Abstract
Neuroblastoma is one of the most common solid tumors in childhood and usually accompanied with poor prognosis and rapid tumor progression when diagnosed with amplification of the proto-oncogene N-Myc. The amplification of N-Myc has major influence on the maintenance of aerobic glycolysis, also known as the Warburg effect. This specific switch in the conversion of pyruvate to lactate instead of the conversion of pyruvate to acetyl-coenzyme A even in the presence of oxygen has important benefits for the tumor, e.g. increased production of enzymes and enzyme substrates that are involved in tumor progression, angiogenesis and inhibition of apoptosis. The antiprotozoal drug nifurtimox, which is generally used for the treatment of infections with the parasitic protozoan Trypanosoma cruzi, has been reported to have cytotoxic properties in the therapy of neuroblastoma. However, its action of mechanism has not been described in detail yet. The presented in vitro study on the neuroblastoma cell lines LA-N-1, IMR-32, LS and SK-N-SH shows an increased production of oxidative stress, a reduced lactate dehydrogenase enzyme activity and reduced lactate production after nifurtimox treatment. Furthermore, nifurtimox leads to reduced mRNA and protein levels of the proto-oncogene protein N-Myc. Thus, the current work gives new insights into the effect of nifurtimox on tumor metabolism revealing a shifted glucose metabolism from production of lactate to oxidative phosphorylation and a reduced expression of the major molecular prognostic factor in neuroblastoma N-Myc, presenting nifurtimox as a possible adjuvant therapeutic agent against (high risk) neuroblastoma.
Abbreviations
| DCF | = | 2′,7′-dichlorodihydrofluorescein |
| DMSO | = | dimethyl sulfoxide |
| HIF | = | hypoxia inducible factor |
| HK2 | = | hexokinase 2 |
| LDH | = | lactate dehydrogenase |
| MTS | = | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MYCN | = | v-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian) |
| nifurtimox | = | (RS)-3-methyl-N-[(1E)-(5-nitro-2-furyl) methylene] thiomorpholin-4-amine 1,1-dioxide |
| N-Myc | = | corresponding protein of MYCN |
| PDH | = | pyruvate dehydrogenase |
| PDK | = | pyruvate dehydrogenase kinase |
| ROS | = | reactive oxygen species |
| SDS | = | sodium dodecyl sulfate |
| topotecan | = | (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione. |
Introduction
Neuroblastoma is the most common extracranial solid cancer in childhood and is despite major progress in medical research often accompanied by rapid tumor progression and high mortality and recurrence rates. It derives from cells of the sympathetic nervous system during the embryonic development with a median age of 19 months at time of diagnosis. Information on 5-year disease-free survival rate range from only 30% up to over 95% for high-risk and non-high-risk neuroblastoma, respectively.Citation1,2 Classification as high-risk or non-high-risk neuroblastoma depends on the stage defined by the International Neuroblastoma Staging System (INSS)Citation3,4 or less common the International Neuroblastoma Pathology Classification (INPC) proposed by Shimada et al.Citation5,6
Over the past decades a multitude of prognosis and risk factors such as genetic mutations (e.g., deletion of chromosome 1p36,Citation7-9 altered expression of ALK,Citation10 PHOX2B) epigenetics,Citation9,11 ploidyCitation12 and ageCitation13 have been identified. In the mid-1980s, the clinical and prognostic role of the proto-oncogene MYCN encoding for the protein N-Myc (v-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian)) was first described.Citation14,15 Since then, its meaning and impact on the malignity of the tumor have been discussed controversially. It still remains unclear how the genetic constellation of the encoding MYCN gene correlates with the mRNA expression of the protein N-myc. Thus, it is possible that hyperploid cell types comprise over 10 chromosomes 2s on which the MYCN gene is located, with no or low amplification of the MYCN gene. On the other hand, euploid cell types with high or very high MYCN amplification exist. Interestingly, the type of MYCN amplification apparently doesn't have an influence on N-Myc mRNA expression. Both, neuroblastoma with low MYCN amplification but high N-Myc mRNA expression and neuroblastoma with high MYCN amplification but low N-Myc mRNA expression have been described.Citation16 Publications in the literature regarding the regulation and role of MYCN and N-Myc in tumor progression and malignancy are often inconsistent, whereby methodical approach and quality control of the practical analytics is often the center of criticism. Nonetheless, the current state of the literature on N-Myc points to decisive upstream regulatory processes that influence activation and inactivation of this transcription factor.
N-Myc like its structurally related analog c-Myc forms binary complexes with the Myc-associated factor X (Max) activating transcription of proteins involved in tumor proliferation, apoptosis, angiogenesis and metabolism such as the glycolytic enzymes hexokinase 2 (HK2), lactate dehydrogenase (LDH) or pyruvate dehydrogenase kinase (PDK).Citation17 PDK inhibits the mitochondrial respiration by phosphorylating and thus inactivating the pyruvate dehydrogenase (PDH), which metabolizes pyruvate to acetyl-coenzyme A. As a result, pyruvate is instead converted to lactate by LDH. This altered metabolism of glucose to lactate, even in the presence of oxygen was described by Otto Warburg as “aerobic glycolysis” and is thus known as the “Warburg effect.”Citation18 A major role in the Warburg metabolism is played by the transcription factor hypoxia inducible factor HIF-1. HIF-1 forms active complexes under hypoxia upregulating a variety of genes that are necessary for the tumor's adaption to these hypoxic conditions.Citation19 HIF-1α has a major effect on Myc-mediated gene expression as it may stimulate binding of Myc to Max enhancing gene expression of the aforementioned shared target proteins.Citation20 The analyses of 101 primary neuroblastoma tumors revealed significantly elevated expressions of HIF-1α and the glycolytic enzymes LDH, HK2 and phosphoglycerate kinase 1 in MYCN-amplified tumor cells compared to non-amplified cell lines. Further, depletion of LDH in 2 MYCN-amplified tumor cell lines via shRNA led to significant reduction of cell proliferation in vitro and tumorigenic capacity in vivo, revealing LDH as an effective target for neuroblastoma therapy.Citation21
In 2006 it was reported that the nitrofurane compound nifurtimox, which is normally used as an antiprotozoal drug against Trypanosoma cruzi, showed antitumor activity in a high-risk N-Myc amplified neuroblastoma patient. Despite intensive chemotherapy cycles with cisplatin, etoposide, vincristine, adriamycin, cyclophosphamide, iphosphamide, and carboplatin the patient received salvage chemotherapy with topotecan and cyclophosphamide due to tumor progression. At this time, the patient acquired infection with Trypanosoma cruzi (Chagas disease) through an infected blood transfusion and was thus started on nifurtimox. The combined treatment of nifurtimox and chemotherapy with topotecan and cyclophosphamide resulted in complete remission of the tumor.Citation22 Subsequently performed in vitro and in vivo studies confirmed the cytotoxic properties of nifurtimox against neuroblastoma cells.Citation23 Further investigations on nifurtimox and the structurally related nitrofurane compound RKS262 confirmed the suspicion that nifurtimox has a similar mechanism of action in neuroblastoma as in Trypanosoma cruzi.Citation24 It could be shown that these two compounds induce the formation of reactive oxygen species (ROS) in neuroblastoma cells and thus lead to apoptotic cell death.Citation25,26 In a phase I dose escalation study, the maximum tolerated dose of nifurtimox in patients with relapsed or refractory high-risk neuroblastoma and medulloblastoma was determined to 30 mg·kg BW−1d−1 (milligram per kilogram bodyweight and day). Mean maximum serum concentrations of nifurtimox were 4.80 ± 3.48 µg/mL (range 1.48 – 10.45 µg/mL) after dosage of 30 mg·kg BW−1d−1.Citation27 Nifurtimox plasma trough concentrations of 3 – 6 µg/mL are recommended for the treatment of trypanosomiasis.Citation29,30 A multi-center phase II study on the efficacy of nifurtimox with a planned enrollment of 100 patients with relapsed or refractory neuroblastoma and medulloblastoma is currently being performed (estimated primary completion date: December 2016).Citation28
Aim of the present study was to further investigate the mechanism of action of nifurtimox and its impact on N-Myc expression and the enzymes involved in aerobic glycolysis in 4 different N-Myc amplified and non-amplified neuroblastoma cell lines in order to evaluate a targeted use of nifurtimox as a combination therapeutic agent in chemotherapy of neuroblastoma.
Results
Nifurtimox reduces cell viability and induces cell cycle arrest and apoptosis in neuroblastoma cells
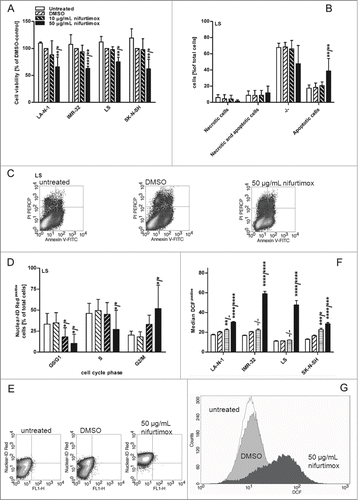
To characterize the cytotoxic impacts of nifurtimox on neuroblastoma, 4 cell lines were subjected to several experiments. As displayed in cell viability was reduced for all 4 neuroblastoma cell lines after 24 h incubation with 50 µg/mL to an average of 66%, 63%, 62% and 75% (LA-N-1, IMR-32 LS and SK-N-SH, respectively). The reduction was significant compared to the untreated control (P < 0.01) and the vehicle control with DMSO (P < 0.05) for all cell lines. Plasma trough concentrations of nifurtimox should reach 3 – 6 µg/mL for the treatment of trypanosomiasis in adults with a dosage of 5 mg per kg bodyweight 3 times a day.Citation29,30
To clarify, if this observed cell death was mediated by apoptosis or necrosis, a flow cytometric analysis with PI and Annexin-V-FITC was performed. The analyses revealed cell death by apoptosis as shown exemplarily for cell lines LS after 24 h incubation with 10 and 50 µg/mL nifurtimox (). Similar results were obtained for cell lines LA-N-1, IMR-32 and SK-N-SH (data not shown). One major mechanism for tumor progression and survival is the prevention from cell cycle arrest by blocking several control mechanisms that regulate cell cycle progression. Thus, reconstruction of these regulatory mechanisms by modulating agents is a desirable effect in tumor treatment. To identify such an effect of nifurtimox on neuroblastoma cell lines, cell cycle progression was assessed after 24 h incubation with nifurtimox exemplarily for cell line LS (). The amount of cells in G0/G1 phase were significantly lower after incubation with 10 (P < 0.05) and 50 µg/mL nifurtimox (P < 0.05) as well as in S phase after incubation with 50 µg/mL nifurtimox (P < 0.05) compared to untreated and vehicle control, whereas cells in the G2/M phase were significantly higher after incubation with 50 µg/mL nifurtimox (P < 0.05). The results for cell lines LA-N-1, IMR-32 and SK-N-SH were similar with a reduction of G0/G1 cells (P < 0.05) and an increase of G2/M cells (P < 0.05) after treatment with 50 µg/mL nifurtimox (data not shown).
Nifurtimox induces formation of reactive oxygen species
It is known, that the major cytotoxic effect of nifurtimox on Trypanosoma cruzi and possibly neuroblastoma as well is attributed to the formation of reactive oxygen species due to activation by nitroreductases.Citation24,25 The induction of ROS in neuroblastoma after nifurtimox treatment could be shown for all neuroblastoma cell lines used in the present study with a DCF flow cytometric assay. It could be shown that nifurtimox increases significantly the amount of ROS after 24 h incubation with 50 µg/mL nifurtimox for all 4 cell lines (P < 0.0001/P < 0.001) and with 10 µg/mL nifurtimox for LA-N-1 (P < 0.01/P > 0.05, insignificant) and SK-N-SH (P < 0.001/P < 0.05) compared to the untreated control or vehicle control, respectively ().
Combination of nifurtimox and topotecan in low nifurtimox doses effectively reduces cell viability
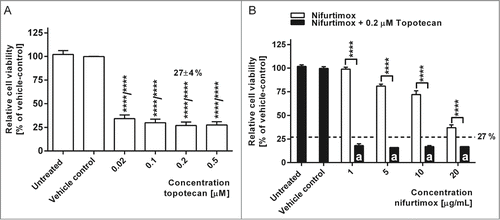
Topoisomerase I inhibitors relax DNA supercoils and thus play a pivotal role in DNA-dependent processes, especially when it comes to DNA damage. To prevent cancer cells from DNA repair after chemotherapeutic DNA damage, topoisomerase I inhibitors such as topotecan as additional chemotherapeutic agents have become attractive in cancer therapy.Citation31 A synergistic effect on the cytotoxicity of nifurtimox and topotecan on neuroblastoma cell lines would be favorable, not least to reduce dosages of each agent. Topotecan target plasma concentration for the treatment of high-risk neuroblastoma in pediatric patients lies between 80 – 120 ng·mL−1·h (≡0.2 – 0.3 µM; molecular weight: 421.45 g/mol).Citation32
A dose escalation of topotecan for 48 h treatment was performed for all neuroblastoma cell lines (exemplarily shown for cell line LS in ) with similar results. The lower target plasma concentration of 0.2 µM (84,3 ng/mL) topotecan resulted in a significantly reduced mean cell viability of 27±4 % compared to the vehicle control (P <0.0001). The combination of nifurtimox in increasing dosages from 1 to 20 µg/mL and 0.2 µM topotecan could further significantly reduce the cell viability compared to treatment with nifurtimox or topotecan alone (P < 0.0001). This is especially of interest in the low concentration range of 1 µg/mL nifurtimox and 0.2 µM topotecan. Here, the average relative cell viability was significantly (P < 0.0001) reduced to 18 % of the vehicle control (). Nifurtimox might thus be attractive for combination therapy.
Figure 2. Cell viability after nifurtimox and/or topotecan treatment. (A) Data show cell viability of cell line LS (N-Myc amplified) in reference to vehicle control after 48h incubation with topotecan as indicated, growth medium alone (untreated) or vehicle control tested with a MTS cell viability assay. Sample size n = 4. Data show mean ± standard deviation. Significant changes vs. vehicle control tested via ANOVA indicate P < 0.0001 (****). (B) Data show cell viability of cell line LS (N-Myc amplified) in reference to vehicle control after 48h incubation with nifurtimox as indicated or nifurtimox + 0.2 µM topotecan tested with a MTS cell viability assay. Sample size n = 4. Dotted line indicates cell viability after 48h incubation with 0.2 µM topotecan alone. Significant differences were tested with an ANOVA and Sidak-Bonferroni correction for multiple comparison (****: P < 0.0001); “a” indicates significant difference to treatment group 0.2 µM topotecan alone.
Expression of the proto-oncogene MYCN is reduced by nifurtimox
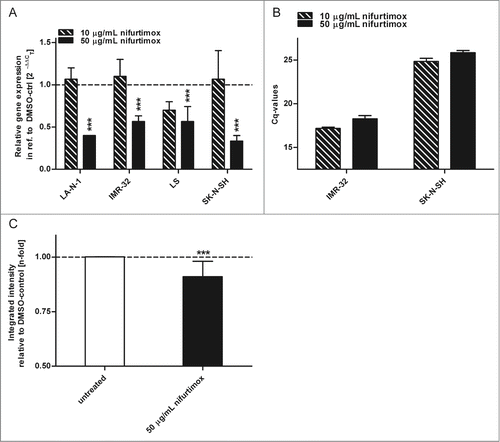
Malignancy of high-risk neuroblastoma is mostly ascribed to amplification and over-expression of the proto-oncogene MYCN.Citation33 The expression of its protein N-Myc was analyzed after the incubation with nifurtimox on mRNA level via quantitative real time PCR and on protein level via quantitative western blot. As displayed in N-Myc mRNA levels significantly decreased (P < 0.001) after 24h incubation with 50 µg/mL nifurtimox compared to the vehicle control for all neuroblastoma cell lines. To illustrate the absolute N-Myc mRNA levels in N-Myc amplified and non-amplified cell lines Cq-values are shown in by means of 2 examples IMR-32 (amplified) and SK-N-SH (non-amplified). shows the significant reduction (P < 0.001) of the N-Myc protein level of the N-Myc amplified cell line LA-N-1 after 4h incubation with 50 µg/mL nifurtimox.
Figure 3. Expression of N-Myc. (A). Data show mRNA expression levels in reference to vehicle control after 24h incubation with nifurtimox as indicated measured with quantitative real time PCR. Sample size n = 3. (B) Data show absolute Cq-values of the data in to illustrate absolute N-Myc mRNA Expression levels using an N-Myc amplified cell line IMR-32 and the non-amplified cell line SK-N-SH. Sample size n = 3. (C) Data show protein expression levels of N-Myc in reference to vehicle control after 4 h incubation with nifurtimox as indicated measured with quantitative western blot (cell line LA-N-1). Data show mean ± standard deviation. Significant changes versus untreated/DMSO tested via ANOVA (A) or unpaired Student's t-test (B) indicate P <0.001 (***).
Nifurtimox affects glucose consumption and lactate production
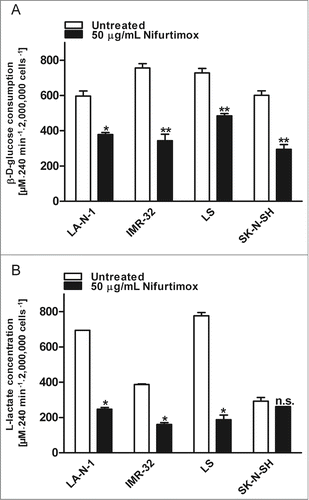
The consumption of glucose and the production of lactate are instructive parameters to illustrate tumor metabolism. To analyze whether nifurtimox has an impact on aerobic glycolysis in neuroblastoma, these 2 parameters were determined after nifurtimox treatment. shows the glucose consumption of the neuroblastoma cell lines LA-N-1 and LS as example cell lines after 4 h nifurtimox-treatment. Nifurtimox leads to a significantly reduced glucose-consumption for cell lines LA-N-1 (P = 0.0101), IMR-32 (P = 0.0057), LS (P = 0.0069) and SK-N-SH (P = 0.0078; ) and a significantly reduced lactate-production for cell lines LA-N-1 (P = 0.0103), IMR-32 (P = 0.0214), LS (P = 0.0242) but not for cell line SK-N-SH (P = 0.2753; ). To detect changes in the glucose metabolism from pyruvate → lactate to pyruvate → acetyl coenzyme A the relative lactate production in relation to the amount of glucose consumed was calculated. As seen in , the ratio glucose consumed/lactate produced increases, indicating that less lactate is produced from the same amount of glucose consumed after nifurtimox treatment compared to untreated cells. These observations were made for MYCN amplified cell lines LA-N-1, IMR-32 and LS but not for SK-N-SH. The ratio decreases in SK-N-SH cells after nifurtimox treatment.
Figure 4. Glycolysis. (A) Data show consumption of β-D-(+)-glucose per 4 h in the supernatant growth medium after incubation with nifurtimox or growth medium alone normalized to the number of cells. Consumption of D-Glucose was significantly reduced for LA-N-1 (P = 0.0101), IMR-32 (P = 0.0057), LS (P = 0.0069) and SK-N-SH (P = 0.0078) after treatment with nifurtimox. Experiments were carried out in duplicates and represent one of 3 independent experiments with similar outcome. (B) Data show production of L-lactate per 4 h in the supernatant growth medium after incubation with nifurtimox or growth medium alone normalized to the number of cells. Production of lactate was significantly reduced for LA-N-1 (P = 0.0103), IMR-32 (P = 0.0214), LS (P = 0.0242) but not for SK-N-SH (P = 0.2753) after treatment with nifurtimox. Experiments were carried out in duplicates and represent one of 3 independent experiments with similar outcome. Data show mean ± standard error of the means. Significant changes vs. untreated tested via ANOVA indicate P < 0.05 (*) and P < 0.01 (**).
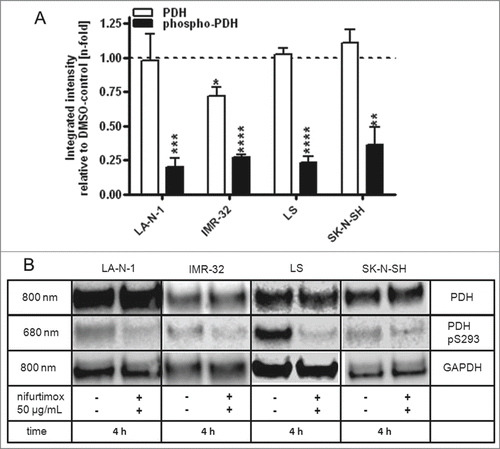
Amount of phosphorylated pyruvate dehydrogenase is reduced by treatment with nifurtimox
The activation state of pyruvate dehydrogenase is essential for the conversion of pyruvate. In its active form, the PDH is able to metabolize pyruvate to acetyl coenzyme A in healthy cells; PDH in its inactive phosphorylated form supports indirectly the conversion of pyruvate to lactate. This effect can be observed in malignant cells and is known as the “Warburg metabolism.”Citation34 A “re-activation” of the PDH would prevent the tumor from this metabolism. displays the results of a quantitative western blot of the mitochondria isolates of all 4 neuroblastoma cell lines after 4 h incubation with 50 µg/mL nifurtimox. It could be shown that nifurtimox significantly reduces the phosphorylation of the pyruvate dehydrogenase to approximately 20–30% of the untreated baseline level of LA-N-1 (P < 0.001), IMR-32 (P < 0.0001), LS (P < 0.0001) and SK-N-SH (P < 0.01). The expression levels of the dephosphorylated PDH remained stable for LA-N-1, LS and SK-N-SH but were significantly reduced for IMR-32 (P < 0.05).
Figure 5. Reduction of phospho-PDH levels after treatment with nifurtimox. (A) Data show protein levels of pyruvate dehydrogenase (PDH) and S293-phosphorylated pyruvate dehydrogenase (phospho-PDH) in reference to control after 4h incubation with nifurtimox as indicated or growth medium alone measured with quantitative western blot. Sample size n = 3. Data show mean ± standard deviation. Significant changes versus untreated control tested via unpaired Student's t-test indicate P < 0.01 (**), P < 0.001 (***) and P < 0.0001 (****). (B) Example blots of each cell line; protein bands are combined accordingly.
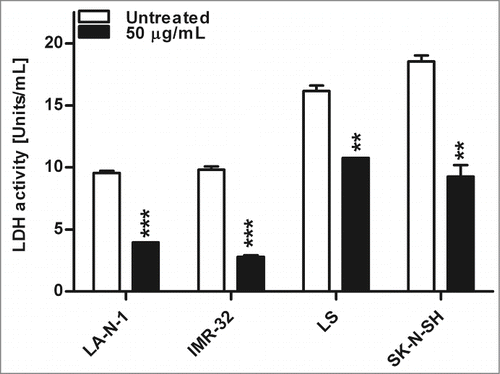
Nifurtimox affects enzyme activity of lactate dehydrogenase
As described above, a reduction of the production of lactate could be observed after treatment with nifurtimox. To differentiate if this effect is a result of a reduced LDH activity or a shift in pyruvate metabolism due to activation of PDH, the enzyme activity of LDH was determined after 4 h treatment with 50 µg/mL nifurtimox (). Compared to the untreated control, the LDH activity was significantly reduced for LA-N-1 (P = 0.005), IMR-32 (P = 0.009), LS (P = 0.0035) and SK-N-SH (P = 0.0065).
Figure 6. Enzyme activity of lactate dehydrogenase after nifurtimox treatment. Data show lactate dehydrogenase enzyme activity after 4 h incubation with nifurtimox as indicated or growth medium alone. One of 3 independent experiments with similar outcome is shown. Data show mean ± standard error of the means. Significant changes vs. untreated control tested via unpaired Student's t-test indicate P < 0.01 (**) and p < 0.001 (***).
Discussion
The cytotoxic effects of nifurtimox on neuroblastoma cell lines and its possible anti-tumor activity in neuroblastoma patients has been reported previously.Citation22,23,27 However, its detailed mechanism of action remains unclear, even in its actual application form against Trypanosomes. The general accepted hypothesis on the mechanism of action is the reduction of the nitro-group forming a nitro-anion radical that induces radical reactions with molecular oxygen resulting in oxidized cell contents such as protein-carbonyls, hydroperoxides and oxidized nucleotides.Citation35,36 On the contrary, Boiani et al. raised reasonable doubts whether induction of ROS was the main effect of nifurtimox treatment and rather proposed the inhibition of dehydrogenases and the affection of the mitochondrial membrane potential as the main cytotoxic property.Citation24 Up to date, only 7 publications address the effect of nifurtimox or related nitrofurane compounds on neuroblastoma or medulloblastoma and still little is known about its mode of action in these malignancies.Citation22,2325–27,37,38
In the present study, the cytotoxic effects and the induction of apoptosis in neuroblastoma cell lines is in accordance with previously published material.Citation23 Combination of 0.2 µM (84,3 ng/mL) topotecan (corresponding to therapeutic serum concentration in neuroblastoma patientsCitation32) and nifurtimox in vitro showed significant reduction of cell viability at low nifurtimox concentration of 1 µg/mL, and significantly lowered cell viability compared to topotecan or nifurtimox alone. Mean maximum serum concentrations of 1 to 5 µg/mL have been reported for pediatric patients with high-risk neuroblastoma or medulloblastoma.Citation27 The additive effect of topotecan and nifurtimox may thus be attractive for combination therapy.
Additionally it could be shown that nifurtimox induces cell cycle arrest at the G2/M phase which is known to be induced by oxidative stress.Citation39 However, further investigations have to be done to explain the detailed mechanisms that lead to the interrupted cell cycle progression after nifurtimox treatment. The presented data shows that oxidative stress is actually induced by nifurtimox but rather in high nifurtimox doses and not to the extent as expected. First significant increase of ROS was seen after approximately 8 hours (cell line LS; data not shown) which is a contradiction to the radical activation of nifurtimox by nitroreductases. The formation of ROS could as well be attributed to a higher oxidative phosphorylation. Highest detectable increases of ROS were always substantially lower with less vital cells than after treatment with positive controls such as tert-Butyl hydroperoxide (data not shown) supporting the suspicion that induction of ROS cannot be the only or preponderant cytotoxic effect of nifurtimox on neuroblastoma cells.
Therefore, further investigations were focused on the neuroblastoma cell metabolism. Major prognostic factor in neuroblastoma remains the proto-oncogene protein N-Myc which forms heterodimers with Max inducing the transcription of target genes involved in tumor proliferation, angiogenesis and apoptosis.Citation40 In the present study it could be shown, that nifurtimox reduced the expression of N-Myc on mRNA and protein levels, which besides the produced oxidative stress might have an impact on the cell cycle arrest.Citation40 Up to date, only few therapeutic agents have been described for targeting the transcription of MYCN (e.g., bromodomain BET inhibitionCitation41), the expression of N-Myc (e.g., cis-retinoic acid), the oncogenic stabilization of N-Myc (e.g., Aurora A kinase, AURKA) or the DNA-binding functions of N-Myc with its binding partner Max (e.g., Omomyc).Citation42 At this point, the detailed mechanism of nifurtimox reducing N-Myc mRNA levels cannot be described but will be the subject of further investigations of the presenting working group.
The upregulation of the glucose transport into malignant cells and their increased lactate-production are decisive factors for tumor cell survival and progression by synthesizing biomass. Therefore, the metabolism of glucose to lactate even under normoxic conditions and its possible metabolic reprogramming has become a field of great interest in oncologic research.Citation34 The presented data show that treatment with nifurtimox leads to a shift of glucose metabolism with reduced glucose consumption and reduced lactate production for the MYCN-amplified cell lines LA-N-1, IMR-32 and LS. Further, a reduced relative lactate-production in relation to the glucose-uptake could be determined after nifurtimox treatment. Interestingly, this relation is vice-versa only in the MYCN non-amplified cell line SK-N-SH, suggesting a relationship between N-Myc and glycolysis. Similar properties of SK-N-SH have been reported previously. mIBG was shown to modulate glycolysis and oxidative phosphorylation and to have cytotoxic effects on MYCN amplified cell lines such as N1E-115 and LS. In contrary cytotoxic effects were far less in SK-N-SH compared to the MYCN amplified cell lines.Citation43-47 These analyses substantiate the hypothesis that N-Myc is related to glycolysis.
An LDH-activity assay revealed that nifurtimox reduces LDH enzyme activity. This observation is in accordance with the hypothesis of Boiani et al. that nifurtimox not only produces oxidative stress but is also capable of inhibiting dehydrogenases in Trypanosomes.Citation24 MYCN amplified neuroblastoma cells have been described to be “addicted” to LDH activity since its depletion causes inhibition of tumorigenesis in vivo.Citation21 A similar mechanism in neuroblastoma cells seems to be imaginable. If the reduced LDH activity or upstream processes e.g. the PDH activation state, or both, are responsible for the reduced lactate-production cannot be decided.
A reduced amount of the mitochondrial phosphorylated PDH could be observed after nifurtimox treatment. Unphosphorylated PDH is the active form of PDH enabling a conversion of pyruvate to acetyl-coenzyme A. These two observed processes, the inactivation of LDH and the activation of PDH force the “classic” tumor metabolism via aerobic glycolysis (pyruvate → lactate) into the normal oxidative phosphorylation (pyruvate → acetyl-coenzyme A) of healthy cells.
A significantly elevated expression of HIF-1α in MYCN-amplified neuroblastoma has been described.Citation21 The transcription factor HIF-1α cooperates with the proto-oncogene c-Myc, inducing an elevated PDK1 (pyruvate dehydrogenase kinase isoenzyme 1) expression for supporting indirectly lactate production.Citation20 A similar interplay of N-Myc and HIF-1α could be possible, thus influencing the activation state or phosphorylation of the PDH.
Taken together, it can be concluded that nifurtimox has a major effect on the shift of the tumor's aerobic glycolysis to the normal oxidative phosphorylation, hindering the tumor metabolism from essential production of biomass and inducing apoptosis-mediated cell death (see Fig. S1). The production of ROS is a helpful cytotoxic effect of nifurtimox, but probably not its main mechanism of action. This effect could be further aggravated by inhibition of the specific enzymes involved in detoxification of oxidative stress. The reduction of N-Myc expression and the early inhibition of LDH activity by nifurtimox-treatment may offer a possible adjuvant therapeutic, not only for N-Myc amplified high-risk neuroblastoma.
Material and Methods
Neuroblastoma cell lines
The commercially available neuroblastoma cell lines IMR-32 (LGC Standards; #CCL−127TM), LA-N-1 (Sigma-Aldrich #06041201) and SK-N-SH (LGC Standards, #HTB-11TM) and the neuroblastoma cell line LS which was established at the University Children's Hospital Tuebingen in 1990Citation48 were grown in RPMI-1640 medium (Merck Millipore #F1215) supplemented with 10% (v/v) fetal calf serum (FCS; Biochrom AG, #S0115), 2 mM L-glutamine (Biochrom AG, #K0282), 100 U/mL penicillin and 100 µg/mL streptomycin (Biochrom AG, #A2213) and incubated at 37°C, 5% CO2 and saturated humidity. All cell lines were regularly tested negative for Mycoplasma spp. (Venor® GeM Classic Mycoplasma PCR detection kit, Minerva Biolabs, #11–1250) and used in laboratory analysis to a maximum of 90 passages. Cell lines IMR-32, LA-N-1 and LS are MYCN-amplified cell lines whereas cell line SK-N-SH is MYCN non-amplified. Additional information on the neuroblastoma cell line characteristics are displayed in .
Table 1. Cell line characteristics
Table 2. Ratios of glucose consumption and lactate production after nifurtimox-treatment
Nifurtimox
(RS)-3-methyl-N-[(1E)-(5-nitro-2-furyl) methylene] thiomorpholin-4-amine 1,1-dioxide (nifurtimox; CAS: 23256–30–6; molecular weight: 287,293 g/mol) was a gift from Bayer HealthCare AG, Berlin, Germany. Stock solutions were made at 40 mg/mL (≡ 139.2 mM) in dimethyl sulfoxide (DMSO, Carl Roth GmbH & Co. KG, #A994) and stored at −20°C. In solution, nifurtimox is critically unstable when exposed to light. Therefore, all stock solutions and standard solutions containing nifurtimox were handled in light-proof containers and reaction tubes. Nifurtimox solutions were discarded after 7 d.
Topotecan
(S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione (topotecan; CAS: 123948-87-8; molecular weight 421.45 g/mol) was purchased from Sigma-Aldrich (St. Louis, MO USA). Stock solutions were made at 0.8 mM in DMSO in light-proof containers and reaction tubes and stored at −20°C until use.
Cell viability – MTS assay
To assess the cell viability after incubation with nifurtimox at different concentrations (10 µg/mL up to 50 µg/mL or 34.8 µM to 174 µM, respectively in the supernatant growth medium) or the vehicle control with according concentrations, all neuroblastoma cell lines were subjected to an MTS assay. Stock solutions of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTS, Carl Roth, #4022) were made at 480 µM in sterile filtered deionized water and stored at −20°C. Cells were grown to approximately 50% confluency, treated with nifurtimox, and incubated for 1 h with fresh media containing 12 µM MTS. The supernatant was subsequently removed and the cells were lysed with DMSO containing 10% (w/v) sodium dodecyl sulfate (SDS; Carl Roth, #0183) and 1% (v/v) glacial acetic acid (Carl Roth, #3738). Purple formazan contents of each cell lysate were photometrically analyzed in triplicates at 570 nm (630 nm reference wave length) in 96 microtiter plates.
Differentiation of apoptosis and necrosis
50% confluent neuroblastoma cells were treated with nifurtimox as described and dyed with Annexin V (apoptotic cells) and Ethidium homodimer III (necrotic cells) using the Apoptotic/Necrotic Cells Detection Kit (Promokine, PromoCell GmbH, #PK-CA707–30017) according to the manufacturer's instructions.
Analysis of cell cycle profiles
50% confluent neuroblastoma cells were treated with nifurtimox as described. Cells were stained using the Nuclear-ID® Green Cell Cycle Kit for flow cytometry according to the manufacturer's protocol (Enzo Life Sciences, Inc.., #ENZ-51014–100). Dyed cells were analyzed in a flow cytometer (FACSCaliburTM, BDBiosciences).
Reactive oxygen species – DCF assay
Neuroblastoma cells were treated with nifurtimox as described and were subjected to a flow cytometric analysis of intracellular ROS; 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) is taken up into the cells and metabolized to the fluorescent 2′,7′-dichlorofluorescein (DCF) by intracellular ROS. Cells were dyed using the Cellular Reactive Oxygen Species Detection Kit (Abcam plc., #ab113851) according to the manufacturer's protocol and the intracellular amount of ROS was quantified with a flow cytometer (FACSCaliburTM, BDBiosciences).
Western Blot
Neuroblastoma cells were treated with nifurtimox as described and indicated. For separation of mitochondrial (determination of (phosphorylated) pyruvate dehydrogenase (PDH, PDH-P)) and cytosolic fraction (determination of N-Myc), 5 × 107 treated cells were homogenized with a dounce tissue grinder (Sigma-Aldrich, #D8938). Mitochondria were subsequently extracted using the Mitochondria/Cytosol fractionation kit (Enzo Life Sciences, #ALX-850–276-KI01) according to the manufacturer's instructions.
The protein content of the according cell lysate was determined using a PierceTM BCA protein assay kit (Thermo Fisher Scientific, #23227) according to the manufacturer's protocol; 15 µg protein were denatured with NuPAGE® LDS 4× Sample Buffer (Life Technologies, #NP0008) for 5 min at 95°C and loaded onto 4–20% Tris-HEPES precast 12 well gels (Life Technologies, #25224). The proteins were separated in a SDS gel electrophoresis in Tris-HEPES SDS running buffer (Life Technologies, #28398) at 120 V const. for approximately 50 min and transferred onto low fluorescent Immobilon-FL 0.45 µm PVDF membranes (Merck Millipore, #IPFL00010) using Tris-Glycine transfer buffer (Life Technologies, #LC3675) in a semi-dry blot. Membranes were blocked with OdysseyTM Blocking Buffer (Li-Cor Inc.., #927–40100) and the proteins were probed with the following antibodies: mouse anti-GAPDH IgG1 clone 1D4 (Abcam, #200575), rabbit anti-human N-Myc polyclonal (Thermo Fisher Scientific, #PA5–17403), mouse anti-PDH and rabbit anti-phospho S293 PDH (Abcam, #ab110416 and #ab177461). For quantitative determination, primary antibodies were probed with the fluorescent dyes goat anti-mouse IRDye 800CW IgG and goat anti-rabbit IRDye 680RD IgG (both Li-Cor Inc.., #926–32210 and #925–68071) and analyzed with the Li-Cor OdysseyTM Fc Dual Imaging System and the Image StudioTM Software Version 3.1 for windows (Li-Cor Inc.).
Quantitative real time PCR
Neuroblastoma cells were treated as described and total RNA was extracted using the RNeasy mini kit (Qiagen, #74104). First-strand cDNA was synthesized from 500 ng of total RNA using the QuantiTect Reverse Transcription Kit (Qiagen, #205311) according to the manufacturer's protocol. MYCN gene was amplified using KAPA SYBR® Fast qPCR MasterMix for Bio-Rad iCycler (Peqlab Biotechnologie GmbH, #KK4600) and the following primers:
Forward: 5′-ACCCTGAGCGATTCAGATGAT-3′ and
Reverse: 5′-GTGGTGACAGCCTTGGTGTT-3′.
Primers were used in a 2-step protocol with 2 min at 95°C pre-heating, 40 cycles at 95°C for 15 s followed by 60°C for 1 min using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories). For quantification of the relative gene expression, ct-values were normalized to GAPDH expression levels and calculated using the 2−ΔΔCT method as described by Livak and Schmittgen.Citation49,50
Glycolysis assay
For evaluation of glucose consumption and lactate-production after 4 h treatment with 50 µg/mL nifurtimox, cells were grown in 250 mL cell culture flasks in growth medium and treated with nifurtimox as described above. L-lactate production and glucose consumption in the cell culture's supernatant medium was determined using the Cell based Glycolysis Assay Kit (Cayman Chemical, #600450) and the Glucose Colorimetric Assay Kit (BioVision, #K606–100). Fresh growth medium was subjected to lactate and glucose determinations as blank value. To reflect bias of the results due to the cytotoxicity of nifurtimox, cells were counted when aliquots for lactate and glucose determination were taken. The same treated cells were lysed and subjected to enzyme activity analyses of LDH using the Lactate Dehydrogenase Activity Assay Kit (Sigma-Aldrich, #MAK066) according to the manufacturer's protocol.
Statistical Analysis
Independent experiments were carried out at least 3 times in duplicates or triplicates and comparisons of the treatment groups were made via one-way ANOVA analysis of variance and Bonferroni correction for multiple testing or an unpaired student's t test each with a 95% confidence interval. Cell viability experiment for comparison of “Nifurtimox” vs. “Nifurtimox + Topotecan” was analyzed for statistical significance with the Šidák-Bonferroni method with α=5.000. P-values <0.05 were considered as statistically significant (*: P < 0.05; **: P < 0.01; ***: P < 0.001; ****: P < 0.0001). The level of significance was adjusted according to Bonferroni as necessary. Statistical analyses and graphs were created using GraphPad Prism software for Windows, version 5.03 (GraphPad Software, San Diego, CA USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figure S1
Download MS Power Point (121.7 KB)Acknowledgments
The authors would like to thank Maren Lübke for performing parts of the real time PCR experiments. The nifurtimox used in this study was a gift from Bayer Health Care AG, Berlin, Germany.
Funding
This work was supported by the non-profit foundation Jürgen Manchot Stiftung, Düsseldorf, Germany.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Philip T, Ladenstein R, Lasset C, Hartmann O, Zucker JM, Pinkerton R, Pearson AD, Klingebiel T, Garaventa A, Kremens B, et al. 1070 myeloablative megatherapy procedures followed by stem cell rescue for neuroblastoma: 17 years of European experience and conclusions. European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party. Eur J Cancer 1997; 33:2130-5; PMID:9516868; http://dx.doi.org/10.1016/S0959-8049(97)00324-9
- Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KK, Hogarty M. Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatric Blood Cancer 2013; 60:985-93; PMID:23255319; http://dx.doi.org/10.1002/pbc.24433
- Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993; 11:1466-77; PMID:8336186
- Castleberry RP, Shuster JJ, Smith EI. The Pediatric Oncology Group experience with the international staging system criteria for neuroblastoma. Member Institutions of the Pediatric Oncology Group. J Clin Oncol 1994; 12:2378-81; PMID:7964953
- Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 1999; 86:364-72; PMID:10421273; http://dx.doi.org/10.1002/(SICI)1097-0142(19990715)86:2%3c364::AID-CNCR21%3e3.0.CO;2-7
- Ikeda H, Iehara T, Tsuchida Y, Kaneko M, Hata J, Naito H, Iwafuchi M, Ohnuma N, Mugishima H, Toyoda Y, et al. Experience with International Neuroblastoma Staging System and Pathology Classification. Bri J Cancer 2002; 86:1110-6; PMID:11953858; http://dx.doi.org/10.1038/sj.bjc.6600231
- Zage PE, Sirisaengtaksin N, Liu Y, Gireud M, Brown BS, Palla S, Richards KN, Hughes DP, Bean AJ. UBE4B levels are correlated with clinical outcomes in neuroblastoma patients and with altered neuroblastoma cell proliferation and sensitivity to epidermal growth factor receptor inhibitors. Cancer 2013; 119:915-23; PMID:22990745; http://dx.doi.org/10.1002/cncr.27785
- Yu F, Gao W, Yokochi T, Suenaga Y, Ando K, Ohira M, Nakamura Y, Nakagawara A. RUNX3 interacts with MYCN and facilitates protein degradation in neuroblastoma. Oncogene 2014; 33:2601-9; PMID:23851507; http://dx.doi.org/10.1038/onc.2013.221
- Domingo-Fernandez R, Watters K, Piskareva O, Stallings RL, Bray I. The role of genetic and epigenetic alterations in neuroblastoma disease pathogenesis. Pediatr Surg Int 2013; 29:101-19; PMID:23274701; http://dx.doi.org/10.1007/s00383-012-3239-7
- Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008; 455:930-5; PMID:18724359; http://dx.doi.org/10.1038/nature07261
- Haruta M, Kamijo T, Nakagawara A, Kaneko Y. RASSF1A methylation may have two biological roles in neuroblastoma tumorigenesis depending on the ploidy status and age of patients. Cancer Lett 2014; 348:167-76; PMID:24680815; http://dx.doi.org/10.1016/j.canlet.2014.03.022
- Kaneko Y, Kanda N, Maseki N, Sakurai M, Tsuchida Y, Takeda T, Okabe I, Sakurai M. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res 1987; 47:311-8; PMID:3791218
- Mosse YP, Deyell RJ, Berthold F, Nagakawara A, Ambros PF, Monclair T, Cohn SL, Pearson AD, London WB, Matthay KK. Neuroblastoma in older children, adolescents and young adults: a report from the International Neuroblastoma Risk Group project. Pediatr Blood Cancer 2014; 61:627-35; PMID:24038992; http://dx.doi.org/10.1002/pbc.24777
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc sequences in primary human neuroblastomas: correlation with advanced disease stage. Prog Clin Biol Res 1985; 175:105-13; PMID:3991726
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Sci (New York, NY) 1984; 224:1121-4; http://dx.doi.org/10.1126/science.6719137
- Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med 1985; 313:1111-6; PMID:4047115; http://dx.doi.org/10.1056/NEJM198510313131802
- Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol 2007; 27:7381-93; PMID:17785433; http://dx.doi.org/10.1128/MCB.00440-07
- Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res 2006; 66:8927-30; PMID:16982728; http://dx.doi.org/10.1158/0008-5472.CAN-06-1501
- Stubbs M, Griffiths JR. The altered metabolism of tumors: HIF-1 and its role in the Warburg effect. Adv Enzyme Regul 2010; 50:44-55; PMID:19896967; http://dx.doi.org/10.1016/j.advenzreg.2009.10.027
- Huang LE. Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ 2008; 15:672-7; PMID:18188166; http://dx.doi.org/10.1038/sj.cdd.4402302
- Qing G, Skuli N, Mayes PA, Pawel B, Martinez D, Maris JM, Simon MC. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res 2010; 70:10351-61; PMID:20961996; http://dx.doi.org/10.1158/0008-5472.CAN-10-0740
- Saulnier Sholler GL, Kalkunte S, Greenlaw C, McCarten K, Forman E. Antitumor activity of nifurtimox observed in a patient with neuroblastoma. J Pediat Hematol/Oncol 2006; 28:693-5; PMID:17023833; http://dx.doi.org/10.1097/01.mph.0000212994.56812.f2
- Saulnier Sholler GL, Brard L, Straub JA, Dorf L, Illeyne S, Koto K, Kalkunte S, Bosenberg M, Ashikaga T, Nishi R. Nifurtimox induces apoptosis of neuroblastoma cells in vitro and in vivo. J Pediat Hematol/Oncol 2009; 31:187-93; PMID:19262245; http://dx.doi.org/10.1097/MPH.0b013e3181984d91
- Boiani M, Piacenza L, Hernandez P, Boiani L, Cerecetto H, Gonzalez M, Denicola A. Mode of action of nifurtimox and N-oxide-containing heterocycles against Trypanosoma cruzi: is oxidative stress involved? Biochem Pharmacol 2010; 79:1736-45; PMID:20178775; http://dx.doi.org/10.1016/j.bcp.2010.02.009
- Singh RK, Dorf L, DeMartino A, Illenye S, Koto K, Currier EA, Ashikaga T, Kim KK, Brard L, Sholler GL. Oral RKS262 reduces tumor burden in a neuroblastoma xenograft animal model and mediates cytotoxicity through SAPK/JNK and ROS activation in vitro. Cancer Biol Ther 2011; 11:1036-45; PMID:21532338; http://dx.doi.org/10.4161/cbt.11.12.15706
- Singh RK, Lange TS, Kim KK, Brard L. A coumarin derivative (RKS262) inhibits cell-cycle progression, causes pro-apoptotic signaling and cytotoxicity in ovarian cancer cells. Invest New Drugs 2011; 29:63-72; PMID:19865799; http://dx.doi.org/10.1007/s10637-009-9335-4
- Saulnier Sholler GL, Bergendahl GM, Brard L, Singh AP, Heath BW, Bingham PM, Ashikaga T, Kamen BA, Homans AC, Slavik MA, et al. A phase 1 study of nifurtimox in patients with relapsed/refractory neuroblastoma. J Pediat Hematol/Oncol 2011; 33:25-30; PMID:21063221; http://dx.doi.org/10.1097/MPH.0b013e3181f47061
- Saulnier Sholler GL. Study of Nifurtimox to Treat Refractory or Relapsed Neuroblastoma or Medulloblastoma. US. National Institutes of Health, 2008; https://clinicaltrials.gov/ct2/show/NCT00601003?term=nifurtimox+neuroblastoma&rank=2; access 21/Jul/2015
- Padro JM, Marson ME, Mastrantonio GE, Altcheh J, Garcia-Bournissen F, Reta M. Development of an ionic liquid-based dispersive liquid-liquid microextraction method for the determination of nifurtimox and benznidazole in human plasma. Talanta 2013; 107:95-102; PMID:23598198; http://dx.doi.org/10.1016/j.talanta.2012.12.050
- Garcia-Bournissen F, Altcheh J, Panchaud A, Ito S. Is use of nifurtimox for the treatment of Chagas disease compatible with breast feeding? A population pharmacokinetics analysis. Arch Dis Child 2010; 95:224-8; PMID:19948512; http://dx.doi.org/10.1136/adc.2008.157297
- Basili S, Moro S. Novel camptothecin derivatives as topoisomerase I inhibitors. Exp Opin Ther Pat 2009; 19:555-74; PMID:19441934; http://dx.doi.org/10.1517/13543770902773437
- Panetta JC, Schaiquevich P, Santana VM, Stewart CF. Using pharmacokinetic and pharmacodynamic modeling and simulation to evaluate importance of schedule in topotecan therapy for pediatric neuroblastoma. Clin Cancer Res 2008; 14:318-25; PMID:18172284; http://dx.doi.org/10.1158/1078-0432.CCR-07-1243
- Schleiermacher G, Janoueix-Lerosey I, Delattre O. Recent insights into the biology of neuroblastoma. Int J Cancer 2014; 135:2249-61; PMID:25124476; http://dx.doi.org/10.1002/ijc.29077
- Chen X, Qian Y, Wu S. The Warburg Effect: Evolving Interpretations Of An Established Concept. Free Radic Biol Med 2014; 79:253-63; PMID:25277420; http://dx.doi.org/10.1016/j.freeradbiomed.2014.08.027
- Maya JD, Cassels BK, Iturriaga-Vasquez P, Ferreira J, Faundez M, Galanti N, Ferreira A, Morello A. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp Biochem Physiol A Mol Integr Physiol 2007; 146:601-20; http://dx.doi.org/10.1016/j.cbpa.2006.03.004
- Maya JD, Repetto Y, Agosin M, Ojeda JM, Tellez R, Gaule C, Morello A. Effects of nifurtimox and benznidazole upon glutathione and trypanothione content in epimastigote, trypomastigote and amastigote forms of Trypanosoma cruzi. Mol Bioch Parasitol 1997; 86:101-6; PMID:9178272
- Koto KS, Lescault P, Brard L, Kim K, Singh RK, Bond J, Illenye S, Slavik MA, Ashikaga T, Saulnier Sholler GL. Antitumor activity of nifurtimox is enhanced with tetrathiomolybdate in medulloblastoma. Int J Oncol 2011; 38:1329-41; PMID:21399873
- McNeil EM, Ritchie AM, Melton DW. The toxicity of nitrofuran compounds on melanoma and neuroblastoma cells is enhanced by Olaparib and ameliorated by melanin pigment. DNA repair 2013; 12:1000-6; PMID:24070777; http://dx.doi.org/10.1016/j.dnarep.2013.08.017
- Hseu YC, Lee MS, Wu CR, Cho HJ, Lin KY, Lai GH, Wang SY, Kuo YH, Kumar KJ, Yang HL. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J Agric Food Chem 2012; 60:2385-97; PMID:22324429; http://dx.doi.org/10.1021/jf205053r
- Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 2013; 3:a014415; PMID:24086065; http://dx.doi.org/10.1101/cshperspect.a014415
- Puissant A, Frumm SM, Alexe G, Bassil CF, Qi J, Chanthery YH, Nekritz EA, Zeid R, Gustafson WC, Greninger P, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Dis 2013; 3:308-23; PMID:23430699; http://dx.doi.org/10.1158/2159-8290.CD-12-0418
- Barone G, Anderson J, Pearson AD, Petrie K, Chesler L. New strategies in neuroblastoma: Therapeutic targeting of MYCN and ALK. Clin Cancer Res 2013; 19:5814-21; PMID:23965898; http://dx.doi.org/10.1158/1078-0432.CCR-13-0680
- Loesberg C, Van Rooij H, Nooijen WJ, Meijer AJ, Smets LA. Impaired mitochondrial respiration and stimulated glycolysis by m-iodobenzylguanidine (MIBG). Int J Cancer 1990; 46:276-81; PMID:2384275; http://dx.doi.org/10.1002/ijc.2910460223
- Cornelissen J, Wanders RJ, Van den Bogert C, Van Kuilenburg AB, Elzinga L, Voute PA, Van Gennip AH. Meta-iodobenzylguanidine (MIBG) inhibits malate and succinate driven mitochondrial ATP synthesis in the human neuroblastoma cell line SK-N-BE(2c). Eur J Cancer 1995; 31a:582-6; PMID:7576973; http://dx.doi.org/10.1016/0959-8049(95)00045-K
- Cornelissen J, Wanders RJ, Van Gennip AH, Van den Bogert C, Voute PA, Van Kuilenburg AB. Meta-iodobenzylguanidine inhibits complex I and III of the respiratory chain in the human cell line Molt-4. Biochem Pharmacol 1995; 49:471-7; PMID:7872952; http://dx.doi.org/10.1016/0006-2952(94)00450-Z
- Larcher JC, Vayssiere JL, Lossouarn L, Gros F, Croizat B. Regulation of c- and N-myc expression during induced differentiation of murine neuroblastoma cells. Oncogene 1991; 6:633-8; PMID:2030913
- Hampel T, Bruns M, Bayer M, Handgretinger R, Bruchelt G, Brückner R. Synthesis and biological effects of new hybrid compounds composed of benzylguanidines and the alkylating group of busulfan on neuroblastoma cells. Bioorg Med Chem Lett 2014; 24:2728-33; PMID:24814532; http://dx.doi.org/10.1016/j.bmcl.2014.04.030
- Rudolph G, Schilbach-Stuckle K, Handgretinger R, Kaiser P, Hameister H. Cytogenetic and molecular characterization of a newly established neuroblastoma cell line LS. Hum Genet 1991; 86:562-6; PMID:2026421; http://dx.doi.org/10.1007/BF00201542
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001; 25:402-8; http://dx.doi.org/10.1006/meth.2001.1262
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3:1101-8; PMID:18546601; http://dx.doi.org/10.1038/nprot.2008.73
- Tumilowicz JJ, Nichols WW, Cholon JJ, Greene AE. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res 1970; 30:2110-8; PMID:5459762
- Seeger RC, Rayner SA, Banerjee A, Chung H, Laug WE, Neustein HB, Benedict WF. Morphology, growth, chromosomal pattern and fibrinolytic activity of two new human neuroblastoma cell lines. Cancer Res 1977; 37:1364-71; PMID:856461
- Rostomily RC, Bermingham-McDonogh O, Berger MS, Tapscott SJ, Reh TA, Olson JM. Expression of neurogenic basic helix-loop-helix genes in primitive neuroectodermal tumors. Cancer Res 1997; 57:3526-31; PMID:9270024
- Kato H, Okamura K, Kurosawa Y, Kishikawa T, Hashimoto K. Characterization of DNA rearrangements of N-myc gene amplification in three neuroblastoma cell lines by pulsed-field gel electrophoresis. FEBS Lett 1989; 250:529-35; PMID:2753147; http://dx.doi.org/10.1016/0014-5793(89)80790-2
- Thiele CJ. Neuroblastoma: In (Ed.) Masters. J Hum Cell Culture 1998; 1:21-53; http://dx.doi.org/10.1007/0-306-46872-7_2
- Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 1973; 33:2643-52; PMID:4748425