Abstract
Multidrug resistance and tumor migration and invasion are the major obstacles to effective breast cancer chemotherapy, but the underlying molecular mechanisms remain unclear. This study investigated the potential of transgelin 2 and salvianolic acid A to modulate the resistance and the migration and invasion abilities of paclitaxel-resistant human breast cancer cells (MCF-7/PTX). MCF-7/PTX cells were found to exhibit not only a high degree of resistance to paclitaxel, but also strong migration and invasion abilities. Small interfering RNA-mediated knockdown of TAGLN2 sensitized the MCF-7/PTX cells to paclitaxel, and inhibited their migration and invasion abilities. In addition, we also observed that combined salvianolic acid A and paclitaxel treatment could reverse paclitaxel resistance, markedly inhibit tumor migration and invasion, and suppress the expression of transgelin 2 in MCF-7/PTX cells. These findings indicate that salvianolic acid A can reverse the paclitaxel resistance and inhibit the migration and invasion abilities of human breast cancer cells by down-regulating the expression of transgelin 2, and hence could be useful in breast cancer treatments
Abbreviations
| MDR | = | multidrug resistance |
| PTX | = | paclitaxel |
| SAA | = | salvianolic acid A |
| EMT | = | epithelial-mesenchymal transition. |
Introduction
Breast cancer has become the first common malignancy in women, with about 1.3 million newly reported cases of breast cancer diagnosed each year worldwide.Citation1 Chemotherapy plays a crucial role in breast cancer treatments. However, the phenomenon of multidrug resistance (MDR) emerges frequently, which is triggered by the continuous use of chemotherapeutic drugs, and it seriously impedes the clinical efficacy of anti-tumor drugs and enhances the occurrence of tumor metastasis and invasion.Citation2,3 Approximately 500,000 women die of breast cancer per year due to MDR and tumor metastasis and invasion.Citation4 However, the mechanisms underlying MDR and tumor migration and invasion are extremely complicated due to the involvement of multiple pathways and targets. Although there are many reports on the appearance of MDR and tumor migration and invasion in breast cancer, their underlying molecular mechanisms are not yet clearly understood. It is therefore urgent to gain further insight into the mechanisms of MDR and tumor migration and invasion in breast cancer and develop reversal reagents with high efficacy but low toxicity to overcome these problems, which is of great significance for improving the quality of life breast cancer patients.
Recently, emerging studies of breast cancer,Citation5 ovarian cancer,Citation6 hepatocellular carcinomaCitation7 and lung adenocarcinomaCitation8 have demonstrated that drug-resistant cancer cells display features of epithelial-mesenchymal transition (EMT). It is well-known that EMT is an essential physiological process in which epithelial cells change into mesenchymal cells through a specific signaling pathway. As a dynamic and reversible process, EMT often occurs at the invasive front of many metastatic cancers. In particular, when the EMT process is triggered, epithelial cells not only undergo a remarkable morphological transition from an epithelial cobblestone phenotype to fibroblast-like characteristics, but also lose their expression of epithelial markers, such as E-cadherin, and gain the expression of mesenchymal markers, including N-cadherin, Vimentin, and fibronectin.Citation9 These alterations contribute markedly to the loss of cell-cell adhesion and the enhancement of the migratory and invasive properties of cancer cells.Citation10 As a consequence, chemotherapy-induced resistance in tumor cells is associated with EMT, metastasis, and invasion.
Paclitaxel (PTX), which has been widely applied in first-line chemotherapies for treating breast cancer, causes cell death by promoting the polymerization of tubulin and preventing depolymerization, thereby blocking mitosis.Citation11 Our previous study demonstrated that the expression of transgelin 2 was 15.58-fold higher in paclitaxel-resistant human breast cancer cells (MCF-7/PTX) established by our laboratory than in breast cancer drug-sensitive cells (MCF-7/S),Citation12 indicating that transgelin 2 might act as a novel biomarker of paclitaxel resistance in breast cancer.Citation13 Furthermore, we demonstrated that transgelin 2 could regulate the expressions of classic resistance protein P-glycoprotein, multidrug resistance-associated protein 1, and breast cancer resistance protein, and that its level of expression was positively correlated with the drug resistance of MCF-7/PTX cells.Citation14 The overexpression of transgelin 2 in various human malignancies and its enhancement of tumor metastasis and invasion have been reported.Citation15,16 However, the effects of transgelin 2 on the paclitaxel resistance and the migration and invasion abilities of human breast cancer cells are still enigmatic and need to be explored further.
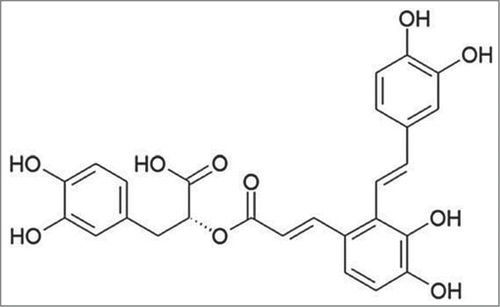
Considerable efforts are now being made into exploiting natural compounds originating from plant that possess strong anti-tumor activity. Their features of high efficacy, low toxicity, and low cost make them highly favored among researchers. Salvianolic acid A (SAA, ), a major phenolic activecomponent extracted from Salvia miltiorrhiza, exhibits numerous pharmacological actions, such as antithrombotic activity, antioxidant, anti-inflammatory and antifibrotic effects.Citation17 Our previous study verified that SAA enhanced the chemosensitivity of paclitaxel in human breast cancer cells by targeting transgelin 2 to accelerate the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and then inactivated the phosphatidylinositol-3-kinase/proteinkinase B (PI3K/Akt) pathway, thereby increasing cell apoptosis.Citation18 It was also documented that SAA could inhibit the migration and proliferation of vascular smooth muscle cells via suppressing platelet-derived growth factor-BB.Citation19 We therefore suppose that SAA has the potential to modulate the paclitaxel resistance and the migration and invasion abilities of human breast cancer cells, and so it was used as a reversal agent in the present study
.In the present study, we aimed to detect and explore the role of transgelin 2 in regulating the paclitaxel resistance and the migration and invasion abilities of MCF-7/PTX cells. The effects of SAA on the paclitaxel resistance and the migration and invasion abilities of MCF-7/PTX cells were also investigated, along with the underlying mechanisms.
Results
Paclitaxel-resistant MCF-7/PTX cells appeared morphological transformation
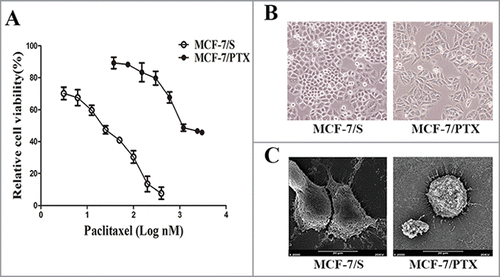
We successfully established the paclitaxel resistant MCF-7/PTX cell line; the IC50 values of paclitaxel for MCF-7/S and MCF-7/PTX cells were (20.0 ± 0.9) and (2,290.9 ± 125.2) nM, respectively. The resistance factor of the MCF-7/PTX cell line was 115, indicating that the cells achieved a high degree of resistance to paclitaxelCitation12 (). Furthermore, the morphology of MCF-7/PTX cells differed from that of MCF-7/S cells in both light microscopy and scanning electron microscopy. We observed that MCF-7/PTX cells displayed elongated, fusiform features with numerous filaments and protuberances, whereas MCF-7/S cells had an epithelial cobblestone appearance with few filaments and protuberances ()
Figure 2. The effect of paclitaxel on MCF-7/S with MCF-7/PTX cells viability and morphological observation of cells. (A) The effect of paclitaxel on MCF-7/S and MCF-7/PTX cells viability was tested by MTT assay. Data were presented as mean ± SD from independent 3 experiments. (B) Cells were observed under inverted microscope (original magnification ×200). (C) Cells were observed under scanning electron microscope (original magnification ×2000).
EMT process existed in MCF-7/PTX cells
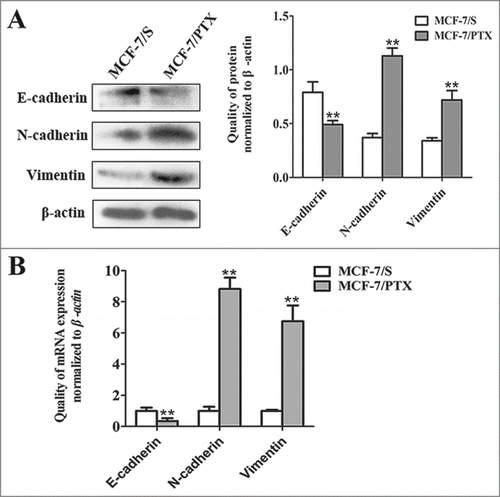
Chemoresistance is thought to be strongly correlated with EMT, combining with the morphological transformation.Citation20 To confirm whether MCF-7/PTX cells had EMT features, the expression of EMT makers—including E-cadherin, N-cadherin and Vimentin—were examined by western blot and qRT-PCR methods. The obtained data showed that the expression of the epithelial marker E-cadherin was significantly lower in MCF-7/PTX cells than in MCF-7/S cells. However, the levels of mesenchymal markers N-cadherin and Vimentin were higher in MCF-7/PTX cells than in MCF-7/S cells (). Taken together, these observations strongly indicate that the EMT process exists in MCF-7/PTX cells
Figure 3. The expression levels of E-cadherin, N-cadherin and Vimentin in MCF-7/S and MCF-7/PTX cells were analyzed. (A) Western blot assay was performed to determine the expression level of E-cadherin, N-cadherin and Vimentin in MCF-7/S and MCF-7/PTX cells. (B) The expression of E-cadherin, N-cadherin and Vimentin were compared by qRT-PCR assay in MCF-7/S and MCF-7/PTX cells. Results were expressed as mean ± SD from 3 experiments. **P< 0.01 by ANOVA vs. MCF-7/S.
MCF-7/PTX cells increased migratory and invasive capability
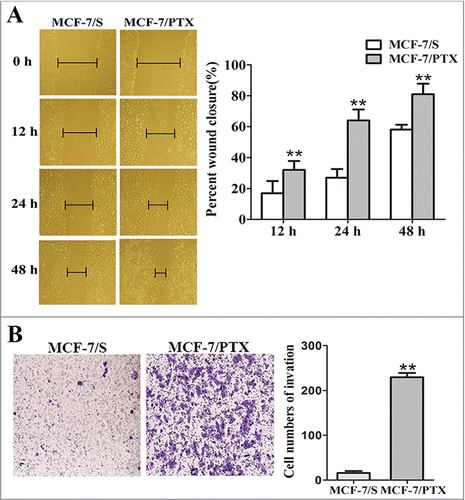
The acquisition of EMT can enhance the migration and invasion abilities of cancer cells.Citation21 The different migration and invasion characteristics of MCF-7/S and MCF-7/PTX cells were investigated by performing wound healing scratch and Transwell invasion assays, respectively. As shown in , MCF-7/PTX cells exhibited significantly enhanced migratory abilities at 0, 12, 24 and 48 h. (). Transwell invasion assay also revealed the strong invasiveness of these cells: the number of invasive cells increased was about 13-fold higher for MCF-7/PTX cells than for MCF-7/S cells (). The data confirm that MCF-7/PTX cells have enhanced migration and invasion abilities
Figure 4. The abilities of migration and invasion were detected in MCF-7/S and MCF-7/PTX cells. (A) Wound-healing assay was used to measure migration ability of cells (original magnification ×100). (B) Transwell invasion assay shown invasion ability of cells (original magnification ×100). Results were expressed as mean ± SD from 3 experiments. **P< 0.01 by ANOVA vs. MCF-7/S.
Knockdown of transgelin 2 inhibited paclitaxel resistance, migration and invasion in MCF-7/PTX cells
Our previous study demonstrated that transgelin 2 was ectopically overexpressed in MCF-7/PTX cells,Citation13 (). To further determine whether transgelin 2 was involved in the paclitaxel resistance and the migration and invasion abilities of MCF-7/PTX cells, TAGLN2 was knocked down in MCF-7/PTX cells by transfection with TAGLN2 siRNA (0.1 nM). qRT-PCR, western blot, MTT, wound healing scratch and Transwell invasion assays were then performed with cells transfected with siRNA of TAGLN2 and its negative control. At 48 h post-transfection, mRNA and protein expression levels of transgelin 2 were both prominently reduced, by >70% compared with the siRNA control. Meanwhile, TAGLN2 siRNA treatment changed the EMT property of MCF-7/PTX cells, attenuated N-cadherin and Vimentin, and increased the expression of E-cadherin ()
Figure 5. Transient knockdown of transgelin 2 by siRNA sensitized MCF-7/PTX cells to paclitaxel and inhibited migration and invasion abilities. (A) The expression of transgelin 2 in MCF-7/S and MCF-7/PTX cells was tested by western blot assay. (B) TAGLN2, E-cadherin, N-cadherin and Vimentin gene expression levels were compared in MCF-7/PTX cells transfected with TAGLN2 siRNA (0.1 nM) or control siRNA (0.1 nM) for 48 h. (C) The protein expression of transgelin 2, E-cadherin, N-cadherin and Vimentin were detected in MCF-7/PTX cells transfected with TAGLN2 siRNA or control siRNA. (D) MCF-7/PTX cells transfected with TAGLN2 siRNA or control siRNA for 48 h, were treated with various concentrations of paclitaxel and cell viability was examined by MTT method. (E) Migration of MCF-7/PTX cells transfected with TAGLN2 siRNA or Control siRNA was measured by wound healing assay (original magnification ×100). (F) Invasiveness of MCF-7/PTX cells transfected with TAGLN2 siRNA or control siRNA was detected by Transwell invasion assay (original magnification ×100). Data were presented as mean ± SD from 3 experiments. **P < 0.01 vs. control.
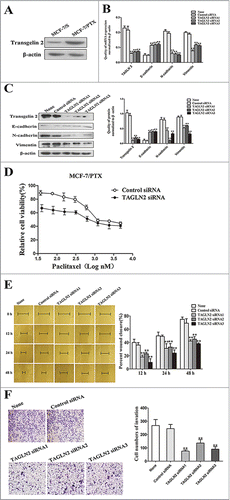
On the other hand, reducing TAGLN2 by siRNA increased the chemosensitivity to paclitaxel in MCF-7/PTX cells, and decreased the IC50 values of paclitaxel for MCF-7/PTX cells from (2,362.3 ± 76.1) to (814.2 ± 13.5) nM (). Additionally, the cellular migration and invasion abilities were clearly inhibited after depleting transgelin 2 (). The above data indicate that the knockdown of transgelin 2 expression by siRNA could contribute to reversing paclitaxel resistance and inhibiting the migration and invasion abilities of MCF-7/PTX cells, which leads us to propose that targeting transgelin 2 could be a useful strategy for increasing the sensitivity of breast cancer cells to paclitaxel and preventing tumor migration and invasion.
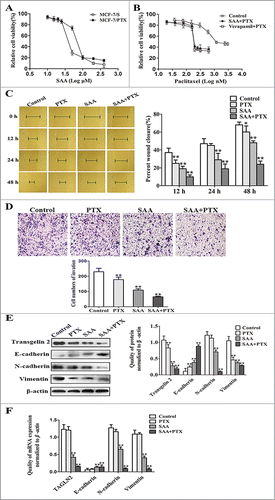
SAA reversed resistance to paclitaxel and inhibited migration, invasion in MCF-7/PTX cells
SAA reportedly exhibits a promising profile as an anti-tumor candidate, but whether or not SAA is capable of reversing the paclitaxel resistance and inhibiting tumor migration and invasion in breast cancer still needed to be determined. First of all, the cytotoxicity and effect of resistance reversal of SAA toward cells were evaluated using the MTT assay. As shown in , SAA inhibited the growth of both MCF-7/S and MCF-7/PTX cells in a dose-dependent manner. The IC10 values of paclitaxel for MCF-7/S cells and MCF-7/PTX cells were (11.9 ± 1.6) and (13.3 ± 2.2) nM, respectively,Citation18 (), indicating that MCF-7/PTX cells did not produce resistance to SAA, and hence that this might be a candidate agent for reversing drug resistance. Therefore, a non-toxic concentration of SAA (12 μM, which produced an inhibition of <10%) was chosen for the subsequent experiments. The reversal index of verapamil (10 μM), used as a positive control, was 10.2-fold. The growth curves showed that SAA augmented the sensitivity of MCF-7/PTX cells to paclitaxel by 9.1-fold, close to the effect of verapamil (), suggesting that SAA has a strong ability to reverse paclitaxel resistance in MCF-7/PTX cells
.To further assess the effect of SAA on the migration and invasion abilities, MCF-7/PTX cells were treated with paclitaxel (0.5 μM) alone, SAA (12 μM) alone, or these 2 drugs in combination. Comparing with the control group, SAA in combination with paclitaxel treatment significantly inhibited the migration () and invasion abilities () of MCF-7/PTX cells.
Since previous studies have confirmed that transgelin 2 and EMT markers exhibit abnormal expression in MCF-7/PTX cells, we used western blot and qRT-PCR assays to determine if these factors are modulated by SAA. As expected, in contrast with the control group, the transgelin 2 level was dramatically reduced in MCF-7/PTX cells following treatment with SAA combined with paclitaxel. Simultaneously, the expression of E-cadherin was markedly elevated, whereas N-cadherin and Vimentin were both clearly reduced, along with the reduction of transgelin 2 (). In brief, these findings indicate that SAA is able to reverse the resistance and inhibit the migration and invasion abilities of MCF-7/PTX cells. Moreover, SAA also changes EMT markers and inhibits transgelin 2 expression.
Discussion
The current study demonstrates that up-regulation of transgelin 2 is critical for paclitaxel resistance and the metastasis and invasion abilities of breast cancer cells in vitro. Furthermore, SAA combined with paclitaxel reversed the resistance to paclitaxel and inhibited the migration and invasion abilities of MCF-7/PTX cells, which might be relevant to the down-regulation of transgelin 2. These findings provide new insights into the molecular function of transgelin 2 as well as the possible role of SAA in the treatment of breast cancer.
There is strong evidence in the literature of a link between chemoresistance and EMT, which is a critical event in cancer development.Citation22 It was shown that gemcitabine-resistant hepatocellular carcinoma cells acquired EMT characteristics with decreased E-cadherin and increased Vimentin and Snail.Citation23 Similarly, paclitaxel-resistant ovarian cancer cells exhibited the EMT phenotype.Citation24 Liu et al.Citation25 found that cells with the EMT phenotype acquired enhanced metastasis and invasion abilities. Consistent with these reports, the present study found that MCF-7/PTX cells displayed the EMT phenotype with downregulation of E-cadherin and upregulation of N-cadherin and Vimentin, and they also exhibited enhanced migration and invasion abilities. Our findings suggest that paclitaxel resistance is associated with EMT in breast cancer.
Transgelin 2, an actin cross-linking/gelling protein, is mostly distributed in the cytoplasm and cell membrane.Citation26 There is very strong evidence that transgelin 2 plays an oncogenic role in the development of human tumors. In support of the function of transgelin 2, the overexpression of transgelin 2 was found and silencing of TAGLN2 increased the apoptosis of bladder cancer cells.Citation27 Moreover, up-regulated transgelin 2 was also found to be associated with the growth of lung cancer cells.Citation28 Consistent with these findings, our previous study showed that transgelin 2 was critically involved in the growth of MCF-7/PTX cells.Citation13 On the other hand, there is some evidence that transgelin 2 is associated with resistance to chemotherapeutic treatments and with tumor migration and invasion. For example, it was reported that transgelin 2 could modulate the resistance to both chemotherapy- and radiation therapy via the insulin-like growth factor 1 receptor (IGF1R)/PI3K/Akt pathway in lung adenocarcinoma.Citation28,29 Zhang et al.Citation30 demonstrated that the overexpression of transgelin 2 was associated with lymph node and distant metastasis, advanced clinical stage, and shorter overall survival in colorectal cancer. The present study is the first to reveal that TAGLN2 knockdown sensitizes MCF-7/PTX cells to paclitaxel and inhibits tumor migration and invasion. Based on these results, we conclude that transgelin 2 is vitally important for paclitaxel resistance and the migration and invasion abilities of human breast cancer cells.
Recent studies have highlighted the key role of SAA in cancer therapy. For example, Wang et al.Citation31 found that SAA showed higher antitumor activity against multidrug-resistant breast cancer cells and enhanced the apoptosis of tumor cells. It was also revealed that SAA could decrease cell migration by modulating matrix metalloproteinase-9.Citation32,33 Moreover, Yang et al.Citation34 found that SAA inhibited angiotensin-II-induced proliferation of human umbilical vein endothelial cells. In line with these findings, our results showed that SAA reverses the resistance to paclitaxel in MCF-7/PTX cells. At the same time, SAA combined with paclitaxel treatment inhibited the migration and invasion abilities of MCF-7/PTX cells. A possible reason for why the biological behavior of MCF-7/PTX cells is changed by SAA is a decrease in the level of transgelin 2. This leads to the suggestion that transgelin 2 could serve as a new molecular target for breast cancer diagnosis and treatment.
While some research about transgelin 2 in MCF-7/PTX has been reported, future studies are needed to investigate the effects of transgelin 2 in native drug-resistant breast cancer cells instead of in artificially induced cancer cells. In addition, the MCF-7/S cell line is positive for the estrogen receptor. Therefore, in order to further explore the biological function of transgelin 2, future studies should investigate estrogen-receptor-negative breast cancer cells, such as MDA-MB-231 and SK-BR-3 cells.
In summary, our findings have demonstrated that MCF-7/PTX cells are highly resistant to paclitaxel and show strong migration and invasion abilities, in part due to the overexpression of transgelin 2, since down-regulating the expression of transgelin 2 was able to reverse the paclitaxel resistance and inhibit the migration and invasion abilities of these cells. More importantly, SAA could also reverse the resistance to paclitaxel and inhibit the migration and invasion abilities of MCF-7/PTX cells, partly by targeting transgelin 2 expression. It is therefore plausible that inactivation of transgelin 2 could represent a novel strategy for inhibiting cell growth, migration, and invasion in breast cancer. Moreover, due to its low toxicity, the inactivation of transgelin 2 by SAA is likely to be a safer approach for the treatment of breast cancer.
Materials and Methods
Chemicals and antibodies
Paclitaxel and salvianolic acid A (12 μM) were purchased from Sike Pharmaceutical and Tianzhen Pharmaceutical, respectively. Verapamil was obtained from China Pharmaceutical Biological Products Analysis Institute. 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) was from Sigma (M-5655). The human monoclonal anti-N-cadherin was purchased from Abcam (1791-4), the monoclonal anti-Vimentin (2862-1) and anti-transgelin 2 (115082) antibodies were purchased from GeneTex, the monoclonal anti-E-cadherin was purchased from Cell Signaling Technology, the polyclonal anti-β-actin antibody was obtained from Biosynthesis Biotechnology (0061R) and the horseradish- peroxidase-conjugated secondary antibody was from Cwbiotech (CW0103).
Cell lines and cell culture
The MCF-7/S human breast cancer cell line was obtained from the Cell Bank of Shanghai, Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. The paclitaxel-resistant MCF-7/S (MCF-7/PTX) cell line was established as previously described.Citation12 MCF-7/S cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum 1% penicillin and streptomycin at 37°C in a humidified atmosphere of 5% CO2. The other culture conditions of MCF-7/PTX cells were the same as the MCF-7/S cells except that maintaining in 30 nM paclitaxel.
MTT assay
Cell viability was measured on the basis of MTT assay as described previously.Citation35 Cells (5 × 105/mL) were seeded in 96-well plates with different treatment for indicated duration. 20 μL of MTT (5 mg/mL) was added into each well and incubated for 4 h. Then 150 μL of dimethyl sulfoxide was added into each well to solubilize the formazan for 15 min. The absorbance was read at 490 nm on a microplate reader (BioTek). Each treatment was performed in sextuplicate and each experiment was repeated 3 times. Calculate the resistance factor (RF) and reversal index (RI) using the following equation: RF = (IC50 of MCF-7/PTX)/(IC50 of MCF-7/S); RI = IC50 of paclitaxel/IC50 of paclitaxel plus SAA.
Morphological observations
MCF-7/PTX and MCF-7/S cells in exponential phase growth were observed under the inverted light microscope (Olympus). For scanning electron microscope, cells were cultured on cover slip, fixed with 2.5% glutaraldehyde, and post-fixed with 1% osmium tetroxide. Cells were then dehydrated with increasing concentrations of ethanol from 50% to 100% and embedded in isoamyl acetate, natural drying, ion sputtering. Ultimately, the samples were observed using the scanning electron microscope (Hitachi).
Wound healing scratch assay
Cells were grown as monolayers in 6-well plate until confluent. Cells were serum-starved overnight and an artificial scratch wound was created. Cell debris was removed by washing with PBS. Then cells were maintained in serum-free culture at 37 °C in a humidified atmosphere of 5% CO2. Migration photos were captured at 0, 12, 24 and 48 h after scratching. Experiments were repeated in triplicate independently. Calculate percent wound closure using the following equation: percent wound closure (%) = [1-(Lt/L0)] × 100%.
Transwell invasion assay
The invasiveness of cells was evaluated by a Boyden chamber method. The polycarbonate filters (8 μm pore size, Corning) were coated with Matrigel Matrix (BD Biosciences), and incubated at 37°C for 5 h. Next, 5 × 10Citation5 cells suspended in 200 μL serum free RPMI 1640 were added into the upper chamber, while 800 μL of complete media was added to the lower chamber. After 48 h of incubation, the cells migrated through the matrigel and adhere onto the lower chamber was fixed in 4% paraformaldehyde for 30 min, stained with 0.1% crystal violet and counted under upright microscope (5 fields per chamber). Each invasion assay was repeated in 3 independent experiments.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total mRNA of cells was extracted using RNAfast2000 kit (Fastagen). All PCR reactions were performed using the Prime Script RT Master Mix Perfect Real Time kit (DRR036A, TaKaRa) and SYBR Premix Ex Taq II (TaKaRa) according to the manufacturer's instruction. Each sample was run independently in triplicate. The primer sequences and product length are listed in . The experiments were run on the Bio-Rad CFX96™ Real-time system (Bio-Rad): pre-degeneration for 95°C, 30 s, 1 cycle, and PCR reaction, 95°C 5 s followed by 60°C, 30 s, 40 cycles, and 95°C for 15 s, 60°C for 30 s, 95°C for 15 s for dissociation. β-actin was used as an internal control
Table 1. Primer lists of qRT-PCR
Western blot assay
Cells with different treatments were lysed in RIPA buffer containing protease inhibitor on ice. Then equal amount of protein lysates were electrophoretically separated 10% sodium dodecyl sulfate polyacrylamide gelelectrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). After blocking with 5% nonfat dried milk for 2 h, the membranes were incubated with primary antibodies overnight at 4°C. After incubation with a horseradish peroxidase-conjugated secondary antibody for 2 h at 37°C, the protein bands were detected using the Super Signal West Pico kit (Thermo Scientific). All western blot experiments were repeated at least 3 times.
Small interference RNA (siRNA) and transfection
MCF-7/PTX cells were seeded in a 6-well plate at a density of 5 × 10Citation5 cells per well in RPMI-1640 without antibiotics. After 24 h, the double-stranded siRNA against TAGLN2 or nonspecific control siRNA (Shanghai GenePharma) was transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The efficiency of RNA interference was checked by qRT-PCR and western blot assays, respectively.
Statistical analysis
Statistical analysis was performed using one-way ANOVA. Values were expressed as mean ± standard deviation (SD) from triplicate experiments performed in a parallel manner. P values less than 0.05 were considered statistically significance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work is supported by National Natural Science Foundation of China (No. 81473177).
References
- Benson JR, Jatoi I. The global breast cancer burden. Future Oncol 2012; 8:697-702; PMID:22764767; http://dx.doi.org/10.2217/fon.12.61
- Deng Z, Yan F, Jin Q, Li F, Wu J, Liu X, Zheng H. Reversal of multidrug resistance phenotype in human breast cancer cells using doxorubicin-liposome-microbubble complexes assisted by ultrasound. J Control Release 2014; 174:109-16; PMID:24287101; http://dx.doi.org/10.1016/j.jconrel.2013.11.018
- Wang H, Xu C, Kong X, Li X, Wang Y, Ding X, Yang Q. Trail resistance induces epithelial-mesenchymal transition and enhances invasiveness by suppressing PTEN via miR-221 in breast cancer. PloS one 2014; 9:e99067; PMID:24905916; http://dx.doi.org/10.1371/journal.pone.0099067
- Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012; 36:237-48; PMID:22459198; http://dx.doi.org/10.1016/j.canep.2012.02.007
- Qu C, Zhang W, Zheng G, Zhang Z, Yin J, He Z. Metformin reverses multidrug resistance and epithelial-mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol Cell Biochem 2014; 386:63-71; PMID:24096736; http://dx.doi.org/10.1007/s11010-013-1845-x
- Li S, Xie Y, Zhang W, Gao J, Wang M, Zheng G, Yin X, Xia H, Tao X. Interferon alpha-inducible protein 27 promotes epithelial-mesenchymal transition and induces ovarian tumorigenicity and stemness. J Surg Res 2015; 193:255-64; PMID:25103640; http://dx.doi.org/10.1016/j.jss.2014.06.055
- Wang F, Dai W, Wang Y, Shen M, Chen K, Cheng P, Zhang Y, Wang C, Li J, Zheng Y, et al. The synergistic in vitro and in vivo antitumor effect of combination therapy with salinomycin and 5-fluorouracil against hepatocellular carcinoma. PloS one 2014; 9:e97414; PMID:24816638; http://dx.doi.org/10.1371/journal.pone.0097414
- Li L, Han R, Xiao H, Lin C, Wang Y, Liu H, Li K, Chen H,Sun F, Yang Z, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res 2014; 20:2714-26; PMID:24644001; http://dx.doi.org/10.1158/1078-0432.CCR-13-2613
- Liu H, Zhang X, Li J, Sun B, Qian H, Yin Z. The biological and clinical importance of epithelial-mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol 2015; 141:189-201; PMID:24965746; http://dx.doi.org/10.1007/s00432-014-1752-x
- Li J, Wang Y, Song Y, Fu Z, Yu W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol Cancer 2014; 13:193; PMID:25128483; http://dx.doi.org/10.1186/1476-4598-13-193
- Murray S, Briasoulis E, Linardou H, Bafaloukos D, Papadimitriou C. Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat Rev 2012; 38:890-903; PMID:22465195; http://dx.doi.org/10.1016/j.ctrv.2012.02.011
- Chen SY, Hu SS, Dong Q, Cai JX, Zhang WP, Sun JY, Wang TT, Xie J, He HR, Xing JF, et al. Establishment of paclitaxel-resistant breast cancer cell line and nude mice models, and underlying multidrug resistance mechanisms in vitro and in vivo. Asian Pac J Cancer Prev 2013; 14:6135-40; PMID:24289639; http://dx.doi.org/10.7314/APJCP.2013.14.10.6135
- Chen S, Dong Q, Hu S, Cai J, Zhang W, Sun J, Wang T,Xie J, He H, Xing J, et al. Proteomic analysis of the proteins that are associated with the resistance to paclitaxel in human breast cancer cells. Mol Biosyst 2014; 10:294-303; PMID:24292090; http://dx.doi.org/10.1039/C3MB70428A
- Cai J, Chen S, Zhang W, Hu S, Lu J, Xing J, Dong Y. Paeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2. Phytomedicine 2014; 21:984-91; PMID:24680370; http://dx.doi.org/10.1016/j.phymed.2014.02.012
- Kawakami K, Enokida H, Chiyomaru T, Tatarano S, Yoshino H, Kagara I, Gotanda T, Tachiwada T, Nishiyama K, Nohata N, et al. The functional significance of miR-1 and miR-133a in renal cell carcinoma. Eur J Cancer 2012; 48:827-36; PMID:21745735; http://dx.doi.org/10.1016/j.ejca.2011.06.030
- Zhou L, Zhang R, Zhang L, Sun Y, Yao W, Zhao A, Li J, Yuan Y. Upregulation of transgelin is an independent factor predictive of poor prognosis in patients with advanced pancreatic cancer. Cancer Sci 2013; 104:423-30; PMID:23331552; http://dx.doi.org/10.1111/cas.12107
- Li YJ, Duan CL, Liu JX. Salvianolic acid A promotes the acceleration of neovascularization in the ischemic rat myocardium and the functions of endothelial progenitor cells. J Ethnopharmacol 2014; 151:218-27; PMID:24189032; http://dx.doi.org/10.1016/j.jep.2013.10.019
- Cai J, Chen S, Zhang W, Zheng X, Hu S, Pang C, Lu J, Xing J, Dong Y. Salvianolic acid A reverses paclitaxel resistance in human breast cancer MCF-7 cells via targeting the expression of transgelin 2 and attenuating PI3 K/Akt pathway. Phytomedicine 2014; 21:1725-32; PMID:25442283; http://dx.doi.org/10.1016/j.phymed.2014.08.007
- Sun L, Zhao R, Zhang L, Zhang T, Xin W, Lan X, Huang C, Du G. Salvianolic acid A inhibits PDGF-BB induced vascular smooth muscle cell migration and proliferation while does not constrain endothelial cell proliferation and nitric oxide biosynthesis. Molecules 2012; 17:3333-47; PMID:22418933; http://dx.doi.org/10.3390/molecules17033333
- Huanna T, Tao Z, Xiangfei W, Longfei A, Yuanyuan X, Jianhua W, Cuifang Z, Manjing J, Wenjing C, Shaochuan Q, et al. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol Carcinog 2014; PMID:24962947; http://dx.doi.org/10.1002/mc.22186
- Zang M, Zhang B, Zhang Y, Li J, Su L, Zhu Z, Gu Q,Liu B, Yan M. CEACAM6 Promotes Gastric Cancer Invasion and Metastasis by Inducing Epithelial-Mesenchymal Transition via PI3K/AKT Signaling Pathway. PloS one 2014; 9:e112908; PMID:25398131; http://dx.doi.org/10.1371/journal.pone.0112908
- Jiang L, He D, Yang D, Chen Z, Pan Q, Mao A, Cai Y, Li X, Xing H, Shi M, et al. MiR-489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett 2014; 588:2009-15; PMID:24786471; http://dx.doi.org/10.1016/j.febslet.2014.04.024
- Wu Q, Wang R, Yang Q, Hou X, Chen S, Hou Y, Chen C, Yang Y, Miele L, Sarkar FH, et al. Chemoresistance to gemcitabine in hepatoma cells induces epithelial-mesenchymal transition and involves activation of PDGF-D pathway. Oncotarget 2013; 4:1999-2009; PMID:24158561
- Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol 2007; 31:277-83; PMID:17611683
- Liu L, Dai Y, Chen J, Zeng T, Li Y, Chen L, Zhu YH, Li J, Li Y, Ma S, et al. Maelstrom promotes hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition by way of Akt/GSK-3beta/Snail signaling. Hepatology 2014; 59:531-43; PMID:23929794; http://dx.doi.org/10.1002/hep.26677
- Prinjha RK, Shapland CE, Hsuan JJ, Totty NF, Mason IJ, Lawson D. Cloning and sequencing of cDNAs encoding the actin cross-linking protein transgelin defines a new family of actin-associated proteins. Cell Motil Cytoskeleton 1994; 28:243-55; PMID:7954852; http://dx.doi.org/10.1002/cm.970280307
- Yoshino H, Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N, Nakagawa M. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer 2011; 104:808-18; PMID:21304530; http://dx.doi.org/10.1038/bjc.2011.23
- Kim TR, Cho EW, Paik SG, Kim IG. Hypoxia-induced SM22alpha in A549 cells activates the IGF1R/PI3K/Akt pathway, conferring cellular resistance against chemo- and radiation therapy. FEBS letters 2012; 586:303-9; PMID:22245152; http://dx.doi.org/10.1016/j.febslet.2011.12.036
- Rho JH, Roehrl MH, Wang JY. Tissue proteomics reveals differential and compartment-specific expression of the homologs transgelin and transgelin-2 in lung adenocarcinoma and its stroma. J Proteome Res 2009; 8:5610-8; PMID:19848416; http://dx.doi.org/10.1021/pr900705r
- Zhang Y, Ye Y, Shen D, Jiang K, Zhang H, Sun W, Zhang J, Xu F, Cui Z, Wang S. Identification of transgelin-2 as a biomarker of colorectal cancer by laser capture microdissection and quantitative proteome analysis. Cancer Sci 2010; 101:523-9; PMID:19930159; http://dx.doi.org/10.1111/j.1349-7006.2009.01424.x
- Wang X, Wang C, Zhang L, Li Y, Wang S, Wang J, Yuan C, Niu J, Wang C, Lu G. Salvianolic acid A shows selective cytotoxicity against multidrug-resistant MCF-7 breast cancer cells. Anti-Cancer Drugs 2015; 26:210-23; PMID:25419632; http://dx.doi.org/10.1097/CAD.0000000000000184
- Zhang T, Xu J, Li D, Chen J, Shen X, Xu F, Teng F,Deng Y, Ma H, Zhang L, et al. Salvianolic acid A, a matrix metalloproteinase-9 inhibitor of Salvia miltiorrhiza, attenuates aortic aneurysm formation in apolipoprotein E-deficient mice. Phytomedicine 2014; 21:1137-45; PMID:24916705; http://dx.doi.org/10.1016/j.phymed.2014.05.003
- Jiang B, Li D, Deng Y, Teng F, Chen J, Xue S, Kong X, Luo C, Shen X, Jiang H, et al. Salvianolic acid A, a novel matrix metalloproteinase-9 inhibitor, prevents cardiac remodeling in spontaneously hypertensive rats. PLoS One 2013; 8:e59621; PMID:23533637; http://dx.doi.org/10.1371/journal.pone.0059621
- Yang LL, Li DY, Zhang YB, Zhu MY, Chen D, Xu TD. Salvianolic acid A inhibits angiotensin II-induced proliferation of human umbilical vein endothelial cells by attenuating the production of ROS. Acta Pharmacol Sin 2012; 33:41-8; PMID:22101169; http://dx.doi.org/10.1038/aps.2011.133
- Chen S, Cai J, Zhang W, Zheng X, Hu S, Lu J, Xing J, Dong Y. Proteomic identification of differentially expressed proteins associated with the multiple drug resistance in methotrexate-resistant human breast cancer cells. Int J Oncol 2014; 45:448-58; PMID:24736981; http://dx.doi.org/10.3892/ijo.2014.2389