Abstract
The oncofetal antigen – immature laminin receptor protein (OFA/iLRP) has been linked to metastatic tumor spread for several years. The present study, in which 2 highly-specific, high-affinity OFA/iLRP-reactive mouse monoclonal antibodies were examined for ability to suppress tumor cell growth and metastatic spread in the A20 B-cell leukemia model and the B16 melanoma model, provides the first direct evidence that targeting OFA/iLRP with exogenous antibodies can have therapeutic benefit. While the antibodies were modestly effective at preventing tumor growth at the primary injection site, both antibodies strongly suppressed end-organ tumor formation following intravenous tumor cell injection. Capacity of anti-OFA/iLRP antibodies to suppress tumor spread through the blood in the leukemia model suggests their use as a therapy for individuals with leukemic disease (either for patients in remission or even as part of an induction therapy). The results also suggest use against metastatic spread with solid tumors.
Abbreviations
| ADC | = | Antibody-drug conjugate |
| ADCC | = | Antibody dependent cellular cytotoxicity |
| ANOVA | = | Analysis of variance |
| BV | = | Benovus |
| CDC | = | Complement dependent cytotoxicity |
| cDNA | = | complementary DNA |
| ELISA | = | Enzyme-linked immunosorbent assay |
| FBS | = | Fetal bovine serum |
| IgG | = | Immunoglobulin G |
| kD | = | kilo Dalton |
| LRP | = | Laminin receptor protein |
| mRNA | = | messenger RNA |
| OFA/iLRP | = | Oncofetal antigen – immature laminin receptor protein |
| RPMI | = | Roswell Park Memorial Institute |
| SCID | = | Severe combined immune deficiency |
Introduction
In 1983, 3 different groups reported the isolation of a 67 kD laminin receptor protein (LRP) on the surface of human and murine tumor cells and a mouse muscle cell line.Citation1-4
Eventually, it was demonstrated that the protein was expressed on a number of different tumor types. Several studies concluded that the protein was over-expressed at both the protein and mRNA levels on malignant tissues and cells as compared to their normal counterparts (reviewed in 5). Expression levels correlated with tumor progression – i.e., increased expression correlated with tumor stage and/or grade and with poorer prognosis.Citation5 While the consensus of the published work was that expression level in cancer tissues and cells was higher, reactivity with normal tissues and cells was acknowledged.
Rao et al Citation6 used a monoclonal antibody to the 67 kD LRP to isolate a full-length cDNA. The encoded protein had a molecular size of 32 kD. The authors assumed that the 32 kD protein was the precursor of the 67 kD mature LRP. Subsequent pulse-chase experiments established the relationship between the 2 entities.Citation7 Eventually, preparatory amounts of the 67 kD protein were isolated and characterized. The protein was found to be covalently bound to fatty acids, presumably membrane components, and had an amino acid profile consistent with it being a dimer of the 32 kD precursor.Citation8 Other studies confirmed acylation with membrane fatty acids, but suggested that the membrane-bound material consisted of a heterodimer between the 32-kD protein and another moiety.Citation9
During this same period in which the LRP was being intensively investigated, Coggin and his colleagues began a series of studies that culminated in the identification of a 37–44 kD protein they termed oncofetal antigen (OFA). This protein was widely expressed through early fetal growth, lost from the cell surface at later stages of development, and then re-expressed in a variety of malignant tumors.Citation10-15 It was eventually confirmed by sequence analysis that the 37 kD protein referred to as OFA and the immature form of the laminin receptor were identical proteins,Citation16 and now commonly referred to as OFA/iLRP.
There is evidence that targeting OFA/iLRP can influence tumor growth and/or metastatic spread. Specifically, it has been shown that dendritic cells primed with OFA/iLRP or transfected with RNA specific to OFA/iLRP can induce a T-cell immune response against OFA/iLRP – positive hematological malignancies.Citation17,18
There is also evidence (albeit indirect) of a humoral response when OFA/iLRP is re-expressed in cancer. Friedrichs et al Citation19–21 detected the presence of pre-existing antibodies to OFA/iLRP in the serum of some leukemia patients. Serum from patients with auto-antibodies to OFA/iLRP was cytotoxic for OFA/iLRP-positive tumor cells in vitro, while serum from subjects without detectable antibody was not. Most importantly, individuals who mounted a humoral response had a more favorable prognosis than those who did not. These findings are consistent with a role for antibody-mediated anti-tumor activity. In spite of this, however, there is still no direct evidence in the scientific literature that targeting OFA/iLRP with exogenous antibody has therapeutic potential. To begin addressing this question, we developed a series of monoclonal antibodies to a recombinant form of OFA/iLRP and characterized these antibodies with respect to in vivo activities in 2 tumor models. Anti-metastatic activity was observed in both the A20 B-cell leukemia model and the B16 melanoma model.
Results
Characteristics of anti-OFA/iLRP antibodies
Affinity data for 5 anti-OFA/iLRP monoclonal antibodies are shown in . All five antibodies demonstrate Kd values in the picomolar range. demonstrates reactivity of the same 5 monoclonal antibodies against epitopes in overlapping 12-amino acid peptides from the C-terminal region of the OFA/iLRP molecule. It can be seen that BV-15 reacts with a peptide sequence that is distinct from the peptide sequence in which epitopes for BV-27, BV-06 and BV-12 are located. It can also be seen from the figure, that unlike each of the other antibodies, BV-19 demonstrates reactivity with multiple distinct amino acid sequences.
Figure 1. (A) Binding of anti-OFA/iLRP antibodies to 12-amino acid peptides from OFA/iLRP. The overlapping 12-AA sequences are shown at left and antibody reactivity by ELISA is shown in the bar graphs to the right. (B) Interaction of anti-OFA/iLRP monoclonal antibodies with cells by confocal fluorescence microscopy. Top row: HCC38 triple-negative human breast carcinoma; Middle row: human epidermal keratinocytes; Bottom row: human dermal fibroblasts. Cells were fixed in 2% paraformaldehyde but not permeabilized prior to addition of the primary antibody. (C) Interaction of anti-OFA/iLRP monoclonal antibodies with cell lysates by protein gel blotting (lower panels). In each panel: Lane a: K562; Lane b: HL60; Lane c: HCC38 Breast carcinoma; Lane d: Normal fibroblasts; Lane e: Normal keratinocytes; Lane f: Immortalized keratinocytes (HaCat).
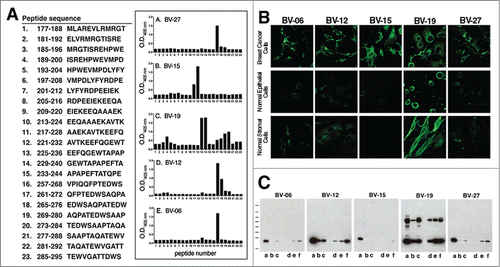
Table 1. Affinity values of 5 anti-OFA/iLRP monoclonal antibodies
Confocal immunofluorescence microscopy was used to assess antibody binding by HCC38 (human breast cancer cells) as well as normal human epidermal keratinocytes and dermal fibroblasts. All five antibodies react with the tumor cells (, upper panels), but of the 5 antibodies, only BV-19 demonstrates strong staining of normal keratinocytes and fibroblasts (, middle and lower panels). In additional studies (not shown), a number of other human tumor cell lines were also shown to react with these antibodies. Among these were K562 and HL60 (myeloid leukemia), HCC1937, MCF-7 and MDA-MB-468 (breast carcinoma), NCI-N87 and SNU-5 (gastric carcinoma), SNU-398 and Hep3B2.1-7 (hepatocellular carcinoma) and OE33 (esophageal adenocarcinoma).
Western blotting was used to characterize antibody reactivity with cell lysates. All five antibodies react strongly with moieties in the 37 kD region of the gel while only BV-19 shows reactivity with moieties in both the 37 kD and 67 kD regions (). Based on these findings (i.e, high-affinity, specificity for tumor cells, reactivity with 37 kD OFA/iLRP but not 67 kD mature LRP and non-overlapping epitopes on the OFA/iLRP moledule), 2 antibodies – i.e., BV-15 and BV-27 – were chosen for in vivo experiments.
In vivo anti-tumor activity in the A20 (B cell leukemia) model
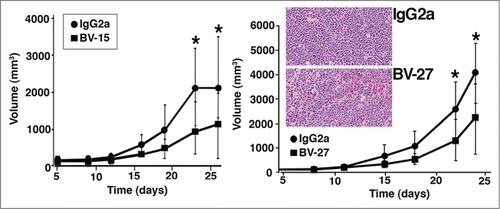
In the first series of studies, BV-15 and BV-27 were assessed for capacity to suppress growth of intramuscular A20 tumor growth in syngeneic (BALB/c) mice. Both antibodies partially inhibited primary tumor growth (). When tumor tissue obtained at the time of sacrifice was examined histologically, tissue sections from both control antibody-treated mice and mice treated with either anti-OFA/iLRP antibody were characterized as sheets of undifferentiated tumor cells with areas of necrosis (, insert). Invasion of tumor cells into the surrounding fascia at the margins was observed in mice treated with IgG2a or either of the therapeutic antibodies. Other than size, there was nothing that distinguished tumors from control and treated animals.
Figure 2. Effects of anti-OFA/iLRP antibodies on Primary A20 tumor growth. Syngeneic BALB/c mice were treated with monoclonal antibody BV-15 vs. IgG2a (n = 15 and n = 12, respectively) and BV-27 versus IgG2a (n = 15 and n = 14, respectively). Values are tumor volume means and standard deviations based on diameter measurements in 2 dimensions. Asterisks denote significance from control at the P < 0.05 level, based on Student t-test. Insert. Histological features of A20 tumor in syngeneic BALB/c mice treated with control IgG2a or BV-27. No significant difference in tumor appearance is noted (Hematoxylin and eosin – stained sections, magnification 66x).
demonstrates effects of BV-15 and BV-27 on liver tumor formation following intravenous tumor cell injection. Both BV-15 and BV-27 antibody decreased liver tumor numbers relative to control IgG2a. Tumor size was also reduced by treatment with either of the 2 antibodies (not shown), although the size of individual liver tumors varied widely in all treatment groups. When examined histologically after staining with hematoxylin and eosin ( insert, upper panels), liver tumors were seen to consist of discrete tumor nodules (arrows) surrounded by healthy liver tissue. Invasion into the surrounding liver tissue was apparent in places, and infiltrating leukocytes could be seen in the tumor sections. Staining with antibodies to T-cells, macrophage/dendritic cells and granulocytes was done in order to identify the infiltrating white blood cells observed in the hematoxylin and eosin-stained sections. There were few granulocytes in any of the sections, but T-cells and macrophage/dendritic cells were present in large numbers. T-cells were seen scattered throughout the tumor ( insert, middle panels) while most of the macrophages/dendritic cells were at the boundary between the tumor cells and the surrounding normal liver tissue (, insert, lower panels). There appeared to be no major difference between tumors from control and antibody-treated mice in regard to either the type of infiltrating cell, pattern of distribution or relative numbers.
Figure 3. Effects of anti-OFA/iLRP antibodies on A20 tumor growth following intravenous tumor cell injection. (A) Liver tumor formation in BALB/c mice injected with A20 cells by the intravenous route and treated with BV-15 vs. IgG2a (n = 15 and n = 14, respectively) and BV-27 versus IgG2a (n = 13 and n = 12, respectively). Values shown are means and standard deviations. Asterisks denote significance from control at the P < 0.05 level based on Student t-test. Insert. Histological and immuno-histological features of liver tumors in BALB/c mice treated with IgG2a or BV-27. Upper panels; Hematoxylin and eosin stained: Tumors are well-defined nodules surrounded by normal liver tissue (arrows). Middle panels: T-cell infiltrate: Tumors from both groups demonstrate infiltrating T-cells in comparable numbers. Lower panels: Macrophages/dendritic cells: These cells are present in liver tissue from both groups, mostly confined to the border between the tumor and the normal tissue surrounding the tumor (arrows). Magnification: Upper panels and lower panels, 66x; middle panels, 130x. (B) Blood tumor levels in BALB/c mice treated with BV-15 (n = 15) and BV-27 (n = 13) vs. IgG2a (n = 55) animals per group. Bars represent the percentage of mice in each group with the blood tumor level shown on the x-axis. Therapeutic antibody and control IgG2a were compared by the Fisher Exact Test for statistical significance. Results with both therapeutic antibodies were statistically different from control at P < 0.001.
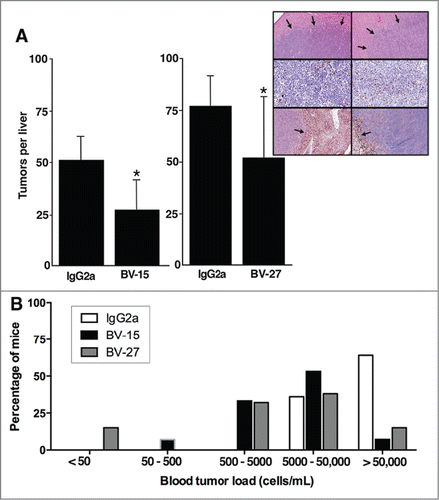
Tumor cell levels in blood obtained from individual mice at the time of sacrifice are shown in . Shown along the x-axis is the blood tumor level based on the limiting dilution endpoint. The percentage of mice is shown in the y-axis. Of the 55 animals receiving control antibody (open bars), 35 animals had >50,000 cells per mL (64%) and the remaining animals had 5000 – 50,000 cells per mL. In contrast, of the mice receiving either of the 2 therapeutic antibodies, in only 3 of 28 mice (11%) did we recover >50,000 viable tumor cells per mL of blood and in 10 of 28 mice (36%) there were 5000–50,000 viable cells. The remaining mice were distributed as follows: 9 of 28 (32%) between 500 and 5000, 1 of 28 (4%) at 50–500 and 2 of 28 (8%) with <50 cells per mL of blood. Each antibody was compared separately to control IgG2a using Fisher's Exact Test and found to be statistically significant (P < 0.001). The two mice in the BV-27 – treatment group with blood tumor levels of less than 50 cells per mL represent mice from which no tumor colonies were established at the highest concentration of blood analyzed (20 μL in 1 mL). These two mice also had no grossly detectable tumor nodules in the liver and no evidence of liver tumors upon microscopic analysis.
In vitro anti-tumor activity in the A20 (B cell leukemia) model
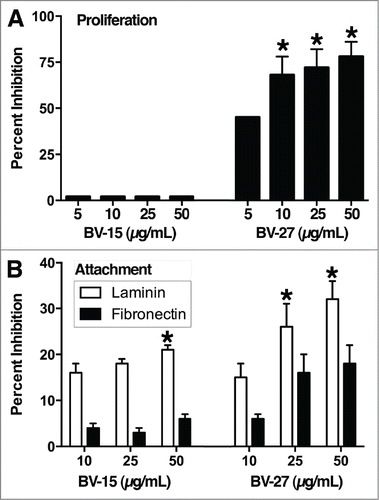
compares the effects of BV-15 and BV-27 on in vitro growth of A20 cells. BV-27 was growth suppressive over the range of antibody concentrations from 5 to 50 μg per mL. Maximal inhibition was approximately 75%. In contrast, BV-15 had no significant effect on A20 proliferation in vitro at concentrations up to 50 μg/mL (highest concentration evaluated). In parallel, the 2 antibodies were examined for effects on A20 attachment to laminin and fibronectin. Both antibodies reduced cell attachment to laminin (). In contrast, neither antibody reduced attachment to fibronectin (not statistically different from control). IgG2a did not affect A20 cell proliferation or attachment to either substrate at concentrations as high as 50 μg per mL (not shown).
Figure 4. Effects of anti-OFA/iLRP antibodies on A20 cells in vitro. (A) In vitro growth of A20 cells: Effects of BV-15 and BV-27 versus IgG2a. Values shown are means and standard errors (percent inhibition of growth) based on n = 4 separate studies with duplicate samples per data point. Asterisks denote significance from control at P < 0.05, determined by ANOVA followed by paired group comparisons. (B) Attachment to laminin and fibronectin. Values shown are means and standard errors (percent inhibition of attachment). Laminin data are based on n = 5 separate experiments with duplicate samples per data point. Results with fibronectin are based on n = 4 experiments with duplicate samples per data point. Asterisks indicate statistical difference from control at P < 0.05 based on ANOVA followed by paired group comparison.
In vivo anti-tumor activity in the B16 (melanoma) model
In an effort to demonstrate that findings with A20 cells were not unique to one particular tumor, studies were conducted in which BV-15 and BV-27 were examined for effects on B16 melanoma growth in syngeneic (C57BL/6) mice. Results from the primary tumor growth studies are shown in . In the primary tumor growth model, BV-27 reduced tumor growth modestly (approximately 22% reduction in volume at day-24). As was the case with A20 leukemic cells, histological examination of primary B16 melanoma tumors revealed no apparent differences between IgG2a control- and anti-OFA/iLRP antibody-treated mice (not shown).
Figure 5. Effects of anti-OFA/iLRP antibodies on B16 melanoma tumor growth in vivo. (A) Primary B16 melanoma tumor volume in syngeneic C57BL/6 mice treated with monoclonal antibody BV-15 and BV-27 vs. IgG2a (n = 9, n = 10 and n = 9 mice, respectively). Values are tumor volume means and standard deviations based on diameter measurements in 2 dimensions. (B) Lung tumor formation in C57BL/6 mice injected with B16 melanoma cells by the intravenous route and treated with BV-15 and BV27 versus IgG2a (n = 15, n = 14 and n = 13 mice, respectively). Values shown are means and standard deviations. Asterisks denote significance from control at the P < 0.05 level, based on ANOVA followed by paired-group comparisons. Insert: Hematoxylin and eosin stained lung tissue: Tumors were well-defined nodules on the lung surface or within the parenchyma (arrows) (Magnification, 66x).
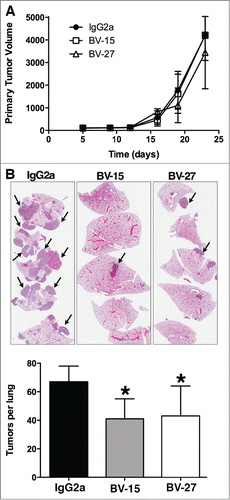
Both antibodies suppressed lung colony formation after intravenous tumor cell injection. The average number of lung tumors per mouse was reduced by either BV-15 or BV-27 relative to control (). In addition, the average size of the tumors was also reduced. In the IgG2a – treated mice, the average tumor size was approximately 1.77 + 1.33 mm (diameter) compared to 1.03 + 0.64 mm and 1.08 + 0.21 mm for animals treated with BV-15 and BV-27, respectively. The differences in average lung tumor size primarily reflected a reduction in the number of larger (greater than 3 mm) tumors in the antibody-treated mice.
Discussion
OFA/iLRP is expressed on a variety of tumor cells, and studies going back to the 1980s have linked expression with metastatic potential.Citation5 Until now, however, there has been no direct evidence to show that targeting this tumor cell surface protein with monoclonal antibodies has therapeutic potential. In the present study, we show that 2 different murine monoclonal antibodies (reactive with 2 different epitopes in the extracellular region of the OFA/iLRP molecule) have anti-tumor efficacy in a highly aggressive, syngeneic, murine model of B-cell leukemia. In this study, both high-affinity IgG antibodies modestly suppressed primary tumor growth. More importantly, both antibodies strongly reduced liver colonization following intravenous injection of the A20 cells. Inhibition of liver tumor formation was associated with clearing of tumor cells from the blood. In addition to studies conducted with a B-cell leukemia line, additional experiments demonstrated that efficacy was not limited to this tumor. In studies with highly aggressive B16 melanoma, both BV-15 and BV-27 suppressed metastasis formation in the lungs. These data provide proof-of-concept that OFA/iLRP can be used as a target for anti-cancer therapy with the appropriate monoclonal antibody. While tumor growth was not completely suppressed with either antibody (except in the case of 2 animals receiving BV-27 in the intravenous model), the conditions of treatment (6 injections with 100 μg of antibody per mouse) were far below levels that can be employed clinically.
Chronic (B cell) lymphocytic leukemia may go untreated for years, but when the disease progresses into an acute form, it has to be treated aggressively. Chemotherapeutic agents and small molecule inhibitors or antibodies targeted to specific signaling pathways (often in combination) are most often used,Citation22,23 as they are in other forms of acute leukemic disease.Citation24-28 A monoclonal antibody that reduces circulating tumor cells could find clinical utility, e.g., as an adjuvant therapy in leukemia/lymphoma patients during acute disease. Alternatively, such an antibody might find use in a regimen for preventing tumor recurrence with patients in remission. Controlled clinical studies will be needed to determine how, and to what extent, BV-15 and BV-27 might be useful in treatment of leukemic disease. While the in vivo studies described here made use of a single line of B-cell leukemia, other B-cell lines, as well as cell lines originating from T-cells and myeloid cells, express surface OFA/iLRP.Citation18 Possible utility against blood-borne spread of solid tumors is also suggested. It is of interest in regard to these animal studies that murine and human OFA/iLRP protein sequences are virtually identical.Citation16 Therefore, the in vivo findings in the syngeneic mouse models are likely to be more predictive of what will be seen in humans than is typically the case where the mouse and human proteins are homologous but structurally and antigenically distinct.
Mechanism(s) underlying efficacy in the animal models is/are not understood. For instance, we observed no significant histological differences in either primary or metastatic tumors that would suggest a mechanism. Furthermore, when liver tumor sections (A20 model) were examined for the presence of infiltrating leukocytes, tumors from control mice (IgG2a – treated) and mice treated with either anti-OFA/iLRP antibody had T cell infiltrates, but the level of infiltrating cells did not appear to be significantly different. Likewise, large numbers of macrophages/dendritic cells were seen at the boundary between liver tumor nodules and normal liver tissue but again, there was no significant difference between livers from IgG2a control and therapeutic antibody-treated animals. In another set of tissue slides (not shown), we stained for activated caspase-3 in both the primary A20 tumors and the liver metastases. Positive (apoptotic) cells were observed scattered throughout the tumor but mice treated with the control antibody and mice treated with either of the therapeutic reagents were not distinguishable in this respect.
In vitro studies also provide no clear understanding of mechanism. A direct growth suppressive effect is not likely to be critical since only one of the 2 antibodies (BV-27) was growth-suppressive in vitro. Alternatively, both antibodies suppressed A20 cell attachment to components of the extracellular matrix especially laminin. Inhibition of laminin attachment is consistent with what has been reported previously with antibodies that targets both the immature and mature forms of the laminin receptor protein Citation29–31 or with treatments that down-regulate expression of the 37 kD/67 kD laminin receptor.Citation30 If such inhibition occurred in the circulation, it might delay tumor cell egress from the blood stream and as a consequence, lead to more efficient intravascular cell killing (by whatever mechanisms).
As BV-15 and BV-27 are mouse IgG2a antibodies, upon binding to tumor cells expressing sufficient levels of plasma membrane-associated OFA/iLRP, both are likely to elicit antibody-dependent cellular cytotoxicity (ADCC) and/or complement-dependent cytotoxicity (CDC). Immune cell-mediated killing of laminin receptor-positive cells in vitro has been described,Citation32 so it is not unreasonable to suggest that antibody-dependent immune cell killing of the tumor cells (with or without CDC) will occur in the circulation, and contribute to the decrease in blood tumor cell levels observed with either of the 2 antibodies. If immune cell killing is a major contributor to the reduced circulating tumor burden, very different results might be observed if in vivo studies of the type conducted here were repeated in immune-compromised animals. Ideally, such studies would make use of beige-SCID mice on a BALB/c background. While beige-SCID mice are commercially available, they are from an unrelated strain. Thus, even a strong response to the antibodies in these mice would not definitively rule out some contribution from the residual host immune system. In addition to carrying out studies in fully immune-compromised mice, studies to assess cell killing in vitro could also be done. Such studies, utilizing unfractionated cells initially and targeted immune populations later (with and without added complement) could help to distinguish between a direct anti-tumor effect versus host immune contribution. In vitro studies on their own, however, are not likely to be definitive, since in vitro anti-tumor activity might not reflect events occurring in vivo.
Nonetheless, even if the mechanism(s) of anti-tumor activity with the naked antibodies were fully understood, the information might not be relevant to how the antibodies are used therapeutically. There are multiple ways in which such antibodies could be used. Currently, there is much interest in antibody – drug conjugates (ADC). As part of our characterization studies, we found that both BV-15 and BV-27 were capable of being internalized after binding to the target cells, and that antibody binding could carry a toxin into the cells (Fig. S1 and Table S1). The demonstrated antibody mediated antigen internalization activity in conjunction with high affinity and tumor specificity makes the ADC therapeutic modality a potential option for both BV-15 and BV-27. As an ADC, both anti-OFA/iLRP antibodies would retain their intrinsic anti-tumor activity through effector function including antibody-dependent cellular cytotoxicity ADCC) and complement-dependent cytotoxicity (CDC) as well as through their respective modes of action, including inhibition of laminin binding, and reduced tumor cell proliferation (BV-27 only). Even further, the finding that BV-15 and BV-27 bind to different, distinct epitopes on the OFA/iLRP molecule () raises the potential to use the 2 antibodies in combination.
Significantly, both BV-15 and BV-27 have specificity for the 37 kD OFA/iLRP – i.e., they do not demonstrably react with the related 67 kD mature LRP (). Consistent with this, neither of the 2 antibodies demonstrated significant reactivity with either normal human epithelial cells or fibroblasts (). This specificity is important because although the 67 kD mature laminin receptor is overexpressed on a variety of tumor cells, it is also expressed on normal cells.Citation5 Reactivity with only the immature form of the receptor would likely provide the requisite measure of selectivity for development as an anti-cancer therapeutic. This is especially important in the context of ADCs.
In summary, OFA/iLRP and the related mature LRP have been associated with malignant disease since they were first described in the 1980s. While anti-tumor activity has been previously demonstrated with a T-cell response to OFA/iLRP, the current study provides the first direct evidence that monoclonal antibodies specific for OFA/iLRP can be utilized to suppress tumor growth and spread. Use as an adjuvant therapy for elimination of OFA/iLRP – positive cells from the blood is yet another potential way in which such a therapeutic may be employed.
Materials and Methods
Mouse monoclonal antibody characteristics
A series of mouse monoclonal antibodies were produced against a recombinant form of OFA/iLRP Citation33 and 5 were chosen for study. Four of the 5 antibodies (designated as BV-06, BV-12, BV-15 and BV-27) were characterized as IgG2a with the fifth antibody (BV-19) identified as IgG2b. The antibodies were screened for reactivity with recombinant OFA/iLRP using a direct ELISA.Citation34 The antibodies were also screened against both the recombinant OFA/iLRP molecule and a recombinant 100-amino acid peptide from the C-terminal end of the OFA/iLRP molecule (Abcam; ab112316) by a surface plasmon resonance biosensor (biacore).Citation35
A series of overlapping 12-amino acid peptides covering the C-terminal region of OFA/iLRP was used to assess epitope specificity. Antibody binding to individual peptides was assessed using a direct ELISA optimized for binding to small peptides as target.Citation36 Briefly, in this procedure, poly-L-lysine was coated onto the surface of the plate and activated with glutaraldehyde. Individual peptides were then added and bound to the activated poly-L-lysine. From this point on, the ELISA was carried out as a direct ELISA.Citation34
Using a combination of flow cytometry, confocal fluorescence microscopy and western blotting, the 5 antibodies were assessed for binding to a wide range of human tumor cell lines. These included K562 and HL60 (myeloid leukemia), HCC38, HCC1937, MCF-7 and MDA-MB-468 (breast carcinoma), NCI-N87 and SNU-5 (gastric carcinoma), SNU-398 and Hep3B2.1-7 (hepatocellular carcinoma) and OE33 (esophageal adenocarcinoma). The tumor cell lines were obtained from the American Type Culture Collection or (OE33) from the European Collection of Cell Cultures. Human epidermal keratinocytes and human dermal fibroblasts served as controls. These cells were prepared freshly as needed from human foreskin tissue (under an exemption from IRB oversight) and used in first-passage culture as described previously.Citation37
Assessment of anti-tumor activity: Primary tumor model
Two of the high affinity antibodies with non-overlapping peptide specificities (BV-15 and BV-27) were chosen for assessment of anti-tumor activity in 2 syngeneic murine tumor models. The murine tumor cell lines (A20 B-cell leukemia and B16 melanoma) were obtained from the ATCC. The A20 cells were maintained in suspension culture using RPMI-1640 culture medium supplemented with 10% fetal bovine serum (FBS) and 0.05 mM 2-mercaptoethanol. Anchorage-dependent B16 cells were grown using the same culture medium as A20 (but without 2-mercaptoethanol). Growth of both cell types was at 37°C in an atmosphere of 95% air and 5% CO2. Past studies have demonstrated that the murine cells used here are positive for OFA/iLRP expression by immunostaining and Western blotting,Citation11 and the present studies confirmed this.
Using a dose of A20 cells that was found in preliminary, range-finding studies to produce tumors in virtually 100% of the injected mice (5 × 105 cells per BALB/c mouse), we carried out tumor growth inhibition studies with BV-15 and BV-27. Mice were injected with tumor cells in log-phase growth by the intra-muscular route on day-zero. Tumor-bearing mice were then treated with each of BV-15 and BV-27 or with isotype-matched (IgG2a) control antibody. One hundred micrograms of antibody per mouse (approximately 4 mg/kg) was injected via the intra-peritoneal route on days 1, 4, 8, 11, 15 and 18 after tumor injection. Tumor size was measured twice weekly and the animals were euthanized on day-25. Tumors with surrounding normal tissue were fixed in 10% buffered formalin and examined histologically after staining with hematoxylin and eosin.
Anti-tumor activity against B16 melanoma was determined in the same manner except that C57BL/6 (syngeneic) mice were used and 1 × 104 cells per mouse was given as inoculum (based on preliminary range-finding studies).
Assessment of anti-tumor activity: Intravenous tumor model
For the intravenous tumor model, BALB/c mice were injected with 5 × 105 A20 cells and C57BL/6 mice were injected with 1 × 104 B16 melanoma cells via the tail vein on day-zero. The treatment schedule consisted of one hundred micrograms of antibody per mouse injected via the intra-peritoneal route on days -1 (one day before tumor injection) and days 1, 5, 8, 12 and 14 after tumor injection. In the intravenous tumor studies, animals were monitored daily for signs of illness and euthanized on day-25 or earlier when signs of illness became apparent (which occurred in 2 control BALB/c mice which were injected with A20 tumor cells).
For the A20 model, livers were removed from animals at euthanasia and examined grossly for the presence of tumors. After formalin-fixation and separation of the 6 liver lobes, the number of tumors per liver was determined by direct counting. Following this, tissue was processed for histology. Blood was obtained via heart puncture at the time of euthanasia. Twenty microliters of blood was immediately mixed with 1 mL of culture medium (RPMI-1640 medium with 10% FBS). Four 10-fold serial dilutions were prepared and wells of a 24-well culture dish were seeded with 1 mL of each dilution. The blood - cell culture medium was incubated at 37°C in an atmosphere of 95% air and 5% CO2. Over the ensuing 2-week period, the cultures were examined microscopically for viable tumor cells, which were established by the formation of a continuously growing/expanding (suspension) culture of A20 tumor cells. Cultures that were sterile after 2-weeks were discarded and scored as containing <50 viable tumor cells per mL. Correspondingly, blood samples were determined to contain 50–500, 500–5000, 5000–50,000 and >50,000 viable cells per mL depending on the limiting dilution from which viable cells were recovered.
B16 melanoma studies were conducted in a similar fashion except that since virtually all of the metastatic tumors were in the lung rather than liver, we counted lung metastatic nodules rather than liver nodules. For this, lungs were formalin-fixed and lobes of individual lungs separated. Individual tumor nodules were then counted and the tissue processed for histology.
All of the procedures involving vertebrate animals were approved by the Committee on Use and Care of Animals.
Immunohistology
Formalin-fixed tissue from tumor-bearing mice was stained by the immunoperoxidase method for presence of T-cells, mononuclear cells and granulocytes. Antibodies used included the following: anti-CD3 as a T-cell marker (#RM9107-s; Thermo Scientific); antibody to F480 as a macrophage/dendritic cell marker (ab6640, Abcam) and antibody to the neutrophil protein Nimp14 for granulocytes (NKp46, Abcam). Evaluation was performed using an Olympus BX45 light microscope at total magnifications ranging from x40 to x600.
In vitro proliferation assay
A20 cells were examined for in vitro proliferation under control conditions and in the presence of each antibody. For these studies, 5 × 104 cells were plated in 0.5 mL of growth medium (RPMI-1640 medium with 10% FBS) in wells of a 24-well dish. Antibodies were added to the wells at desired concentrations. Cells were then incubated at 37°C in an atmosphere of 95% air and 5% CO2 for 2 d and then counted.
Cell attachment to laminin and fibronectin
Laminin from the EHS sarcoma was obtained from Sigma (L2020) and human plasma fibronectin was obtained from Life Technologies (cat # 33016-015. Wells of a 24-well dish were incubated overnight with murine laminin (0.25 μg per well) or fibronectin (1.0 μg per well) diluted in phosphate-buffered saline. The next day, after washing to remove unbound protein, A20 cells (4 × 104 per well) were added to each well in serum-free RPMI-1640 culture medium containing 200 μg per mL bovine serum albumin. Non-attached cells were harvested and counted after incubation for 4 hours at 37°C in an atmosphere of 95% air and 5% CO2. From this, the percentage of cells that attached under each condition was calculated.
Disclosure of Potential Conflicts of Interest
ACM, JS, KJJ and JV have a proprietary interest in the reagents under study.
Supplemental Information and Figure.zip
Download Zip (1.3 MB)Funding
This study was supported in part by Benovus Bio (Atlanta, GA).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Malinoff HL, Wicha MS. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol 1983 96:1475-9; PMID:6302102; http://dx.doi.org/10.1083/jcb.96.5.1475
- Rao NC, Barsky SH, Terranova VP, Liotta LA. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun 1983 111:804-8; PMID:6301485; http://dx.doi.org/10.1016/0006-291X(83)91370-0
- Lesot H, Kuhl U, Mark KV. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J 1983 2:861-65; PMID:16453457
- Terranova VP, Rao CN, Kalebic T, Margulies IM, Liotta LA. Laminin receptor on human breast carcinoma cells. Proc Nat Acad Sci U S A 1983 80:444-8.
- Menard S, Tagliabue E, Colnaghi MI. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treatment 1998 52:137-45; PMID:10066078; http://dx.doi.org/10.1023/A:1006171403765
- Rao CN, Castronovo V, Schmitt MD, Wewer UM, Claysmith AP, Liotta LA, Sobel ME. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry 1989 28:7476-86; PMID:2531008; http://dx.doi.org/10.1021/bi00444a047
- Castronovo V, Taraboletti G, Sobel ME. Functional domains of the 67-kDa laminin receptor precurson. J Biol Chem 1991 266:20440-6; PMID:1834645
- Landowski TH, Dratz EA, Starkey JR. Studies of the structure of the metastasis-associated 67 kDa laminin binding protein: fatty acid acylation and evidence supporting dimerization of the 32 kDa gene product to form the mature protein. Biochemistry 1995 34:11276-87; PMID:7669786; http://dx.doi.org/10.1021/bi00035a037
- Buto S, Tagliabue E, Ardini E, Magnifico A, Ghirelli C, van den Brule F, Castronovo V, Colnaghi MI, Sobel ME, Ménard S. Formation of the 67-kDa laminin receptor by acylation of the precursor. J. Cell Biochem 1998 69:244-51; PMID:9581863; http://dx.doi.org/10.1002/(SICI)1097-4644(19980601)69:3%3c244::AID-JCB2%3e3.0.CO;2-R
- Coggin JH Jr, Rohrer SD, Leinbach ED, Hester RB, Liu PI, Heath LS. Radiation-induced lymphoblastic lymphomas/leukemias and sarcomas of mice express conserved immunogenic 44-kilodalton oncofetal antigen. Amer. Pathol 1988 130:136-46; PMID:3337209
- Payne WJ, Jr. Coggin JH, Jr. Mouse monoclonal antibody to embryonic antigen: Development, cross-reactivity with rodent and human tumors, and preliminary polypeptide characterization. JNCI 1985 75:527-44; PMID:2411994
- Barsoum AL, Coggin JH, Jr. Isolation and partial characterization of a soluble oncofetal antigen from murine and human amniotic fluids. Int J Cancer 1991 48:248-52; PMID:1850386; http://dx.doi.org/10.1002/ijc.2910480216
- Barsoum AL, Coggin JH, Jr. Immunogenicity of a soluble, partially purified oncofetal antigen from murine fibrosarcoma in syngeneic mice. J Biol Response Mod 1989 8:579-92; PMID:2600602
- Gussack GS, Rohrer SD, Hester RB, Barsoum A, Coggin JH, Jr. Human squamous cell carcinoma lines express oncogetal 44-kd polypeptide defined by monoclonal antibody to mouse fetus. Cancer 1988 62:283-90; PMID:3289728; http://dx.doi.org/10.1002/1097-0142(19880715)62:2%3c283::AID-CNCR2820620210%3e3.0.CO;2-O
- Gussack GS, Harris W, Rohrer S, Hester R, Liu P, Coggin JH, Jr. Comparison of cellular and antigenic tumor characteristics between primary head and neck carcinomas and their derived tumor cell lines. In: Head and Neck Oncology Research, Taylor & Francis, Amsterdam, p. 121-31 (1988).
- Coggin JH, Jr, Barsoum AL, Rohrer JW. 37 kiloDalton oncofetal antigen protein and immature laminin receptor protein are identical, universal T-cell inducing immunogens on primary rodent and human cancers. Anticancer Res 1999 19:5532-42
- Rohrer JW, Barsoum AL, Coggin JH, Jr. Identification of oncofetal antigen/immature laminin receptor protein epitopes that activate BALB/c mouse OFA/iLRP-specific effector and regulatory T cell clones. J Immunol 2006 176:2844-56; PMID:16493041; http://dx.doi.org/10.4049/jimmunol.176.5.2844
- Siegel S, Wagner A, Kabelitz D, Marget M, Coggin JH, Jr., Barsoum A, Rohrer J, Schmitz N, Zeis M. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood 2003 102:4416-23; PMID:12869512; http://dx.doi.org/10.1182/blood-2003-01-0198
- Friedrichs B, Siegel S, Kloess M, Barsoum A, Coggin J, Jr., Rohrer J, Jakob I, Tiemann M, Heidorn K, Schulte C, et al. Humoral immune responses against immature laminin receptor protein show prognostic significance in patients with chronic lymphocytic leukemia. J Immunol 2008 180:6374-84; PMID:18424761; http://dx.doi.org/10.4049/jimmunol.180.9.6374
- Siegel S, Friedrichs B, Budde AK, Barsoum A, Coggin J Jr, Tiemann M, Kabelitz D, Zeis M.. In-vivo detectable antibodies directed against the oncofetal antigen/immature laminin receptor can recognize and control myeloma cells - clinical implications. Leukemia 2008 22:2115-8
- Friedrichs B, Siegel S, Reimer R, Barsoum A, Coggin J, Jr., Kabeitz D, Heidorn K, Schulte C, Schmitz N, Zeis M. High expression of the immature laminin receptor protein correlates with mutated IGVH status and predicts a favorable prognosis in chronic lymphocytic leukemia. Leukemia Res 2011 35:721-9; PMID:21055809; http://dx.doi.org/10.1016/j.leukres.2010.10.002
- Kanti RR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, Hines J, Threatte GA, Larson RA, Cheson BD, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. New Eng J Med 2000 343:1750-7; PMID:11114313
- Coiffier B, Lepdretre S, Pdersen LM, Gadeberg O, Fredriksen H, van Oers MH, Wooldridge J, Kloczko J, Holowiecki J, Hellmann A, et al. Safety and efficacy of ofatumumat, a fully human monoclonal anti-CD20 antibody in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood 2008 111:1094-100; PMID:18003886; http://dx.doi.org/10.1182/blood-2007-09-111781
- Hehlmann R, Berger U, Pfirmann M, Heimpel H, Hochhaus A, Hasford J. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood 2007 109:4686-92; PMID:17317858; http://dx.doi.org/10.1182/blood-2006-11-055186
- Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbauum F. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European Leukemia Network. Blood 2006 108:1809-20; PMID:16709930; http://dx.doi.org/10.1182/blood-2006-02-005686
- Hehlmann R, Saussele. Treatment of chronic myeloid leukemia in blast crisis. Haematologica 2008 93:1765-9; PMID:19050065; http://dx.doi.org/10.3324/haematol.2008.001214
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCL-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with Philadelphia chromosome. New Eng J Med 2001 344:1038-42; PMID:11287973; http://dx.doi.org/10.1056/NEJM200104053441402
- Hallek M, Fischer K, Fingerle-Rowson G. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukemia: a randomised, open-label, phase 3 trial. Lancet 2010 376:1164-74; PMID:20888994; http://dx.doi.org/10.1016/S0140-6736(10)61381-5
- Omar A, Reusch U, Knackmuss S, little M, Weiss SFT. Anti-LRP/LR - specific antibody IgG1-iS18 significantly reduces adhesion and invasion of metastatic lung, cervix, colon and prostate cancer cells. J Molec Biol 2012 419:102-9; PMID:22391421; http://dx.doi.org/10.1016/j.jmb.2012.02.035
- Zuber C, Knackmuss S, Zemora G, Reusch U, Vlasova E, Diehl D, Mick V, Hoffmann K, Nikles D, Frohlich T, Arnold GJ, Brenig B, Wolf E, Lahm H, et al. Invasion of tumorigenic HT1080 cells is impeded by blocking or down-regulating the 37-kDa/67-kDa laminin receptor. J Molec Biol 2008 378:530-9; PMID:18387633; http://dx.doi.org/10.1016/j.jmb.2008.02.004
- Khalfaoul T, Grouix J-F, Sabra G, GuezGuez A, Basora N, Vermette P, Beaulieu J-F. laminin receptor 37/67LR regulates adhesion and proliferation of normal human intestinal cells. Plos One 2013 8:e74337; PMID:23991217; http://dx.doi.org/10.1371/journal.pone.0074337
- Ferrarini M, Heltai S, Pupa SM, Menard S, Zocchi MR. Killing of laminin receptor-positive human lung cancers by tumor-infiltrating lymphocytes bearing gd T-cell receptors. J Nat Cancer Inst 1996 88: 436-41; PMID:8618235; http://dx.doi.org/10.1093/jnci/88.7.436
- Barsoum A, Liu B, Rohrer JW, Coggin JH, Jr., Tucker JA, Pannell LK, Schwarzenberger PO. Production, safety and antitumor efficacy of recombinant Oncofetal Antigen/immature laminin receptor protein. Biomaterials 200930:3091-9; PMID:19268360; http://dx.doi.org/10.1016/j.biomaterials.2009.02.022
- Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods 1987: 100:173-9; PMID:2439600; http://dx.doi.org/10.1016/0022-1759(87)90187-6
- Myszka DG, Rich RL. Advances in surface plasmon resonance biosensor analysis. Curr Opin Biotechnol 2000 11:54-61; PMID:10679342; http://dx.doi.org/10.1016/S0958-1669(99)00054-3
- Ball JM, Henry NL, Montelaro RC, Newman MJ. A versatile synthetic peptide-based ELISA for identifying antibody epitopes. J. Immunological Methods 1994 171, 37-44; PMID:7513733; http://dx.doi.org/10.1016/0022-1759(94)90226-7
- Varani J, Perone P, Griffiths CE, Inman DR, Fligiel SE, Voorhees JJ. All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest 1994 94:1747-56; PMID:7962521; http://dx.doi.org/10.1172/JCI117522
