Abstract
Nanotoxicology and nanosafety has been a topic of intensive research for about more than 20 years. Nearly 10 000 research papers have been published on the topic, yet there exists a gap in terms of understanding and ways to harmonize nanorisk assessment. In this review, we revisit critically ignored parameters of nanoscale materials (e.g. band gap factor, phase instability and silver leaching problem, defect and instability plasmonic versus inorganic particles) versus their biological counterparts (cell batch-to-batch heterogeneity, biological barrier model design, cellular functional characteristics) which yield variability and nonuniformity in results. We also emphasize system biology approaches to integrate the high throughput screening methods coupled with in vivo and in silico modeling to ensure quality in nanosafety research. We emphasize and highlight the recommendation regarding bridging the mechanistic gaps in fundamental research and predictive biological response in nanotoxicology. The research community has to develop visions to predict the unforeseen problems that do not exist yet in context with nanotoxicity and public health hazards due to the burgeoning use of nanomaterial in consumer’s product.
Introduction
Nanotoxicology is a subfield of toxicology that is concerned with potentially toxic effects of nanoscale structures or particles with a diameter of less than 100 nm (unique nanotoxicology scale, ) (Liu et al. Citation2011). In general, the gradually enhanced toxicity of some nano-sized chemicals in comparison to the respective bulk materials arises from the fact that nanoparticles have a very high surface-to-volume (S/V) ratio which makes them highly reactive (Moreno-Horn and Gebel Citation2014; Recordati et al. Citation2015). This relates to other chemicals as well as to the physiological environment of different tissues in the human body. Taking advantage of their small size, nanomaterials are hypothesized to penetrate cellular membranes, biological barriers and tissues more efficiently than larger sized materials (Gidwani and Singh Citation2013). However, a large number of biokinetic studies has only revealed a minor influence of particle size on biodistribution (Laux et al. Citation2017b). The field of nanotoxicology is concerned with classifying the conditions that lead to toxic effects and devising ways to prevent and treat them. One of the main concerns of the field of nanotechnology is determining which properties of specific nanoscale particles lead to toxic effects.
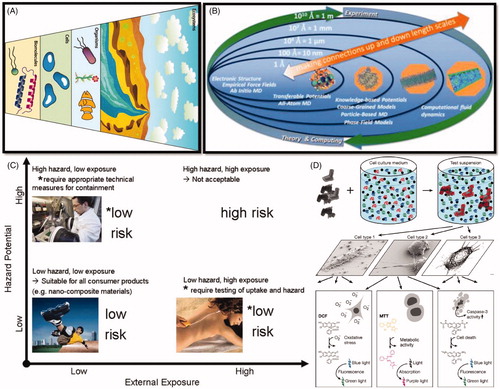
Figure 1. (A) Nanoparticles interact with biological systems at the molecular, cellular, organismal, and ecosystem levels. (B) Computational approaches to understanding the nano–bio interface span orders of magnitude in time and space (A, B reproduced with permission from Murphy et al. Citation2015). (C) The generally accepted principle assesses risk as: Risk = Hazard × Exposure. (D) Workflow of in vitro testing. The nanomaterials are dispersed in a physiological nutrient medium that contains proteins and other macromolecules (coils) and low‐molar‐mass components such as salts (dots). The nanomaterial surface changes by differential adsorption of some of these components, correlated with changes in the state of agglomeration. Only afterwards, the interaction with a multitude of cell species is studied by the (typically optical) readout of a large number of markers and endpoints (C, D reproduced with permission from Landsiedel et al. Citation2015).
In this review article, we revisit a nanosafety conclusion from an emerging trend of a decade of nanotoxicity research. Since the inception of nanotechnology more than three decades ago, there has been a concern that the term ‘nano’ was somehow inherently related to dangerous (Laux et al. Citation2017b). This was majorly propagated by a media hype, science fiction and books seeking attention of public with the term ‘nano’ which introduced imaginary self-replicating nanobots (Joachim Citation2005; McCray Citation2010). Throughout the popular electronic and print media, the scientific results get picked up by the news outlets, often reporting results that did not have the right context (Sitti Citation2009; Sengupta et al. Citation2012). Nanotoxicity is not just about assessing environment and health safety (EHS) exposure levels. There are many other factors that need to be incorporated into an experimental design in order to achieve experimental data that are valuable and reproducible (Bernstein et al. Citation2004; Laux et al. Citation2017b).
The regulatory challenges in incorporating nanomaterial into commercial products: the anomalous biological outcome from nanoscale features
The US Food and Drug Administration (FDA) and Environment Protection Agency (EPA) under the Department of Health and Human Service are the main regulatory bodies which govern the nanosafety issues in the USA (Sandoval Citation2009). The European counterparts work under the European Union (EU), namely ERC and EURO-NanoTox, which is an EU nanosafety cluster () (Gottardo et al. Citation2017). For cosmetic products, a notification together with toxicological data for the respective NM is required by a European regulation (EC 2009) (Buzek and Ask Citation2009). For agriculture, food and feed, the use of nanomaterials is authorized by the EC and member states of the EU. The risk assessment makes use of guidance by the European Food Safety Agency (EFSA) (EFSA Scientific Committee Citation2011). Furthermore, the use of an NM as an ingredient of a biocide is subject to authorization based on a distinct assessment of nanospecific risks (EC 2012) (Regulation Citation2012). Chemicals and their use in products for which no other specific regulation exists are subject to the Regulation EC No. 1907/2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in the EU (EC 2006) (Parliament and Union Citation2006). A key requirement in NM risk assessment is represented by the measurement of particle migration, e.g. pigments from composite materials. The release of free nanoparticles in daily life scenarios is considered as potential concern to human health.
Figure 2. (A) Regulatory bodies in USA and European Union working as watchdog for nanotoxicological assessment. (B) Key scientific considerations to enable understanding of the long-term, medium and short opportunities in nanotoxicology. The boxes on the left hand side detail the tools that are essential towards (i) ensuring that fundamental properties and nanomaterial lifecycle are measured in the prioritization of nanomaterials taken forward into risk testing, and (ii) the efficacious utilization of in vitro, mechanistic methods to predict apical toxic effects. Figure adapted from Burden et al. (Citation2017).
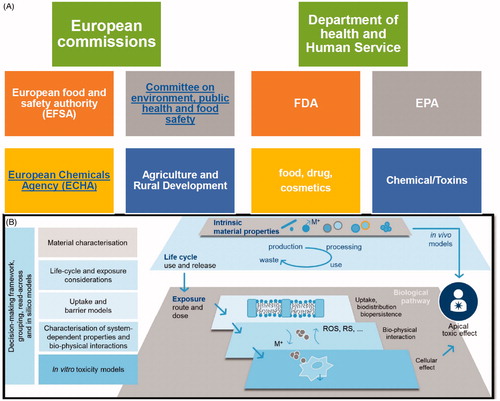
These watchdogs treat nanomaterials from how they have treated every other compound (Laux and Luch Citation2015; Laux et al. Citation2016). The allied animal veterinary and cosmetic products dealing agencies under FDA work with the sense that government and private partners involved in risk assessment need to establish a reproducible process for producing and biosafety testing of these nanomaterials (). Once reproducible fabrication and allied testing is optimized, there is a well-defined protocol to understand the exposure level and how those materials are distributed in the body and on ecosystem level. Such established systems enable us to look forward and make an assessment on whether the material is safe or not. These regulatory bodies keep revising the set of standards to understand maybe some of the nuances of why those analytics and methods may cause adverse reactions before scale up and their potential application in clinics. There is no fundamentally different modus operandi of nanomaterial governance in USA or Europe. The authorities emphasize reproducible studies in context with a correct nanosafety evaluation (Andrione et al. Citation2017; Gottardo et al. Citation2017).
Plasmonic properties
Optically active or plasmonic NPs from noble metals such as gold copper and silver exhibit strong interactions with incident light and can magnify the local electromagnetic field, influencing some of the characteristics of the particles in biology (Zeng et al. Citation2014). These optical NPs can convert light into thermal heat and are therefore used in photothermal therapy, however, during routine characterization of biological system-interaction they are also able to add false positive/negative influence to nanotoxicity results. Nanoparticle compatibility in this size versus plasmonic interaction to evaluate the Nanotoxicity has been critically ignored in the research community (Hassan and Singh Citation2014).
The band gap factor
The band gap is relevant for a select group of materials. Excitation of nanomaterials with an appropriate bandgap can impact their optical properties, the redox properties or the reactive oxygen generation potential of the material () (Singh et al. Citation2010b). As the conduction band energy of metal oxide nanoparticles (MOx NPs) approaches to those of biological molecules or in other words the hydration enthalpy becomes less negative, material toxicity is known to be amplified. In a recent study by Kaweeteerawat et al., authors have shown that the hydration energies and conduction bands of MOx NPs severely affect the growth of E. coli, which is in similar line of evidence of band gap factor of NPs with mammalian cells (Kaweeteerawat et al. Citation2015).
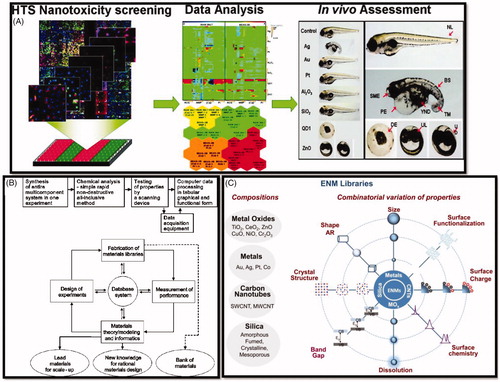
Figure 3. (A) High throughput screening of nanomaterial toxicity in vivo (George et al. Citation2011). (B) Concepts of the combinatorial materials-development workflow. Upper panel with boxes; Initial concept proposed by Hanak (Citation1970). Lower panel demonstrates modern ‘combinatorial materials cycle’ (Potyrailo and Mirsky Citation2008). Reproduced with permission from Hanak (Citation1970) and Potyrailo and Mirsky (Citation2008). (C) Use of compositional and combinatorial ENM libraries including metals, metal oxides, carbon nanotubes, and silica-based nanomaterials, to perform mechanisms-based toxicological screening that links material composition and systematic variation of specific properties to biological outcome (Nel et al. Citation2013). Reproduced with permission from Nel et al. (Citation2013).
Surface coating
The surface coating also impacts the surface charge but has alternative functions and role for nanotoxicity. It can sterically keep the particles stable and in some cases the surface coating can be designed to target or recognize specific binding on surfaces in solution to explore novel functionalities if it is coated within for instance an antibody or some other biological targeting moiety () (Singh et al. Citation2011a, Citation2013).
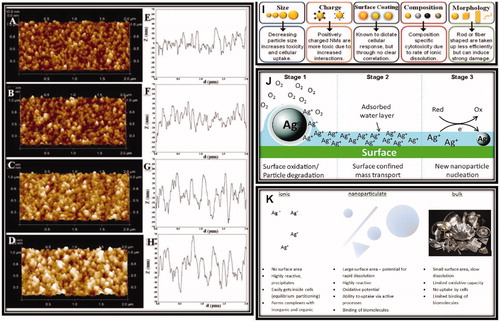
Figure 4. AFM characterization of surface topography and nanoroughness of different TiO2 films. (A–D) Representative height maps in three-dimensional view of ns-TiO2 films with increasing thickness (50, 100, 200, and 300 nm); (E–H) Representative surface profiles exhibiting variations in Rq, Aspec, correlation length, skewness, and kurtosis, as well as in pore width and depth distributions, as discussed in the main text. (I, J) Proposed pathway for new particle formation from parent (nano) particles. (K) Main differences between ionic, nanoparticulate, and bulk silver. Reproduced with permission from Glover et al. (Citation2011), Singh et al. (Citation2011b), Reidy et al. (Citation2013), and Hussain et al. (Citation2015).
Size determines surface area and particle unit mass
The size of particles is the most important and most studied factor, which dominates nanotoxicology. There is a number density and a surface area per unit mass that increases as a function of size as the particles get smaller, and determines acute to toxicity of these NPs in organ specific manner (Schmid and Stoeger Citation2016). The number of particles per unit masses and the surface area has direct correlation with the fate and transport. These nanoparticles are in the order of a size of a virus and biological systems handle the NPs in many aspects like the virus (uptake distribution and efflux) (Goodsell Citation2004). In fact, receptor-mediated viral invasion of living cells by virus particles share the mechanistic similarity for the cellular and endocytic uptake time of a single NP as unified into a general energy-balance framework of NP- membrane deformation and membrane adhesion (Grove and Marsh Citation2011).
Positive versus negative charged particles
There is a surface charge associated with nanoparticles. When they are suspended in solution they have a double layer that keeps them stable and repels them from each other and allows them to remain individual. If the particle is negatively charged, it is going to be attracted to positively charged surfaces and vice versa (). Surface charge is one of the primary criterions which determines how the nanoparticles interact with biological subsystems or membranes in the aqueous environment. In this context, positive nanoparticles are more toxic than negative ones, therefore ammonium compounds are typically used as antimicrobial substances (Alvarez-Paino et al. Citation2017). Being positively charged, they are easily attracted to the negatively charged membrane of bacteria. Once attached to the membrane, they damage the bacterial membrane and interact with bacterial enzymes, proteins, and DNA (Hasan et al. Citation2013). In biological systems, most of the surfaces are negatively charged and so a positively charged particle can actually stick or interact with the element of interest, typically increasing the probability of uptake. Particularly, silver nanoclusters releasing silver ions locally to the surface can induce a higher level of toxicity (Dobias and Bernier-Latmani Citation2013). Silver is relatively dynamic. It has a dissolution constant in water. In the case of silver or also copper, ions are toxic because of the constant ion release to the local environment, which potentially affects the biological environment around them.
Morphology
The morphology is also an important aspect for the toxicology of NPs. Asbestos is a good example of a structure, which became a topic of extensive research because of its fibrous shape, and hazardous nature (). There is a concern in the nanotoxicology space that the same thing would happen with nanomaterials and that was true to some extent but in most cases, it was not as dramatic as the asbestos example. However, while the nanodimension as such does not present a hazard, there is clear evidence for carcinogenicity of some MWCNTs and further biopersistent nanofibers (Porter et al. Citation2010; Donaldson et al. Citation2013; Rittinghausen et al. Citation2014). Furthermore, pulmonary fibrosis has been associated to MWCNT exposure (Vietti et al. Citation2015; Sharma et al. Citation2016). The development of in vitro and early-stage predictive markers for lung cancer or mesothelioma is urgently required. This should however include the consideration of physico-chemical properties and experimental factors (Kuempel et al. Citation2017). Wide range of morphology and shapes can affect stability, transport, surface adsorption and uptake by biological systems (Charu et al. Citation2018). The wide range of morphology and shapes can affect stability, transport, surface adsorption (Singh et al. Citation2018), and uptake by biological systems (Charu et al. Citation2018).
Phase stability and silver leaching problem
Bulk phase diagrams do not predict the phases for NPs as there exists a difference in the phase stability between the bulk and the nanoscale dynamics (Wang and Swihart Citation2015). This is most often seen in sintering of the nanoparticle much below their melting temperature. Applying a thermal change well below the bulk melting point still results in the particles sticking to each other. This effect is caused by the high curvature (Gonzalez Solveyra and Szleifer Citation2016) and the high surface area of the materials themselves.
In terms of mass loading, silver ions or metal ions in general are more toxic than silver nanoparticles, but they are indistinguishable from each other. The silver nanoparticles are a high surface area source for the silver ions (). In biological assays, silver NPs showed a continuous release of silver ions during transport to their final location (Singh et al. Citation2012; Guehrs et al. Citation2017). Nanotoxicological assays are able to give information on the fraction of ions to NPs in the supernatant or permeate from the biological medium supplemented silver NPs using ICP or mass spectrometry (Bohmert et al. Citation2017). Therefore, the conducted investigations provide the actual silver ion concentration released from a given silver nanoparticle concentration in order to set up a proper control for the nanotoxicologists (Bewersdorff et al. Citation2017; Bohmert et al. Citation2017; Laux et al. Citation2017a). The new technique of single particle ICP mass spectrometry can predict individual particles split with the solid particle versus the ion concentration in the solution (Reidy et al. Citation2013).
Instability and defect
Synthetic methods used to produce nanomaterials also produce a wide range of crystal defects and defect densities (O’Brien et al. Citation2016). This effect is influenced by impurities in the material, which then lead to the instability. Due to this instability, small nanoparticles can change with time into bigger particles via Ostwald’s ripening since the pressure inside the particles is inversely related with the diameter of the particles. Therefore, the smallest particles going away at the expense of the larger particles, a concept that is not usually seen in the in the bulk material due to the curvature of the particles (Hansen et al. Citation2013). They can release ions and the particle size distribution or inter-particle connection can change with time via ligand mitigated dissolution, influencing the overall surface area. This sort of level of change or dynamics or range of properties within the nanomaterial systems are unforeseen for a person coming from nanotoxicology background. We have got this idea of the physicochemical properties of the nanospecific things that nanotoxicologists need to worry about or be concerned about when design experiments predicting a biological response from these nanomaterials (Singh et al. Citation2010a, Citation2010b).
Protocols, data gaps, and challenges for safer nanomaterial design
Nano-toxicologists still encounter data gaps and challenges associated with filling those data gaps in regards to instrumentation, analytics and measuring the response of complex biological matrices to particles (Handy et al. Citation2008). Over the last 10 years, the nanotoxicology community has been trying to address further technical questions e.g. dosing issues or new protocols to handle aggregation state of materials as a function of time (Donaldson et al. Citation2013). Results from more than a thousand papers have been published looking into the impact and influence of nanomaterials on biological systems; however, the nanotoxicity community was not able to reach a general consensus (Richardson et al. Citation2007). As explained in the previous section, the SBIR/STTR had some predictions or generalizations on how different characteristics of the nanoparticles affect their ultimate toxicity and how those properties can be used to create guidelines and rules for the application of safer materials in NP design (Hussain et al. Citation2015).
Decreasing the particle size generally increases the toxicity and the amount of cellular uptake.
Positively charged nanomaterials are more toxic due to their increased interactions with primarily negatively charged biological surfaces and entities (Singh et al. Citation2010a, Citation2010b).
From a composition perspective, ionic dissolution definitely correlates with the toxicity index.
Anisotropic morphology or rod-shaped NPs are taken up less efficiently, but once internalized, they exhibit significant damage to NIR plasmonic criterions.
There is a whole sort of secondary fields coming up with nanosafety emphasizing about how to make sustainable nanomaterials for safer design of components that could ultimately end up in nano-therapies and for the cancer treatment. One of the topics for nanotoxicologists and policy makers is the protection of the community and the identification of risks factors. On the other side, they are providing a foundation for the future nanomedicine and nanobiology pinning the right data which are actually very interesting to predict that community wellbeing. Most of the experiments to date have been done with pristine systems. Starting with a very complex material, it is very difficult to determine whether the component of that matrix or the complexity is causing the effect. There have been a lot of pristine studies exposing animals and cells in vivo or in vitro but those experimental setups are not realistic concerning real exposure where the concentration and physiochemical properties of the nanotoxic pollutant are unknown (Andrione et al. Citation2017). In contrary, we are exposed to materials, which are integrated into composites that are potentially manufactured using uncontrolled, unpurified, and very complex processes. It is important to recognize that some of the results that have been published out of those experiments might not be completely relevant to the nanotoxicological community and are even posing an actual risk because the experimentally used artificial exposure route is not natural. If we take that a little further, we can look at the problematic nature of this issue in terms of the studies that have been conducted under specific exposure conditions.
In case, the MWCNTs-SCNTs filled polymers lose their resin, the carbon nanotubes could be individualized, and potentially be an exposure source () (Harper et al. Citation2015). The question is in a composite like environment, what would be the exposure risk with a particular lifecycle of a material? Interestingly, in more than a dozen of those studies with nanoparticles or nanotubes, no evidence of any significant free nanofibre/particulate has been found in any of the reviewed studies, indicating that it is not that common for nanocomposites to release a particulate matter individually. Loosening of nanocomposites often leads to particles aggregate/coalescing to get bigger or deposit on surfaces with natural state. However, in context with new cellular phenomenological studies mimicking in vivo decision making of cells to show chiral based behavior, a new outcome was recently reported (Singh et al. Citation2014). These could be included into nanotoxicology parameters as novel ‘chiral behavior’ trend in nanotoxicology (Raymond et al. Citation2017). depicts important recommendation scenario of ENMs exposure to model organism or in a specific microcosm/ecosystems for the sustainable study (Holden et al. Citation2016).
Figure 5. (A, B) Life cycle of a nanocomposite. (A) Relevance for exposure of professionals or consumers, and environmental emission from nanofiller production to end of life/recycling. (B) Specific life cycle map of sports equipment made of MWCNT-polymer composite, detailing release processes that must be considered with the anticipated relative release probability indicated by the thickness of arrows. (C) Schematic illustration of the competing environmental transformation and organismal uptake processes that occur for a nanomaterial in aquatic environments, illustrated using a silver nanoparticle. (D) Characterization of porous nanoparticles for in vitro or post in vivo exposure in blood. Reprinted with permission from Harper et al. (Citation2015), Malysheva et al. (Citation2015), and Valsami-Jones and Lynch (Citation2015).
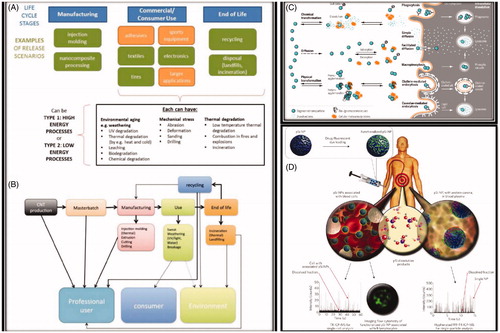
Table 1. Recommendations regarding exposure conditions used in assessing ENM hazard potentials (Holden et al. Citation2016).
Conclusions, new emerging trends and outlook
Reviewing the topic thoroughly, we can agree on some general conclusions that it is difficult to find examples of common processes that will release large quantities of engineered nanomaterials. Partly, this is because there is still a lack of big scale ‘nano’ manufacturing industry that is associated with producing ENMs right now but also because a lot of risky materials are getting integrated into products, which do not have the potential for release, yet. Second, if the particles are released into the environment, they are rapidly aggregated and encapsulated or otherwise sequestered into large safer materials. Third, the risks identified in many in vitro experiments, if extrapolated back in context with the exposure paradigm, appear partly unrealistic. Even the positive results mentioned in literature that something is toxic at a particular dose, the actual risk or hazard associated with the bulk form of exposure perspective is relatively low. The overall conclusion of the research from the field of nanotoxicology struggles with its incredible complexity in regards to the immense effort that has to be made to fully understand the fundamental processes of how those materials interact with biological systems (Singh et al. Citation2015) (). Very subtle differences in the properties can result in surprising changes in vivo. Very slight changes in the size or the surface state can completely change the protein corona that gets absorbed onto a particle once it is introduced in vivo. Huge challenges remain in the post corona transformational characterization to understand how these particles are changing once they are in the biological system. There are complex surface dynamics posing the global problem of extending the results from the pristine materials to much more complex industrial byproducts. Burgeoning use of nanoproducts make nanotoxicology research absolutely essential for the society to develop safer nanomaterials for the future (. This also provides the backbone for nanotherapies in the nanomedicine which is coming down the pipe (Nel et al. Citation2009).
The inherent complexity of biological systems gives a complicated mechanistic response in assays performed in nanotoxicology (. The dynamic transformation of nanoparticle surface corona and batch-to-batch variability imposes further challenges in this context (Singh et al. Citation2015). Lack of modeling instruments, harmonized protocols and theoretical understanding further complicate the way to make reproducible data. In this prospect, lessons can be learnt from recent developments in artificial intelligence and disease modeling (Khare et al. Citation2014; Singh et al. Citation2017). Limited data about the uptake mechanism of NPs, scarcity of biomarkers and a few demonstrative functional analyses which the research community needs to build up potentiate the nanotoxicology research. The lack of standardized dosimetry and materials consideration, and insufficient statistics needs to be worked out to progress in this context. Addressing these issues will widen the scope for the faithful use of nanomaterials for contemporary nanobiotechnology based pharmaceutical design to combat infection, neurological problems and drug delivery in rare disease.
The prioritized goal of system nanotoxicology is endeavoring reproducibility and relevance to experimental nanosafety research, and the discovery of nano-specific toxicity biomarkers, and pathways. Toxicologist also believe that cross-fertilization between human toxicology and environmental is vital, and suggest that the joint efforts could further bring great benefits to these two mutually related fields. The linkages between the direct molecular initiating event in nano(eco)toxicological sciences and an adverse outcome aiming towards the implementation at the biological level of organization relevant to risk assessment will further strengthen this approach (Ankley et al. Citation2010).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor change. This change do not impact the academic content of the article.
Additional information
Funding
References
- Alvarez-Paino M, Munoz-Bonilla A, Fernandez-Garcia M. 2017. Antimicrobial polymers in the nano-world. Nanomaterials (Basel, Switzerland). 7:48.
- Andrione M, Timberlake BF, Vallortigara G, Antolini R, Haase A. 2017. Morphofunctional experience-dependent plasticity in the honeybee brain. Learn Mem. 24:622–629.
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 29:730–741.
- Bernstein JA, Alexis N, Barnes C, Bernstein IL, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB, Bernstein JA. 2004. Health effects of air pollution. J Allergy Clin Immunol. 114:1116–1123.
- Bewersdorff T, Vonnemann J, Kanik A, Haag R, Haase A. 2017. The influence of surface charge on serum protein interaction and cellular uptake: studies with dendritic polyglycerols and dendritic polyglycerol-coated gold nanoparticles. Int J Nanomed. 12:2001–2019.
- Bohmert L, Laux P, Luch A, Braeuning A, Lampen A. 2017. Nanomaterials in foodstuffs – toxicological properties and risk assessment. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 60:722–727.
- Burden N, Aschberger K, Chaudhry Q, Clift MJ, Fowler P, Johnston H, Landsiedel R, Rowland J, Stone V, Doak SH. 2017. Aligning nanotoxicology with the 3Rs: what is needed to realise the short, medium and long-term opportunities? Regul Toxicol Pharmacol. 91:257–266.
- Buzek J, Ask B. 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off J Eur Union L. 342:59–209.
- Charu D, Ishan P, Himanshu P, Sandip P, Bhushan MS, Pandey AC, Zamboni P, Ramteke PW, Vikram SA. 2018. In vivo diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J Biomed Mater Res A. 106:641–651.
- Dobias J, Bernier-Latmani R. 2013. Silver release from silver nanoparticles in natural waters. Environ Sci Technol. 47:4140–4146.
- Donaldson K, Schinwald A, Murphy F, Cho W-S, Duffin R, Tran L, Poland C. 2013. The biologically effective dose in inhalation nanotoxicology. Acc Chem Res. 46:723–732.
- EFSA Scientific Committee. 2011. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 9:2140.
- George S, Xia T, Rallo R, Zhao Y, Ji Z, Lin S, Wang X, Zhang H, France B, Schoenfeld D, et al. 2011. Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano. 5:1805–1817.
- Gidwani M, Singh AV. 2013. Nanoparticle enabled drug delivery across the blood brain barrier: in vivo and in vitro models, opportunities and challenges. CPB. 14:1201–1212.
- Glover RD, Miller JM, Hutchison JE. 2011. Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano. 5:8950–8957.
- Gonzalez Solveyra E, Szleifer I. 2016. What is the role of curvature on the properties of nanomaterials for biomedical applications? Wires Nanomed Nanobiotechnol. 8:334–354.
- Goodsell DS. 2004. Bionanotechnology: lessons from nature. John Wiley & Sons; 227–311.
- Gottardo S, Alessandrelli M, Amenta V, Atluri R, Barberio G, Bekker C, Bergonzo P, Bleeker E, Booth A, Borges T, et al. 2017. NANoREG framework for the safety assessment of nanomaterials. JRC Sci Pol Rep. 28550:22–76.
- Grove J, Marsh M. 2011. The cell biology of receptor-mediated virus entry. J Cell Biol. 195:1701.
- Guehrs E, Schneider M, Gunther CM, Hessing P, Heitz K, Wittke D, Lopez-Serrano Oliver A, Jakubowski N, Plendl J, Eisebitt S, et al. 2017. Quantification of silver nanoparticle uptake and distribution within individual human macrophages by FIB/SEM slice and view. J Nanobiotechnol. 15:21.
- Hanak JJ. 1970. The “multiple-sample concept” in materials research: synthesis, compositional analysis and testing of entire multicomponent systems. J Mater Sci. 5:964–971.
- Handy RD, Owen R, Valsami-Jones E. 2008. The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology. 17:315–325.
- Hansen TW, DeLaRiva AT, Challa SR, Datye AK. 2013. Sintering of catalytic nanoparticles: particle migration or Ostwald ripening? Acc Chem Res. 46:1720–1730.
- Harper S, Wohlleben W, Doa M, Nowack B, Clancy S, Canady R, Maynard A. 2015. Measuring nanomaterial release from carbon nanotube composites: review of the state of the science. J Phys Conf Ser. 617:012026.
- Hasan J, Crawford RJ, Ivanova EP. 2013. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. 31:295–304.
- Hassan S, Singh AV. 2014. Biophysicochemical perspective of nanoparticle compatibility: a critically ignored parameter in nanomedicine. J Nanosci Nanotechnol. 14:402–414.
- Holden PA, Gardea-Torresdey JL, Klaessig F, Turco RF, Mortimer M, Hund-Rinke K, Cohen Hubal EA, Avery D, Barceló D, Behra R, et al. 2016. Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ Sci Technol. 50:6124–6145.
- Hussain SM, Warheit DB, Ng SP, Comfort KK, Grabinski CM, Braydich-Stolle LK. 2015. At the crossroads of nanotoxicology in vitro: past achievements and current challenges. Toxicol Sci. 147:5–16.
- Joachim C. 2005. To be nano or not to be nano? Nature Mater. 4:107.
- Kaweeteerawat C, Ivask A, Liu R, Zhang H, Chang CH, Low-Kam C, Fischer H, Ji Z, Pokhrel S, Cohen Y, et al. 2015. Toxicity of metal oxide nanoparticles in Escherichia coli correlates with conduction band and hydration energies. Environ Sci Technol. 49:1105–1112.
- Khare M, Singh A, Zamboni P. 2014. Prospect of brain machine interface in motor disabilities: the future support for multiple sclerosis patient to improve quality of life. Ann Med Health Sci Res. 4:305–312.
- Kuempel ED, Jaurand M-C, Møller P, Morimoto Y, Kobayashi N, Pinkerton KE, Sargent LM, Vermeulen RC, Fubini B, Kane AB. 2017. Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit Rev Toxicol. 47:1–58.
- Landsiedel R, Ma-Hock L, Kroll A, Hahn D, Schnekenburger J, Wiench K, Wohlleben W. 2010. Testing metal-oxide nanomaterials for human safety. Adv Mater. 22:2601–2627.
- Laux P, Luch A. 2015. The European landscape of national regulations of tattoo inks and businesses. Tattooed skin and health. Berlin: Karger Publishers; p. 196–200.
- Laux P, Riebeling C, Booth AM, Brain JD, Brunner J, Cerrillo C, Creutzenberg O, Estrela-Lopis I, Gebel T, Johanson G, et al. 2017a. Biokinetics of nanomaterials: the role of biopersistence. NanoImpact. 6:69–80.
- Laux P, Tentschert J, Riebeling C, Braeuning A, Creutzenberg O, Epp A, Fessard V, Haas K-H, Haase A, Hund-Rinke K. 2017b. Nanomaterials: certain aspects of application, risk assessment and risk communication. Arch Toxicol. 92:1–21.
- Laux P, Tralau T, Tentschert J, Blume A, Dahouk SA, Bäumler W, Bernstein E, Bocca B, Alimonti A, Colebrook H, et al. 2016. A medical-toxicological view of tattooing. Lancet. 387:395–402.
- Liu R, Rallo R, George S, Ji Z, Nair S, Nel AE, Cohen Y. 2011. Classification NanoSAR development for cytotoxicity of metal oxide nanoparticles. Small (Weinheim an Der Bergstrasse, Germany). 7:1118–1126.
- Malysheva A, Lombi E, Voelcker NH. 2015. Bridging the divide between human and environmental nanotoxicology. Nature Nanotechnol. 10:835.
- McCray P. 2010. How nanotechnology captured the public imagination. Nature. 467:271.
- Moreno-Horn M, Gebel T. 2014. Granular biodurable nanomaterials: no convincing evidence for systemic toxicity. Crit Rev Toxicol. 44:849–875.
- Murphy CJ, Vartanian AM, Geiger FM, Hamers RJ, Pedersen J, Cui Q, Haynes CL, Carlson EE, Hernandez R, Klaper RD, et al. 2015. Biological responses to engineered nanomaterials: needs for the next decade. ACS Cent Sci. 1:117–123.
- Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, Zhang H. 2013. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc Chem Res. 46:607–621.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. 2009. Understanding biophysicochemical interactions at the nano–bio interface. Nature Mater. 8:543.
- O’Brien MN, Jones MR, Mirkin CA. 2016. The nature and implications of uniformity in the hierarchical organization of nanomaterials. Proc Natl Acad Sci USA. 113:11717–11725.
- Parliament E, Union T. 2006. Regulation (EC) no 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155. EEC 93:67.
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S. 2010. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 269:136–147.
- Potyrailo RA, Mirsky VM. 2008. Combinatorial and high-throughput development of sensing materials: the first 10 years. Chem Rev. 108:770–813.
- Raymond MJ, Ray P, Kaur G, Fredericks M, Singh AV, Wan LQ. 2017. Multiaxial polarity determines individual cellular and nuclear chirality. Cell Mol Bioeng. 10:63–74.
- Recordati C, De Maglie M, Bianchessi S, Argentiere S, Cella C, Mattiello S, Cubadda F, Aureli F, D’Amato M, Raggi A. 2015. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: nano-specific and size-dependent effects. Part Fibre Toxicol. 13:12.
- Regulation E. 2012. No. 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. Off J Eur Union L. 167:181–303.
- Reidy B, Haase A, Luch A, Dawson KA, Lynch I. 2013. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials. 6:2295–2350.
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. 2007. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res/Rev Mutat Res. 636:178–242.
- Rittinghausen S, Hackbarth A, Creutzenberg O, Ernst H, Heinrich U, Leonhardt A, Schaudien D. 2014. The carcinogenic effect of various multi-walled carbon nanotubes (MWCNTs) after intraperitoneal injection in rats. Part Fibre Toxicol. 11:59.
- Sandoval BM. 2009. Perspectives on FDA's regulation of nanotechnology: emerging challenges and potential solutions. Comprehens Rev Food Sci Food Saf. 8:375–393.
- Sarkar S, Leo BF, Carranza C, Chen S, Rivas-Santiago C, Porter AE, Ryan MP, Gow A, Chung KF, Tetley TD, et al. 2015. Modulation of human macrophage responses to mycobacterium tuberculosis by silver nanoparticles of different size and surface modification. PLoS One. 10:e0143077.
- Schmid O, Stoeger T. 2016. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J Aerosol Sci. 99:133–143.
- Sengupta S, Ibele ME, Sen A. 2012. Fantastic voyage: designing self‐powered nanorobots. Angew Chem Int Ed. 51:8434–8445.
- Sharma M, Nikota J, Halappanavar S, Castranova V, Rothen-Rutishauser B, Clippinger AJ. 2016. Predicting pulmonary fibrosis in humans after exposure to multi-walled carbon nanotubes (MWCNTs). Arch Toxicol. 90:1605–1622.
- Singh AV, Gailite L, Vyas V, Lenardi C, Forti S, Matteoli M, Milani P. 2011a. Rapid prototyping of nano- and micro-patterned substrates for the control of cell neuritogenesis by topographic and chemical cues. Mater Sci Eng C. 31:892–899.
- Singh AV, Galluzzi M, Borghi F, Indrieri M, Vyas V, Podestà A, Gade WN. 2013. Interaction of bacterial cells with cluster-assembled nanostructured titania surfaces: an atomic force microscopy study. J Nanosci Nanotechnol. 13:77–85.
- Singh AV, Jahnke T, Kishore V, Park BW, Batuwangala M, Bill J, Sitti M. 2018. Cancer cells biomineralize ionic gold into nanoparticles-microplates via secreting defense proteins with specific gold-binding peptides. Acta Biomater. 71:61–71.
- Singh AV, Mehta KK, Worley K, Dordick JS, Kane RS, Wan LQ. 2014. Carbon nanotube-induced loss of multicellular chirality on micropatterned substrate is mediated by oxidative stress. ACS Nano. 8:2196–2205.
- Singh AV, Patil R, Lenardi C, Paolo M, Gade W. 2010a. Nanobiomaterial applications in tissue repair and ulcer management: a new role for nanomedicine. New York: Nova Publishers; p. 117–141.
- Singh AV, Raymond M, Pace F, Certo A, Zuidema JM, McKay CA, Gilbert RJ, Lu XL, Wan LQ. 2015. Astrocytes increase ATP exocytosis mediated calcium signaling in response to microgroove structures. Sci Rep. 5:7847.
- Singh AV, Subhashree L, Milani P, Gemmati D, Zamboni P. 2010b. Interplay of iron metallobiology, metalloproteinases, and FXIII, and role of their gene variants in venous leg ulcer. Int J Lower Extremity Wounds. 9:166–179.
- Singh AV, Vyas V, Montani E, Cartelli D, Parazzoli D, Oldani A, Zeri G, Orioli E, Gemmati D, Zamboni P. 2012. Investigation of in vitro cytotoxicity of the redox state of ionic iron in neuroblastoma cells. J Neurosci Rural Pract. 3:301.
- Singh AV, Vyas V, Patil R, Sharma V, Scopelliti PE, Bongiorno G, Podestà A, Lenardi C, Gade WN, Milani P. 2011b. Quantitative characterization of the influence of the nanoscale morphology of nanostructured surfaces on bacterial adhesion and biofilm formation. PLoS One. 6:e25029.
- Singh SP, Rathee N, Gupta H, Zamboni P, Singh AV. 2017. Contactless and hassle free real time heart rate measurement with facial video. J Card Crit Care. 1:24–29.
- Sitti M. 2009. Miniature devices: voyage of the microrobots. Nature. 458:1121.
- Valsami-Jones E, Lynch I. 2015. NANOSAFETY. How safe are nanomaterials? Science. 350:388–389.
- Vietti G, Lison D, van den Brule S. 2015. Mechanisms of lung fibrosis induced by carbon nanotubes: towards an adverse outcome pathway (AOP). Part Fibre Toxicol. 13:11.
- Wang X, Swihart MT. 2015. Controlling the size, shape, phase, band gap, and localized surface plasmon resonance of Cu2–xS and CuxInyS nanocrystals. Chem Mater. 27:1786–1791.
- Zeng Q, Shao D, Ji W, Li J, Chen L, Song J. 2014. The nanotoxicity investigation of optical nanoparticles to cultured cells in vitro. Toxicol Rep. 1:137–144.
