormetic effect, by which low-intensity stimulation not only causes adaptations in cells (Vaiserman Citation2011) but also stimulates protein activation and as such should represents just another response strategy to eu-/di-stress exposure of a living system (Kim et al. Citation2018). Moreover, it stimulates adaptive-response genes thereby resulting in long-lasting epigenetic memory. Such epigenetic activation can make adaptive responses, including DNA repair, more efficient, and in turn, trigger a positive hormetic feedback loop. Approaches of this kind do represent a fascinating area for further research whereby ‘epigenetic engineering’ by uwf-EM agents (hormetins) will likely play a more significant role in the future. However, the hormesis concept still challenges the biomedical and clinical communities. Thus, to better clarify the therapeutic implications of the hormetic dose-response as treatment success or failure, optimization is still an issue in order to obtain the best effect with the right dose (Calabrese et al., Citation2010).
As a long-term perspective, we envision that what Persinger (Citation1988) already reported three decades ago: i.e. an “injection” of a substance into an in vitro system become manifest before the lag time required for the chemical reaction to set in. This is an unevoquous indicator that the effects of any medication are not primarily associated with the chemical but rather due to its physical property (i.e. electromagnetic oscillations). Practically, one can transmit a drug’s electromagnetic signature onto the body, and still obtain measurable effects just in the same was as if it has been incorporated chemically.
Conclusion
This study demonstrates that by using uwf-EM signals it is possible to increase type-1 collagen and elastin in human fibroblasts cultured for 96 h, while at the same time it reduces MMP-1 – the latter is the primary responsible for ECM degradation, with IL-6 and TNF-α being modulators of MMP-1 and MMP-3 produced by fibroblasts. Based on the results obtained in vitro we could speculate, that uwf-EM signal stimulation leads to modulation of inflammation and promotes proliferation and new collagen. The above-presented approach with its non-invasive nature represents an effective tool to mitigate the process of skin aging, posing minimum side effects yet assuring safe handling to induce these beneficial results. Nonetheless, we want to emphasize that these preliminary results are just the beginning of a much wider investigation, thus further studies are necessary to solidify the underlying mechanisms and to indicate the possible fields of application. Thus, a follow-up investigation shall look at the histone modifications of these genes to establish the exact modulating dynamics at the DNA-level.
Acknowledgments
We are grateful to Prof. Maria Antonietta Tufano (Department of Experimental Medicine, Section of Microbiology and Clinical Microbiology, Second University of Naples, Italy) for enabling us to perform these biophysical trials at her laboratories. We appreciate programming for the FRACTOS processor unit received by Andreas Feichtner (Department of Physics and Biophysics, University of Salzburg, Austria) as well as the 3D-printing support performed by Andreas Schroecker (Design & Product Management, University of Applied Science, Puch, Austria). Additional thanks are expressed to Prof. Giuseppe Vitiello (Dep. of Physics, University of Salerno, Italy) for useful discussions during the compilation of the manuscript. Finally, we dedicate this article to Emilio Del Giudice, our beloved friend and courageous pioneer.
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties. No writing assistance was used in the production of this manuscript. The authors alone are responsible for the content and writing of this article.
References
- Arani, R., I. Bono, E. Del Giudice, and G. Preparata. 1995. QED coherence and the thermodynamics of the water. Int. J. Mod. Phys. B 9:1813–41. doi:10.1142/S0217979295000744.
- Baek, S. B., H. Choi, H. S. Park, B. G. Cho, S. Y. Kim, and S. P. Kim. 2019. Effects of a hypomagnetic field on DNA methylation during the differentiation of embryonic stem cells. Sci. Rep. 9:1333. doi:10.1038/s41598-018-37372-2.
- Barnes, F. S., and B. Greenebaum. 2015. The Effects of Weak Magnetic Fields on Radical Pairs. Bioelectromagnetics 36:45–54. doi:10.1002/bem.21883.
- Beane, W. S., J. J. Morokuma, J. M. Lemire, and M. Levin. 2013. Bioelectric signaling regulates head and organ size during planarian regeneration. Development 140 (2):313–22. doi: 10.1242/dev.086900.
- Bischof, M., and E. Del Giudice. 2013. Communication and the Emergence of Collective Behavior in Living Organisms: A Quantum Approach. Mol. Biol. Int. Article ID 987549, 19. doi:10.1155/2013/987549.
- Bunde, A., and S. Havlin. 1994. A Brief Introduction to Fractal Geometry. In Fractals in Science. Ch. 1, 1-26, Berlin: Springer. doi:10.1007/978-3-642-77953-4.
- Calabrese, E. J., M. P. Mattson, and V. Calabrese. 2010. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Human & Experimental Toxicology 29 (12):980–1015. doi:10.1177/0960327110383625.
- Daamen, W. F., J. H. Veerkamp, J. C. van Hest, and T. H. van Kuppevelt. 2007. Elastin as a biomaterial for tissue engineering. Biomaterials 28(30):4378–98. doi:10.1016/j.biomaterials.2007.06.025.
- Dasdag, S., M. Z. Akdag, M. E. Erdal, N. Erdal, O. I. Ay, M. E. Ay, S. G. Yilmaz, B. Tasdelen, and K. Yegin. 2015a. Long term and excessive use of 900 MHz radiofrequency radiation alter microRNA expression in brain. International Journal of Radiation Biology 91 (4):306–11. doi:10.3109/09553002.2015.997896.
- Dasdag, S., M. Z. Akdag, M. E. Erdal, N. Erdal, O. I. Ay, M. E. Ay, S. G. Yilmaz, B. Tasdelen, and K. Yegin. 2015b. Effects of 2.4 GHz radiofrequency radiation emitted from Wi-Fi equipment on microRNA expression in brain tissue. International Journal of Radiation Biology 91 (7): 555–61. doi:10.3109/09553002.2015.1028599.
- De Ninno, A., and A. Congiu Castellano. 2011. Deprotonation of Glutamic Acid Induced by Weak Magnetic Field: An FTIR-ATR Study. Bioelectromagnetics 32(3):218–25. doi:10.1002/bem.20631.
- De Ninno, A., and A. Congiu-Castellano. 2014. Influence of Magnetic Fields on the Hydration Process of Amino Acids: Vibrational Spectroscopy Study of L-Phenylalanine and L-Glutamine. Bioelectromagnetics 35 (2):129–35. doi:10.1002/bem.21823.
- De Ninno, A., and M. De Francesco. 2018. ATR-FTIR study of the isosbestic point in water solution of electrolytes. Chemical Physics 513:266–72. doi:10.1016/j.chemphys.2018.08.018.
- De Ninno, A., and M. Pregnolato. 2016. Electromagnetic homeostasis and the role of low-amplitude electromagnetic fields on life organization. Electromagnetic Biology and Medicine 36 (2):115–22. doi:10.1080/15368378.2016.1194293.
- Debelle, L. and A.J. Alix. 1999. The structures of elastins and their function. Biochimie 81:981–94. doi:10.1016/S0300-9084(99)00221-7.
- Del Giudice, E., and G. Preparata. 1988. A New QED Picture of Water: Understanding a Few Fascinating Phenomenon. In Macroscopic Quantum Phenomenon, ed. E. Sassaroli, Y. Strivastava, J. Swain, and A. Widom, Ch.9, 108–29. Singapore: World Scientific. doi:10.1142/9789812833068
- Del Giudice, E., A. De Filippis, N. Del Giudice, M. Del Giudice, I. d’Elia, L. Iride, E. Menghi, A. Tedeschi, V. Cozza, B. Adone, and M.A. Tufano. 2015. Evaluation of a Method Based on Coherence in Aqueous Systems and Resonance-Based Isotherapeutic Remedy in the Treatment of Chronic Psoriasis Vulgaris. Topics in Medicinal Chemistry 15 (6):542–48. doi:10.2174/1568026615666150225103903.
- Del Giudice, E., and A. Tedeschi. 2009. Water and autocatalysis in living matter. Electromagnetic Biology and Medicine 28(1):46–52. doi:10.1080/15368370802708728.
- Del Giudice, E., A. Tedeschi, G. Vitiello, and V. Voeikov. 2013. Coherent structures in liquid water close to hydrophilic surfaces. Journal of Physics: Conference Series 442:012028. doi:10.1088/1742-6596/442/1/012028.
- Del Giudice, E., and G. Preparata. 1995. Coherent dynamics in water as a possible explanation of biological membranes formation. Journal of Biological Physics 20:105–16. doi:10.1007/BF00700426.
- Del Giudice, E., M. Fleischmann, G. Preparata, and G. Talpo. 2002. On the “unreasonable“ effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics 23 (7):522-30. doi:10.1002/bem.10046.
- Del Giudice, E., P. Stefanini, A. Tedeschi, and G. Vitiello. 2011. The interplay of biomolecules and water at the origin of the active behavior of living organisms. Journal of Physics: Conference Series 329:012001. doi:10.1088/1742-6596/329/1/012001.
- Del Giudice, E., P. R. Spinetti, and A. Tedeschi. 2010. Water dynamics at the root of metamorphosis in living organisms. Water 2(3):566–86. doi:10.3390/w2030566.
- Dlask, M., J. Kukal, M. Poplova, P. Sovka, M. Cifra, and M. A. Sánchez Granero. 2019. Short-time fractal analysis of biological autoluminescence. PLoS ONE 14 (7):e0214427.doi:10.1371/journal.pone.0214427.
- Fisher, G. J., H.-C. Choi, Z. Bata-Csorgo, Y. Shao, S. Datta, Z.-Q. Wang, S. Kang, and J. J. Voorhees. 2001. Ultraviolet Irradiation Increases Matrix Metalloproteinase-8 Protein in Human Skin In Vivo. Journal of Investigative Dermatology 117 (2):219–26. doi:10.1046/j.0022-202x.2001.01432.x.
- Fröhlich, H. 1967. Theoretical physics and biology. In Proceedings of the 1st Int. Conference on Theoretical Physics and Biology, ed. M. Marois., Ch. 1, 12–22. Versailles, Amsterdam: North Holland. doi.org/10.1007/978-3-642-73309-3_1
- Giuliani, L., E. D’Emilia, S. Grimaldi, A. Lisi, N. Bobkova, and M. N. Zhadin. 2009. Investigating the ICR Effect in a Zhadin’s Cell. Int. J. Biomed. Sci. 4:181–85.
- Kim, S.A., Y.-M. Lee, J.-Y. Choi, D. R. Jacobs, and D.H. Lee. 2018. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environmental Pollution 233:725–34. doi:10.1016/j.envpol.2017.10.124.
- Kobahashi, M. 2005. Two-Dimensional Imaging and spatio-temporal Analysis of Biophoton. In Biophotonics – Optical Science and Engineering for the 21st Century, ed. X. Shen and R. van Wijk, Ch. 12, 155-171. New York: Springer. doi:10.1007/b106475
- Leone, L., S. Fusco, A. Mastrodonato, R. Piacentini, S. A. Barbati, S. Zaffina, G. B. Pani, M. V. Podda, and C. Grassi. 2014. Epigenetic Modulation of Adult Hippocampal Neurogenesis by Extremely Low-Frequency Electromagnetic Fields. Mol. Neurobiol. 49:1472–86. doi:10.1007/s12035-014-8650-8.
- Marchettini, N., E. Del Giudice, V. Voeikov, and E. Tiezzi. 2010. Water: A medium where dissipative structures are produced by a coherent dynamics. J. Theor. Biol. 265:511–16. doi:10.1016/j.jtbi.2010.05.021.
- Mehta, R. C., and R. E. Fitzpatrick. 2007. Endogenous growth factors as cosmeceuticals. Dermatol. Ther. 20:350–59. doi:10.1111/j.1529-8019.2007.00149.x.
- Montagnier, L., J. Aıssa, E. Del Giudice, C. Lavallee, A. Tedeschi, and G. Vitiello. 2011. DNA waves and water. J Phys Conf Ser 306. article ID 012007. doi:10.1088/1742-6596/306/1/012007.
- Mosman, T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1:55–63. doi:10.1016/0022-1759(83)90303-4.
- Orringer, J. S., C. Hammerberg, T. Hamilton, T. M. Johnson, D. Sachs, G. Fisher, and J. J. Voorhees. 2008. Molecular effects of photodynamic therapy for photoaging. Arch. Dermatol. 144:1296–302. doi:10.1001/archderm.144.10.1296.
- Pazur, A. 2004. Characterisation of weak magnetic field effects in an aqueous glutamic acid solution by nonlinear dielectric spectroscopy and voltammetry. Biomagn Res Technol 2 (1):8. doi:10.1186/1477-044X-2-8.
- Persinger, M. 1988. The Modern Magnetotherapies. In Modern Bioelectricity, Ch.18, ed. A. A. Marino, 407–34. New York: Dekker.
- Preparata, G. 1995. QED coherence in matter - Dynamics and Thermodynamics of Water, Ch.10, 195-217. Singapore: World Scientific. doi:10.1142/2738
- Presman, A. S. 1970. Electromagnetic Fields and Life, 1–14. New York: Plenum Press. doi:10.1007/978-1-4757-0635-2
- Prigogine, I., and G. Nicolis. 1997. Self-Organization in Non- Equilibrium Systems, Ch.4, 49–62. New York: John Wiley & Sons. doi:10.1007/978-94-009-6239-2_1
- RadBio. (2017) What role does electromagnetic signaling have in biological systems? Exploring Cell-to-Cell Communications. Arlington: Defense Advanced Research Project Agency. http://microwavenews.com/news-center/darpa-radiobio (accessed: 12th May 2020)
- Roschger, P., and H. Klima (1985) Untersuchungen von NOx-Schaedigung an Wasserlinsen mit Hilfe der ultraschwachen Photonenemisison. Atominstitut der Universität Wien, AIAU- Bericht No. 85501.
- Rossi, C., A. Foletti, A. Magnani, and A. Lamponi. 2011. New perspectives in cell communication: Bioelectromagnetic interactions. Seminars in Cancer Biology 21 (3): 207–14 doi: 10.1016/j.semcancer.2011.04.003
- Sage, C., and E. Burgio. 2017. Electromagnetic Fields, Pulsed Radiofrequency Radiation, and Epigenetics: How Wireless Technologies May Affect Childhood Development. Child Dev 89:129–36. doi:10.1111/cdev.12824.
- Smith, C. W. 1994. Electromagnetic and magnetic vector potential bio-information and water. In Ultra High Dilution – Physiology and Physics, Part 3, ed. P. C. Endler and J. Schulte, 187–201. Dordrecht: Kluwer Academic. doi:10.1007/978-94-015-8342-8
- Tedeschi, A. 2010. Is the Living Dynamics able to Change the Properties of Water? Int. J. Design. Nat. Ecodyn 5:60–67. doi:10.2495/DNE-V5-N1-60-67.
- Trukhanand, E. M., and V. N. Anosov. 2007. Vector potential as a channel of informational effect on living objects. Biofizika 52:376–81.
- Vaiserman, A. M. 2011. Hormesis and epigenetics: Is there a link? Ageing Res. Rev. 10:413–21. doi:10.1016/j.arr.2011.01.004.
- Ventura, C. (2013) Effetti del trattamento con le piastrine frattali WHITE sulle dinamiche cellulari e molecolari di fibroblasti umani (Effects of fractal WHITE treatment on cellular and molecular dynamics of human fibroblasts); personal communication.
- Vitiello, G. 2008. Topological defects, fractals and the structure of quantum field theory. In Vision of Oneness, ed. I. Licata and A. J. Sakaji, Aracne Editrice, Rome, Ch. 9, 155–80. https://arxiv.org/abs/0807.2164.
- Vitiello, G. 2009. Coherent states, fractals and brain waves. New Math. Nat. Comp. 5 (1):245–64. doi:10.1142/S1793005709001271.
- Vitiello, G. 2012. Fractals, coherent states and self-similarity induced noncommutative geometry. Phys. Lett. A. 376 (37):2527–32. doi:10.1016/j.physleta.2012.06.035.
- Voeikov, V. 2003. Mitogenetic radiation, biophotons and non-linear oxidative processes in aqueous media. In Integrative Biophysics - Biophotonics, ed. F. A. Popp and L. V. Beloussov. Ch. 10, 331-59, Heidelberg: Springer. doi:10.1007/978-94-017-0373-4
- Voeikov, V., and E. Del Giudice. 2009. Water respiration: The base of the living state. Water 1:52–75. doi:10.14294/WATER.2009.4.
- Yinnon, T. A. 2017. Very Dilute Aqueous Solutions - Structural and Electromagnetic Phenomena. Water 9:28–66. doi:10.14294/WATER.2017.4.
- Zhadin, M., V. Novikov, F. Barnes, and N. Pergola. 1998. Combined action of static and alternating magnetic fields on ionic current in aqueous glutamic acid solution. Bioelectromagnetics 19(1):41–45. doi:10.1002/(sici)1521-186x(1998)19:1<41::aid-bem4>3.0.co;2-4.
- Zheng, J.-M., W.-C. Chin, E. Khijniak, E. Khijniak, and G. H. Pollack. 2006. Surfaces and interfacial water: Evidence that hydrophilic surfaces have long-range impact. Advances in Colloid and Interface Science 127 (1):19–27. doi:10.1016/j.cis.2006.07.002.
Appendix
Working Hypothesis on how uwf-EM exposure interacts with the aqueous phase of living matter
The nature of the biological effects due to weak electromagnetic fields (EMFs), whose intensities are lower than that of the geomagnetic field and whose frequencies range from a few to 10000 s of Hertz, are still questioned by many scientists. The main objections regard: (1) the very small amount of energy carried by these EMFs is much lower (by as much as an order of magnitude) than the energy associated with the thermal motion kBT. This is known as “kT paradox”. (2) even if an excited state is induced by the perturbation, according to the generally accepted models of molecular dynamics, it should rapidly disappear as a result of thermal collisions (De Ninno and Congiu-Castellano, Citation2011).
Already in the 1960 s, Presmann (Citation1970) in his ground-breaking work on EM-bio-interaction argued that environmental EMFs not only play a central role in the evolution of life but are also involved in the regulation of vital organismic processes. His conclusions were based on the observation that living beings reveal specialized and highly sensitive antenna systems for diverse parameters of weak fields that are similar in strength as ambient, respectively, natural fields. While recent studies suggest that an applied EM field affects free radical reactions and oxidative status in cells (Barnes and Greenebaum Citation2015), the crucial role of endogenic EMFs demonstrated (1) how communication and coordination of physiological processes within living organisms are mediated (Beaene et al., 2013) and (2) how the interconnection between organisms and their environment is governed by these fields (RadBio Citation2017).
Within the framework of QFT, every physical object (either a material particle or a field) is assigned an intrinsically fluctuating nature. This is of particular relevance in the case of water – in biologically active systems it contributes about 70% of the total mass and 99% of the total numerical molecular cell components (Del Giudice and Tedeschi Citation2009) – where levels of coherence can be found and described. The basic unit of coherence starts with the elementary domain, the coherence domain (CD), whose size is ≅100 nm (which is the wavelength corresponding to an EM-mode of 12.06 eV) and reaches much higher scales corresponding to “super-domains”. These domains extend and thereby organise the hierarchical levels in biology (Bischof and Del Giudice Citation2013).
One of the CD-properties regard their ability to release electrons, either by quantum tunnelling or by mild external excitations. These electrons are able to “tap” the noisy (kT) energy from the environment and transform it into high-grade coherent energy (Del Giudice and Tedeschi Citation2009). This high-grade energy seems to activate biomolecules resonating with the water CDs and turns them into dissipative structures – much in the sense of Prigogine (Citation1997) and Fröhlich (Citation1967) – as these are able to oscillate and couple coherently among them.
Based on QFT-calculations, water comes in two fractions – a coherent fraction (FC), the ensemble constituting the CDs and a non-coherent fraction (FnC), the latter is present at the boundary of a CD (De Ninno and De Francesco Citation2018). The separation of coherent and incoherent water in space yields a RedOx-pile with a potential gradient up to hundreds of millivolts. Here the coherent water representing the negative pole (electron donor, reducing element) and the incoherent water being the positive pole (oxidizing element) (Marchettini et al. Citation2010). Obviously, their ratio is temperature dependent, in that H2O is 90% coherent at 200 K, 20% at 300 K and about 5% at 400 K (Preparata Citation1995). A difference of electric potential in the order of about 100 mV (equivalent to the membrane potential) has been estimated to be present across the interface at the boundary of water CD’s (Marchettini et al. Citation2010). This parametric characterization needs to be described in more detail, as these are relevant for this paper:
a) through the hierarchical scale of nested CDs, water is able to store energy in the coherent electron vortices in the CD’s whose lifetime can be quite long (Del Giudice and Preparata Citation1988). The water CD appears as a device able to collect energy from the environment as an ensemble of many independent low-energy excitations (high entropy) and to transform them into a single excitation of high energy (low entropy) (Voeikov and Del Giudice Citation2009). When the energy stored in a CD changes the frequency of the CD (as to match the frequency of one non-aqueous molecule present in its surrounding), this molecule can be “accepted” as a partner of the CD. Only then does it receive via resonant interaction the CD’s stored energy, becomes chemically activated and ultimately able to become engaged in a biochemical reaction. The exergonic energy released in that process is in turn picked up by the CD and converted back into a resonant state. The uptake of this excess energy induces an altered resonance frequency, which enables the CD to attract different molecular species to trigger subsequent biochemical reactions. The range of attraction between CD’s and co-resonating molecules corresponds to the physical extension of the CD and assures the long-range selective attraction among molecules (Del Giudice et al. Citation2010).
b) a second property yet coupled with the first one, plays a crucial role in the correlation at a long distances (Zheng et al. Citation2006). An ensemble of nested time-dependent CDs, such as those possible in aqueous systems (synonymous with living organisms), exhibits an extended phase-field evolving over time. Within this field, a correlation at a large distance among coherently coupled parts sets in; such kind of entanglement must be envisioned as an energetic event (e.g., a chemical reaction) occurring at some place of the coherent region (Giuliani et al. Citation2009; Pazur Citation2004; Zhadin et al. Citation1998), whose exergonic output energy induces a change of phase within the host CD. The concomitant em-potential of the CD reaches out to far-away regions whose phase will be correspondingly altered, implying a change of the local molecular dynamics. An actual transfer of energy and/or matter does not take place at any point in this sequence of events, removing therefore the challenge of relocating molecules at high speed (something that diffusion over long distances does not allow).
Given the rather lengthy compilation on the “mechanism of action” we follow Marchettini’s interpretation in that once a CD is immersed into an energy field, it becomes recharged again and a permanent oscillation is created in which charging is electromagnetic and discharging is chemical (Marchettini et al. Citation2010). The relevance of these properties for our investigation becomes clearer when confronting the electromagnetic nature of the biological dynamics and the central role played by water. Such experiments demonstrate that serial dilutions yield low-frequency em-fields (500–3000 Hz) when exposed to very low-frequency (a few Hz) em-noise as regularly found in nature (Montagnier et al. Citation2011). A number of other experiments performed over the last years (Smith Citation1994; Trukhanand and Anosov Citation2007; Yinnon Citation2017) gives additional evidence thereby corroborating these phenomena. As the observed effect does not depend on the frequency only, the structure of the water is able to mediate EM-effect via long-lived excitations of water structures, at least in part and must be considered in part being responsible for the electromagnetic properties of living systems (De Ninno and Congiu Castellano Citation2011).
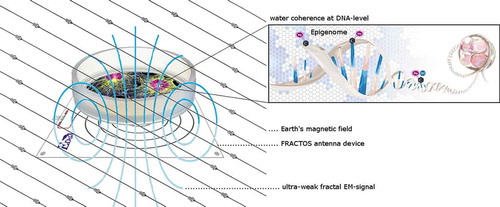
In order to comprehend the mode of action when a biological system is exposed to an uwf-EM signal, it may be helpful to recapture our previous work in this field, which has been done using pristine biomass (Materia prima). We used an “analogue” approach, whereby coupling of coherent oscillations into the aqueous medium was obtained by suspending triturated vegetal leaves and algae in bi-carbonated water (Tedeschi Citation2010). After a suitable physical treatment (see Del Giudice and Tedeschi Citation2009 for details) the trituration of the suspended vegetal matter induces highly enhanced coherent activity. We assume that the so-called “instinct of survival” triggered by “irritation” or distress events (similar to measured auto-luminescence intensities of injured bio-matter (Kobahashi Citation2005; Roschger and Klima Citation1985)) augments coherent activity for a limited amount of time. The EM-signals related to this process would then, as in Montagnier’s experiment, imprint the surrounding water and trigger the formation of coherence among CDs, namely, super-coherence. These ultra-low photon density fluxes as observed during autoluminescence differ from high-intensity radiation (bio-luminescence) in that the statistical and temporal properties of at least the so-called delayed luminescence (induced by irradiation of living organisms with a flash of light) results from the relaxation of an intrinsic coherent electromagnetic field (Voeikov Citation2003). Yet recent statistical analysis of biological autoluminescence revealed that there is a fractal character associated to it (Dlask et al. Citation2019); i.e. when one cut a piece of fractal and magnifies it isotropically to the size of the original, both the original and the magnification coincide (Bunde and Havlin Citation1994), which in the quantum optics scheme denotes holographic properties. This coincides with theoretical predictions that the macroscopic nature of fractals emerges from microscopic coherent local deformation processes; i.e. a fractal property is rooted in quantum dissipation, through squeezing of coherent states. It is the resulting “geometry” that characterizes coherent states by exhibiting self-similarity properties (Vitiello Citation2012). This property is fundamental if uwf-EM is intended to have any effect on living samples; in other words, the fractal EM-signal employed in our experimental setting needs to have a proper loudness-frequency relation, which implies that the various frequencies and the signal strengths must reveal fractality in order to become effective. In addition, the FRACTOS antenna device is based on a logarithmic coil progression (likewise revealing fractal properties), as this is intrinsic to many biological phenomena, ranging from botany to physiological and functional properties in animals (Vitiello Citation2012). Thus, the current set-up of the FRACTOS prototype accesses a digital fractal EM signal, converts it into an analogue signal and feeds it to the log-antenna ().
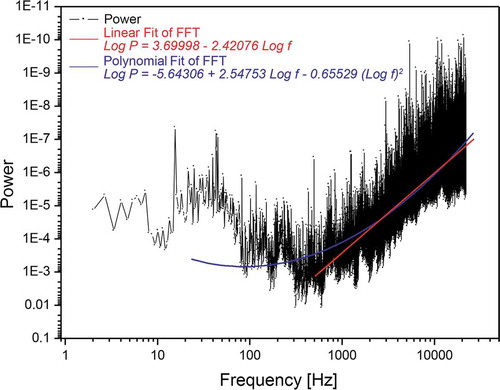
The “digitalized” approach using pre-recorded fractal signals standardizes the approach by increasing reproducibility. In this way, the coupling of the biological sample with the fractal inductance to induce coherent oscillations and thereby producing an ensemble of quasi-free electrons to establish the redox-pile is easier to achieve. This however requires that the audio file used is present in an uncompressed format; i.e. to maintain the integrity in its digitalized form, the wav-format was used. The emitted and measured signal intensities are shown in . Field intensity data have been recorded using an NFA-1000 data logger (GHz-Solutions, FRG) along with their software packages (NFAsoft v.1.72 from same supplier). Due to the weak signals measurements have been done in a mu-metal test-chamber (Marchandise-Tech, FRG) especially conceptualized for this investigation. Analysing the used audio-file (S1_20-24_bit48k_with_cutoff_500 Hz.wav - in short: whiteHBfractal.wav) via Fast Fourier Transformation (FFT) and further processing it via Power Spectrum Density yields the signals relative frequency density – as shown in reveals a self-similar mathematical structure (fractality). The pattern of the signal power as a function of the frequency (in log-log scale), shows the presence of a linear regime beyond a frequency threshold value of >500 Hz. The non-self-similar, non-fractal value below the lower frequency threshold is due to experimental signal recording limits. This experimental result, according to Vitiello’s theorem, is proof of coherence (Vitiello Citation2008, Citation2009, Citation2012).
Table 3. Magnetic flux densities of background and FRACTOS logarithmic antenna in three repeats. Background values denote signal strength with full set-up yet at min. signal intensity, whereas 100% measurements denote maximum intensity of the emitted signal originating from the log-antenna.
Figure 6. Linear regime (red segment of fitting) between power logarithm and frequency logarithm in the frequency window of 0.5 - 20 kHz of the used audio signal (whiteHBfractal.wav). The polynomial fit includes also the sub-500 Hz regime down to 20 Hz (see text for details).
Fractality and coherence therefore refer to each other, or conversely, whenever one recognizes a fractal mathematical structure in a system one can infer that a coherent pattern is present within the system. A self-similar signal therefore can be an electromagnetic oscillation as well as an acoustic one – a property frequently observed in biological systems where it is possible to record the presence of both electromagnetic and sounds signals. The coupling between the fractal uwf-EM signal and the sample takes place via the aqueous phase of the exposed specimen. Since biological systems are foremost aqueous systems, coherence and fractality are mediated by water (Del Giudice et al. Citation2011; Tedeschi Citation2010).
Summarizing, water molecules, which account for the vast majority of molecular components of the organism, have peculiar properties as (1) the effective dielectric constant decreases dramatically to 10 (from 80 of bulk water) – particularly of “vicinal water” near the surface of an organic macromolecule and (2) the entropy content is lower than in bulk phase (De Ninno and Pregnolato, Citation2016). As a result, water molecules organize themselves into extended CDs where EM-fields with a well-defined frequency are trapped inside. These CDs are able to collect chaotic energy (high entropy) from the environment and store it in the form of coherent excitations (low entropy), which in turn changes the frequency of the trapped EM-field (Del Giudice et al. Citation2002). When this frequency matches the frequency of some non-aqueous molecules present in its surrounding, those molecules are attracted towards the boundaries of the water CD’s, coating them and possibly arranging themselves in a membrane-like manner (Del Giudice and Preparata Citation1995). The attractive force becomes very large when the three frequencies (νCD, νBiomolecule, νEM,) do not differ more than the thermal noise kT. Hence, when molecules satisfy that constraint (only those molecules engage in a biochemical reaction that comply with the above resonance principles), then the kT-energy is used for the coherent dynamics. The estimated CD’s frequencies of oscillation are located in the infrared range (0.2–0.3 eV) (Arani et al. Citation1995). Del Giudice et al. (Citation2015) have demonstrated that such a non-chemically driven method can successfully modulate cytokines in plasma of patients affected by pathogenesis of psoriasis. Our observations are thus in line with a recent study that assessed the mechanism of interaction between uwf-EM-signals and biomolecular structures using hypomagnetic field exposure during differentiation of embryonic stem cells (Baek, Citation2019).