ABSTRACT
Treatment of metastatic melanoma has changed dramatically in the past 5 years with the approval of six new agents (vemurafenib, dabrafenib, trametinib, ipilimumab, pembrolizumab, and nivolumab) by the US Food and Drug Administration (FDA). This review will compare the immunotherapies recently approved by the FDA (ipilimumab, nivolumab and pembrolizumab) with the long-approved immunotherapy, interleukin-2. Additional consideration will be given to the evolving landscape, including the opportunities for combination regimens. Immunotherapies have distinct mechanisms of action and unique response kinetics that differ from conventional cytotoxic and targeted therapies, and have a range of adverse events that can be safely managed by experienced health-care providers. Data suggest immunotherapies can result in long-term survival in a proportion of patients. This dynamic and evolving field of immunotherapy for melanoma will continue to offer challenges in terms of optimal patient management for the foreseeable future.
Introduction
Skin cancer is the most commonly diagnosed malignancy, and melanoma is the most serious form of skin cancer. The annual incidence of melanoma has been increasing at an alarming rate for the past 50 years.Citation1Current projections estimate a 400% increase in the incidence of melanoma in men and a nearly 800% increase in women under the age of 39 years compared with historic incidence rates.Citation2 The lifetime risk of developing melanoma is now 1 in 50 white Americans, and it is anticipated that there will be 76,100 new cases of melanoma and 9710 melanoma-related deaths in the United States in 2014.Citation3,Citation4 Early detection and surgical management of early-stage melanoma are associated with 5-year survival rates of over 90%, but once the disease metastasizes to regional lymph nodes, the 5-year survival drops to 50%; metastatic disease in visceral organs is associated with a median survival of 9–12 months and an estimated 3-year survival rate of 15%.Citation5
The treatment of metastatic melanoma has changed dramatically in the past few years with the approval by the US Food and Drug Administration (FDA) of six new agents since 2011 (vemurafenib, dabrafenib, trametinib, ipilimumab, pembrolizumab, and nivolumab). Currently, the systemic management of metastatic melanoma may include agents classified as cytotoxic chemotherapy (dacarbazine), molecularly targeted therapy (vemurafenib, dabrafenib, trametinib), or immunotherapy (aldesleukin or interleukin-2 [IL-2], ipilimumab, nivolumab, and pembrolizumab). Although chemotherapy is still used to treat melanoma, the low overall survival (OS), reported as 6 months or less, makes this option less appealing in most cases.Citation6 Targeted therapies (vemurafenib, dabrafenib, and trametinib) are only approved for use in melanoma harboring a mutation in BRAF V600E or V600K. While these drugs have been associated with a relatively high objective response rate (ORR) in patients (approximately50%),Citation7,Citation8 resistance and eventual disease recurrence are common.Citation9 In contrast, immunotherapy agents have been associated with durable long-term survival in a proportion of patients, although response rates, particularly with IL-2 and ipilimumab, are relatively low (11–40% across all approved immunotherapies) compared with targeted therapy.Citation10–17
Ipilimumab, a monoclonal antibody directed against the immune checkpoint receptor cytotoxic T-lymphocyte antigen 4 (CTLA-4), was initially approved by the FDA in 2011, and was subsequently approved in many other countries worldwide. The success of ipilimumab, which was the first agent to improve the overall survival of patients with advanced melanoma in a phase III trial,Citation18,Citation19 encouraged the development of agents designed to inhibit other immune checkpoint pathways such as programmed cell death-1 (PD-1).Citation20 Pembrolizumab and nivolumab, two different monoclonal antibodies directed against PD-1, were approved by the FDA in 2014 for the treatment of metastatic melanoma in patients who had failed ipilimumab and, if they had a BRAF V600 mutation, had also progressed on BRAF inhibitor therapy.Citation21,Citation22 On June 19, 2015, nivolumab was approved in the European Union for the treatment of unresectable or metastatic melanoma (untreated or previously treated), regardless of BRAF mutation status; pembrolizumab was subsequently approved in the European Union on July 22, 2015, for the same indication. All three checkpoint inhibitors, as well as high-dose (HD) IL-2, are recommended for first- and second-line treatment of advanced melanoma by the National Comprehensive Cancer Network (NCCN) guidelines, with treatment choice depending on evaluation of the individual patient.Citation23
This review will compare the pharmacology of all currently approved immunotherapy agents, with a focus on IL-2, ipilimumab, nivolumab and pembrolizumab. These four agents have distinct mechanisms of action, unique kinetics of response, and a range of adverse events (AEs) associated with their mechanism of action that can be safely managed by experienced health-care providers. Since the clinical management of melanoma patients receiving immunotherapy differs from that of patients receiving conventional cytotoxic and targeted therapies, we discuss clinical management guidelines for safe administration and patient monitoring of these novel agents. With an increasing array of treatments available for advanced melanoma, we consider factors that influence choice of treatment for an individual patient. Finally, we discuss the evolving treatment landscape of melanoma, including the opportunities for combination therapy.
Pharmacology of immunotherapy agents used to treat melanoma mechanism of action
Tumor development and the scope for immunotherapy
Tumors develop when the immune system fails to eradicate cells which, having acquired numerous somatic mutations, no longer have a normal phenotype and can expand uncontrollably. Dysregulation of the immune system, which may be caused in part by immunogenic changes in the tumor cells, can play a major role in tumor growth, allowing the tumor cells to avoid immune-mediated detection and destruction.Citation24–26 In addition to lack of immunological recognition, tumors may escape immunosurveillance by the induction of central or peripheral immune tolerance. The range of mechanisms employed by tumors to evade immune destruction lends itself to different approaches for immunotherapy.
IL-2
IL-2 was initially identified in 1976 as a soluble factor in the supernatant of activated lymphocyte cultures that selectively stimulated the in vitro proliferation and survival of normal human T cells.Citation27 The first use of IL-2 first as immunotherapy for metastatic cancer was to generate and expand ex vivo populations of lymphokine-activated killer cells for adoptive T-cell transfer.Citation28 However, it soon became clear that IL-2 alone was capable of mediating tumor regression,Citation29 which ultimately led to the clinical trials that support the current use of high-dose (HD) IL-2 monotherapy for advanced melanoma.Citation30
Although the precise mechanism of IL-2 -mediated tumor regression has not been characterized, it seems to involve the induction and augmentation of the inherent antitumor activity of CD8+ cytotoxic T lymphocytes and the cytolytic activity of natural killer cells.Citation31 However, IL-2 also has a critical role in the activation of immunosuppressive regulatory T cells (Tregs).Citation32 These cells normally act to preserve peripheral T-cell tolerance and prevent autoimmunity, but they can also blunt lymphocyte-mediated tumor rejection. Thus, although HD IL-2 is capable of bolstering the activity of antitumor effector T cells, this effect may be counteracted in some patients by the concomitant activation of Tregs. Patients who respond to IL-2 seem to experience a transient reduction in circulating Tregs, whereas the Treg levels among nonresponders remain stable or increase after treatment.Citation33
Immune checkpoint inhibitors
From a therapeutic perspective, the checkpoint receptors are some of the most promising elements recently implicated in cancer immune system dysregulation. These receptors play a major role in maintaining self-tolerance and limiting the extent of immune responses to infection, but can be exploited by tumors as an important immune resistance mechanism.Citation20
The process of T-cell activation is a complex balance of stimulatory and inhibitory signals. In brief, T-cell activation requires that a T-cell receptor recognizes and binds to antigen on a major histocompatibility complex molecule, and that there is a costimulatory signal from a B7 molecule on the surface of the antigen-presenting cell binding to CD28 on the T cell. CTLA-4, a homolog of CD28, is expressed on the T-cell surface and also binds B7, but results in T-cell inhibition. Ipilimumab is a fully human, anti-CTLA-4 monoclonal antibody designed to block the CTLA-4 immune checkpoint and thereby augment antitumor T-cell responses ().Citation37,Citation38 Additional data also suggest that depletion or blockade of the activity of Tregs may contribute to the antitumor effects of ipilimumab.Citation38
Figure 1. Blockade of CTLA-4 and PD-1: distinct immune checkpoint molecules. Upon antigen presentation by APCs (eg, dendritic cell) via the TCR, T cells become activated or suppressed depending on secondary signaling through CD28 or CTLA-4, respectively. Ipilimumab is a fully human, monoclonal antibody designed to block the immune checkpoint inhibitor, CTLA-4, permitting increased signaling through CD28 and sustained T-cell activation. Unlike CTLA-4 expression which occurs early during T cell activation, the PD-1 receptor is typically upregulated after prolonged T cell receptor stimulation during an ongoing immune response.Citation34 While CTLA-4 limits T cell activation and clonal expansion, the main function of PD-1, when bound to one of its ligands (PD-L1 or PD-L2), is to limit effector T cell function in the tumor microenvironment.Citation20,Citation35,Citation36 Anti-PD-1 antibodies block the interaction between PD-1 and its ligands, thereby interfering with inhibitory signaling between tumor cells and T cells within the tumor microenvironment. APC, antigen presenting cell; MHC, major histocompatibility complex; TCR, T cell receptor. Adapted from an oral presentation at the 2013 Annual Meeting of the American Society of Clinical Oncology [Callahan MK Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Clin Oncol 31, 2013 (suppl; abstr 3003)]
![Figure 1. Blockade of CTLA-4 and PD-1: distinct immune checkpoint molecules. Upon antigen presentation by APCs (eg, dendritic cell) via the TCR, T cells become activated or suppressed depending on secondary signaling through CD28 or CTLA-4, respectively. Ipilimumab is a fully human, monoclonal antibody designed to block the immune checkpoint inhibitor, CTLA-4, permitting increased signaling through CD28 and sustained T-cell activation. Unlike CTLA-4 expression which occurs early during T cell activation, the PD-1 receptor is typically upregulated after prolonged T cell receptor stimulation during an ongoing immune response.Citation34 While CTLA-4 limits T cell activation and clonal expansion, the main function of PD-1, when bound to one of its ligands (PD-L1 or PD-L2), is to limit effector T cell function in the tumor microenvironment.Citation20,Citation35,Citation36 Anti-PD-1 antibodies block the interaction between PD-1 and its ligands, thereby interfering with inhibitory signaling between tumor cells and T cells within the tumor microenvironment. APC, antigen presenting cell; MHC, major histocompatibility complex; TCR, T cell receptor. Adapted from an oral presentation at the 2013 Annual Meeting of the American Society of Clinical Oncology [Callahan MK Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Clin Oncol 31, 2013 (suppl; abstr 3003)]](/cms/asset/5bedf010-8dbc-4733-ac33-ff7a24fa9e48/kcbt_a_1095401_f0001_oc.jpg)
In addition to the CTLA-4 pathway, the immune system has a number of other inhibitory or checkpoint pathways that regulate T-cell activity in different ways to provide a natural counterbalance to costimulatory pathways, limiting the size and duration of a T-cell response and preventing damage to normal tissue.Citation39 One of these, the PD-1 receptor, is also expressed on activated T cells; however, unlike CTLA-4 expression which occurs early during T cell activation, PD-1 is typically upregulated after prolonged T cell receptor stimulation during an ongoing immune response ().Citation34 While CTLA-4 limits T cell activation and clonal expansion, the main function of PD-1, when bound to one of its ligands (PD-L1 or PD-L2), is to limit effector T cell function in the tumor microenvironment.Citation20,Citation35,Citation36 PD-L1 and PD-L2 are expressed on a variety of hematopoietic and non-hematopoietic cells; PD-L1 is also commonly expressed by melanomas and many other tumor types.Citation20,Citation40,Citation41 PD-L1 expression by tumor cells appears to facilitate immune evasion, inhibiting T cell activation and lysis of tumor cells and, in some cases, leading to increased tumor-specific T cell death; increased PD-1 expression on tumor infiltrating lymphocytes may also contribute to tumor immunosuppression.Citation42 Like CTLA-4, PD-1 is also expressed by TregsCitation35,Citation42 and may be required for the suppressive function of these cells.Citation43
Nivolumab, a fully human IgG4 anti-PD-1 monoclonal antibody, has been shown in preclinical studies to potently enhance cytokine production and stimulate antigen-specific T-cell responses.Citation44 Pembrolizumab, a humanized anti-PD-1 IgG4-kappa isotype monoclonal antibody, showed preclinical antitumor activity in a range of tumor types.Citation45
Clinical efficacy and response patterns
Key efficacy data for each of the immunotherapy agents approved by the FDA for the treatment of advanced melanoma are summarized in .
Table 1. Key clinical efficacy data for FDA-approved immunotherapy agents for advanced melanoma
High dose IL-2
HD IL-2 was approved by the FDA in 1998 for the treatment of metastatic melanoma based on its ability to induce a durable objective response in a small subset of patients treated in phase II studies.Citation30 No phase III trials comparing HD IL-2 to other treatments, providing an assessment of relative impact on OS, have been done. In a pooled analysis of 270 patients with metastatic melanoma treated with HD IL-2 in eight phase II clinical trials, the overall ORR was 16% (95% confidence interval [CI], 12–21%), with 6% achieving a complete response and 10% achieving a partial response.Citation30 The median duration of response was 8.9 months among all responders, and had not been reached but exceeded 59 months for complete responders.Citation46 None of the patients responding for over 30 months progressed at any point thereafter, suggesting that treatment was, effectively, curative. Long-term follow-up data show that over 80% of complete responders remain disease-free at 17 to 253 months of follow-up, with median duration of response more than 176 months.Citation47 Most responses to IL-2 usually occur early; in one report, 90% of responders to IL-2 demonstrated some tumor regression after the first course of therapy.Citation48
Ipilimumab
Approval of ipilimumab for the treatment of advanced melanoma was based on data from two phase III studies that demonstrated significant improvements in OS. MDX010-20 was a placebo-controlled, randomized trial of 676 patients with previously treated unresectable or metastatic melanoma who received ipilimumab 3 mg/kg alone, ipilimumab 3 mg/kg in combination with the peptide vaccine gp100, or gp100 alone. Median OS was significantly greater with ipilimumab plus gp100 compared with gp100 alone (10.0 months vs 6.4 months; hazard ratio [HR] = 0.68; P< .001) and with ipilimumab monotherapy compared with gp100 alone (10.1 months vs 6.4 months; HR = 0.66; P= .003).Citation18 Follow-up analysis from this study showed the survival rate was 25% at both 2 and 3 years for patients who received ipilimumab alone.Citation12
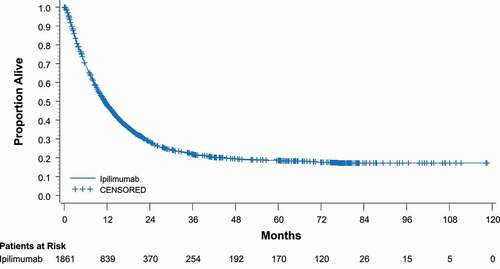
In study CA184-024, dacarbazine plus ipilimumab (10 mg/kg) was compared with dacarbazine alone in treatment-naive patients with advanced melanoma. OS rates were higher with the combination at 1 year (47.3% vs 36.3%), 2 years (28.5% vs 17.9%), and 3 years (20.8% vs 12.2%) (HR for death = 0.72; P< .001).Citation19 The most recent data from this study show that twice as many people who received the ipilimumab combination were alive at 5 years as those receiving dacarbazine only (18.2% vs 8.8%; P= .002).Citation49 A recent pooled analysis of data from 1861 patients across 12 ipilimumab studies showed the median OS was 11.4 months, with 22% of the patients alive after 3 years, and most remaining in remission thereafter with follow-up of up to 10 years ().Citation13
Figure 2. Overall survival with ipilimumab in a pooled analysis. In a group of 1861 patients treated with ipilimumab, median OS was 11.4 months and the 3-year OS rate was 22%.Citation13 CI, confidence interval. Reprinted with permission by the American Society of Clinical Oncology
Patients treated with ipilimumab have shown heterogeneous response patterns, some of which differ from responses seen with chemotherapy and HD IL-2, presumably due to the unique mechanism of action of ipilimumab and the time it can take for a patient to develop a clinically effective immune response.Citation50,Citation51 Responses may be delayed for many weeks and may even occur after apparent disease progression. In patients with advanced melanoma, the median time to response was 2.86 months in a phase III trial.Citation16 So far, four distinct variations have been recorded: (1) response in baseline (index) lesions; (2) a slow, steady decline in tumor burden; (3) response after an increase in tumor burden; and (4) response in index and new lesions accompanied by the appearance of other new lesions. While all four patterns have been associated with favorable survival, patterns (3) and (4) may be unfamiliar to many treating oncologists and also are not well-characterized by standard response assessment criteria; instead, ‘immune-related’ response criteria have been proposed.Citation50,Citation51
The potential for these unconventional patterns of response may lead to inaccurate response assessment using conventional tumor response criteria, or if assessment occurs too soon. Initial assessment is currently recommended after the 12-week induction phase of treatment. Scans should be repeated 2 weeks after the last treatment and again a month later to confirm response.Citation52
Nivolumab and pembrolizumab
Nivolumab and pembrolizumab were both approved for the treatment of unresectable or metastatic melanoma on the basis of data from phase I trials showing relatively high response rates and long duration of responses, lasting more than two year in some cases.Citation53,Citation54 Recent landmark analysis of the nivolumab phase I trial showed 1-, 2-, and 3-year survival rates of 63%, 48%, and 41%, and median OS of 17.3 months for patients with advanced melanoma (n = 107).Citation14 Similarly, a pooled analysis of 411 patients treated with pembrolizumab in a phase I study showed a 1-year OS rate of 69%.Citation55
Several phase III trials with nivolumab and pembrolizumab have now been reported, with the results confirming the early promise of both agents. In a phase III trial of patients previously treated with ipilimumab, the objective response rate (ORR) was approximately 32% in patients randomized to nivolumab (3 mg/kg) and 11% in those receiving chemotherapy; overall survival data were not mature at the time of analysis.Citation17 In patients with previously untreated metastatic melanoma without BRAF mutation, nivolumab (3 mg/kg) significantly improved OS compared with dacarbazine (HR for death = 0.42; P < .001).Citation15 The OS rate at 1 year for nivolumab vs dacarbazine was 72.7% vs 42.1%; median OS had not been reached in the nivolumab group and was 10.8 months in the dacarbazine group. The study was terminated early after this interim analysis so that patients receiving dacarbazine could be switched to nivolumab.
A phase III trial has now compared the efficacy of two different dosing schedules of pembrolizumab (10 mg/kg) with ipilimumab (3 mg/kg) in advanced melanoma.Citation16 Patients randomized to pembrolizumab had significantly improved OS compared with those randomized to ipilimumab, regardless of which schedule was used. The HR for death for pembrolizumab given every two weeks (Q2W) when compared with ipilimumab was 0.63 (95% CI: 0.47, 0.83; P< .0005) and for pembrolizumab given every three weeks (Q3W) was 0.69 (95% CI: 0.52, 0.90; P= .036). One-year overall survival rates were 74.1%, 68.4%, and 58.2% in the pembrolizumab Q2W, pembrolizumab Q3W, and ipilimumab groups, respectively; median OS was not reached in any group. Pembrolizumab benefited all subgroups analyzed, with the exception of patients with PD-L1-negative tumors.
As with ipilimumab, anti-PD-1 therapy has been associated with unconventional or ‘immune-related’ responses in patients with melanoma, although the majority of responses meet traditional RECIST criteria.Citation15,Citation17,Citation54 Most responses have been detected at the first assessment (8–12 weeks after starting treatment), but responses occurring as late as 8 months after starting treatment have also been reported.Citation15–17 Because unconventional responses may occur, trial protocols with nivolumab or pembrolizumab typically allowed continued treatment in patients with possible tumor progression provided other clinical parameters were favorable.Citation15–17
Dosing and patient selection
IL-2
IL-2 is administered in an inpatient setting as a high-dose (600,000 or 720,000 IU/kg) intravenous bolus infusion given over 15 minutes via a centrally placed catheter. Distribution and elimination are relatively rapid, and the drug is ultimately metabolized and excreted by the kidneys.Citation56 A course of therapy generally consists of 2 inpatient treatment cycles separated by 9–14 days of rest. During each cycle, up to 14 doses are administered every 8 hours, typically until the onset of significant toxicity – most of which quickly resolves upon treatment cessation because IL-2 has a short half-life. Doses may be held for significant toxicity. Reduced doses are not generally given because IL-2 is maximally effective when delivered with a high-dose, bolus administration regimen.Citation57 Progressive disease after a course of HD IL-2 represents treatment failure and is generally a contraindication to further therapy.
The success of IL-2 is partly determined by careful selection of patients who can tolerate treatment. Contraindications have traditionally included poor performance status (Eastern Cooperative Oncology Group [ECOG] performance status ≥2), untreated brain metastases, infection, pleural effusions, ascites, and the presence of significant cardiac, pulmonary, or autoimmune disease.
Ipilimumab, nivolumab and pembrolizumab
Ipilimumab is administered as a 90-min intravenous infusion at a dose of 3 mg/kg every 3 weeks for 4 doses over a total period of 12 weeks (induction phase).Citation52 Ipilimumab can be safely administrated in the ambulatory setting. Ipilimumab retreatment is an option in progressing patients who experienced an objective response or stability lasting at least 3 months and had no serious treatment-related toxicity with their initial ipilimumab treatment.Citation23
Nivolumab is administered as an intravenous infusion over 60 minutes every 2 weeks at a dose of 3 mg/kg, and pembrolizumab is administered at a dose of 2 mg/kg as an intravenous infusion over 30 minutes every 3 weeks; both drugs are continued until disease progression or unacceptable toxicity.Citation21,Citation22 No pre-medication is needed for infusion of these agents and infusion-related reactions are rare.Citation21,Citation22
There are no specific contradictions to ipilimumab, nivolumab or pembrolizumab use in their respective FDA labels. However, like IL-2, none of these agents has been studied in patients with autoimmune diseases.Citation21,Citation22,Citation52 All immunotherapies need to be given with caution in patients with underlying autoimmune disease.
Safety
IL-2
Most HD IL-2 toxicities are preventable, are easily managed by an experienced provider, and are nearly all reversible. Comprehensive screening guidelines and management algorithms have reduced the risk of treatment-related mortality to virtually zero.Citation58 The main AE associated with HD IL-2 is capillary leak syndrome (CLS), which manifests clinically with persistent hypotension, edema, and low urine output. CLS develops within hours of the first infusion and is caused by a combination of systemic vasodilation and increased vascular permeability resulting in plasma extravasation and third space fluid sequestration. Management involves careful fluid replacement with the goal of maintaining adequate intravascular volume without causing pulmonary edema. If adequate volume cannot be maintained through fluid replacement, vasopressor support may be necessary.
Other common systemic AEs, such as fevers, myalgias, and nausea, are managed prophylactically and almost always subside within hours after treatment is stopped. One of the few potential long-term AEs of IL-2 is autoimmune thyroiditis leading to hypothyroidism, which occurs in up to one-third of patients and may require hormone replacement therapy.Citation59 Autoimmune vitiligo has also been seen following IL-2 therapy, although this is often not reported or sought by clinicians as part of the post-treatment assessment.Citation30,Citation59–64
Ipilimumab, nivolumab and pembrolizumab
Most toxicities associated with immune checkpoint inhibitors are of mild to moderate severity and reversible, but serious AEs and, rarely, fatalities can also occur.Citation15–18,Citation65 Phase III data suggest that, overall, the incidence and severity of treatment-related AEs reported with ipilimumab are slightly higher than those reported with nivolumab;Citation15,Citation17,Citation18,Citation65 rates with ipilimumab may also be higher than those reported with pembrolizumab, but phase III data using the approved dose of pembrolizumab (2 mg/kg) are lacking. Reported rates of any-grade and grade 3/4 treatment-related AEs range from 73% to 86% and 20 to 27%, respectively, for ipilimumab,Citation16,Citation18,Citation65 and from 59% to 82% and 9 to 16%, respectively, for nivolumab.Citation15,Citation17,Citation65 Using pembrolizumab 10 mg/kg, the reported rate of any treatment-related AEs was 76% and of grade 3/4 AEs was 12%.Citation16
The immune activation induced by each of the three approved immune checkpoint inhibitors has been associated with various immune-related AEs (irAEs). These irAEs include, most notably, cutaneous, gastrointestinal, hepatic, and endocrine AEs, as well as (more rarely) pulmonary and renal toxicity ().Citation15–18,Citation65–71 In addition, ipilimumab can cause severe neurological irAEs, including sensory and motor neuropathy, Guillain-Barré syndrome, and myasthenia gravis.Citation52,Citation72 However, most irAEs in clinical trials were of mild or moderate severity. The incidence and severity of gastrointestinal irAEs, and in particular of colitis, appear higher with ipilimumab than with either nivolumab or pembrolizumab, while the incidence (but not severity) of endocrine irAEs is slightly higher with the anti-PD-1 antibodies than with ipilimumab (). In nivolumab clinical trials, AEs were similar in treatment-naïve patients and those who had received prior ipilimumab,Citation15,Citation17 suggesting that the safety profile of nivolumab is unaffected by previous exposure to ipilimumab therapy.
Table 2. Comparison of key immune-mediated adverse events with ipilimumab, nivolumab and pembrolizumab as reported in phase III trials, by organ category
Guidelines for management of irAEs are available for approved immune checkpoint inhibitors,Citation15,Citation17,Citation18,Citation73–82 and are summarized in for ipilimumab and nivolumab, respectively. Although precise recommendations vary with irAE and agent, general principles for management include vigilance with close patient monitoring, prompt intervention, withholding treatment until symptoms subside, and use of corticosteroids when appropriate.Citation15,Citation17,Citation72–74 In the event of a severe irAE, prompt treatment with systemic corticosteroids (for example, prednisone typically administered at a dose of 1 to 2 mg/kg/d or equivalent) is recommended. For some grade 3 and all grade 4 irAEs, permanent discontinuation of the relevant immune checkpoint inhibitor is recommended. In the case of ipilimumab, treatment should also be permanently discontinued if symptoms persist such that the full treatment course cannot be completed within 16 weeks of initiation.Citation72 Any irAEs would generally emerge before the last dose of the treatment course, although hepatitis and endocrinopathies have been reported after the last dose of drug.Citation70
Table 3. Monitoring and management of common or potentially severe immune-related adverse events associated with ipilimumab
Table 4. Management and follow-up of immune-related adverse events associated with nivolumab (adapted from Robert 2015a15 and Bristol-Myers Squibb 201574)
The available data from nivolumab and ipilimumab phase III trials showed that 70–100% of grade 3/4 irAEs resolved successfully using recommended management guidelines and immune modulatory medication.Citation15,Citation17,Citation65 Unlike most irAEs observed with immune checkpoint inhibitors, endocrinopathies associated with treatment may be irreversible, but can be managed with hormone replacement therapy (without corticosteroids); usually, no interruption in immune checkpoint inhibitor therapy should be necessary.Citation15,Citation17,Citation18,Citation73,Citation74
Considerations for immunotherapy patient selection and sequencing strategies
Patients who may be unsuited for immunotherapy
Immunotherapy has the potential for long-term survival in patients with advanced melanoma. Patients may not be ideal candidates for IL-2 or ipilimumab, if they have rapidly progressive disease;Citation23 such patients may not tolerate treatment well, or may have insufficient predicted survival in which to achieve a response. In this type of patient, nivolumab and pembrolizumab are options or, if a BRAF mutation is present, one might consider attempting to halt the progression with a BRAF inhibitor initially, with the goal of switching to immunotherapy.Citation23 Other patients who are not good candidates for immunotherapy include those with underlying autoimmune diseases, patients with uncontrolled brain metastases, and patients with poor performance status (ECOG 3–4).
Patients who may be unsuited for IL-2
In addition to the patients mentioned above, patients with poor lung function (less than 75% of predicted forced expiratory volume in 1 second [FEV1]), poor renal function, ascites, or pleural effusions should not receive IL-2. The presence of reversible cardiac ischemia is also a contraindication for IL-2 therapy, so patients with a smoking history, those aged >50 years, and those with a family history of coronary disease should have a nuclear stress test or stress echocardiogram before therapy. Lack of access to an experienced IL-2 center may additionally prevent treatment.
Patients who may be unsuited for ipilimumab, nivolumab or pembrolizumab
Overall, more patients are candidates for ipilimumab therapy than for HD IL-2. Patients must be able to wait 9–12 weeks for a tumor response, tolerate the potentially severe immune-related side effects, and tolerate high doses of steroids for several weeks. For example, a frail elderly patient may be unable to tolerate severe colitis, even if identified and treated promptly.
Apart from individuals with autoimmune diseases, no specific types of patient are known to be unsuited to treatment with nivolumab or pembrolizumab. The decision to treat should be made on an individual patient basis taking potential risk factors into account. No dose adjustment of either agent is required in patients with renal or hepatic impairment.Citation21,Citation22 For all three agents, use in pregnant women bears risk to the fetus, and safety has not been established in pediatric populations.Citation21,Citation22,Citation52
Which Immunotherapy to Use First?
The choice of which immunotherapy to use first must be made with each individual patient, taking into account all of the factors discussed above. Clearly, more patients may be suited to treatment with immune checkpoint inhibitors than with HD IL-2, with its requirement for extremely careful patient selection to minimize risk of excessive toxicity. Furthermore, based on the data currently available, the chances of long-term benefit appear greater with immune checkpoint inhibitors than with HD IL-2, based on much more rigorous testing in phase III trials. With survival curves for ipilimumab plateauing at 3 years, approximately 20% of patients may expect long-term survival.Citation13,Citation49 Furthermore, long-term survival appears higher with treatment-naïve patients (26%) than previously treated patients (20%),Citation13 which would favor using ipilimumab (or nivolumab or pembrolizumab, if long-term survival data sustain the early promise of these agents) rather than HD IL-2. With HD IL-2, long-term survival seems dependent on achieving a complete response, reported in only approximately 6% of patients.Citation30,Citation46 In contrast, some guidelines have advocated for IL-2 treatment as first-line therapy in appropriate patients because of the higher complete response rate and better outcomes in patients with a better underlying performance status.Citation10
One potential disadvantage of using immune checkpoint inhibitors before IL-2 is the longer time to response. With HD IL-2, response is evident within approximately 6 weeks, but with all three immune checkpoint inhibitors, 8–12 weeks, or sometimes longer, may be needed. Certain irAEs with immune checkpoint inhibitors (with a bigger risk of grade 3/4 irAEs associated with ipilimumab than with nivolumab or pembrolizumabCitation83) may affect subsequent therapy with HD IL-2 or other immunotherapy and may limit enrollment in clinical trials. Any toxicity requiring a prolonged course of steroids would delay or prevent the initiation of an alternative immunotherapy, and further immunotherapy would be complicated for patients with hypophysitis (secondary to use of ipilimumab) requiring long-term steroids. Even if an irAE on ipilimumab resolves, patients may be predisposed to experience similar or more severe irAEs with IL-2.Citation84 In contrast, prior IL-2 therapy does not seem to affect tolerability or response to ipilimumab.Citation85,Citation86 Neither clinical response nor progression-free survival with IL-2 has been able to predict a benefit with subsequent ipilimumab.Citation87
Immunotherapy for patients with noncutaneous melanoma
Information regarding IL-2 activity in metastatic, noncutaneous melanoma is sparse. Recently, data from multiple studies have shown improved OS with ipilimumab in patients with metastatic uveal melanoma.Citation88–92 The largest study with the most patients with metastatic uveal melanoma (n = 82) showed a 1-year survival rate of 31%.Citation88 Data also suggest ipilimumab has activity in patients with metastatic mucosal melanoma.Citation92–94 An analysis of 71 patients with metastatic mucosal melanoma treated with ipilimumab showed a 1-year survival rate of 35%.Citation93 To date, the use of anti-PD-1 antibodies in noncutaneous melanoma has been limited, but the available data suggest antitumor activity with a similar toxicity profile to that previously reported.
Among seven patients with metastatic uveal melanoma who received pembrolizumab as part of Merck’s expanded access program, one patient each had a complete response, a partial response, and stable disease; median progression-free survival (PFS) at data-cutoff was 12.2 weeks and two patients were still receiving therapy without progression.Citation95 A case report of a patient with metastatic mucosal melanoma treated with pembrolizumab showed that this patient discontinued treatment with severe hypothyroidism, rhabdomyolysis, and acute kidney injury, but remained in near complete response 14 months after stopping treatment.Citation96 A phase II study to investigate the role of pembrolizumab in patients with advanced uveal melanoma is planned (NCT02359851), while another phase II study of nivolumab in combination with ipilimumab for uveal melanoma is currently recruiting patients (NCT01585194).
Evolution of immunotherapy for advanced melanoma
The evidence for long-term survival with HD IL-2 and, more recently, ipilimumab reinvigorated the development and evaluation of immunotherapies for a range of tumors types, including melanoma. PD-1 checkpoint inhibitors are now clinically proven, with pembrolizumab and nivolumab approved for the treatment of advanced melanoma, Two other agents targeting the PD-1 pathway (MEDI4736, and MPDL3280A) are in phase III clinical development for different tumor types, with additional agents in earlier stages of development.
The development of agents designed to inhibit different immune checkpoints provides the opportunity to evaluate the safety and activity of sequential or concurrent combination regimens. In theory, combining immunotherapies with the goal of overcoming more than one immunosuppressive mechanism has the potential to provide a more comprehensive antitumor immune response than might be achieved with single-agent therapy.Citation20,Citation97 In a phase Ib/II clinical trial, patients with metastatic melanoma treated with standard HD IL-2 and escalating doses of ipilimumab reported an initial ORR of 22%.Citation98 On further follow-up, the response rate increased to 28%, likely reflecting the delayed kinetics of response observed with ipilimumab, and 17% of patients achieved a complete response, suggesting potential benefit for combining IL-2 and ipilimumab.Citation99
Phase III data have recently confirmed preclinical and early phase clinical results which suggested that sequential or concurrent combination therapy with immune checkpoint inhibitors might be superior to monotherapy treatment.Citation65,Citation100–102 In a double-blind, placebo-controlled phase III trial, treatment with a combination of nivolumab and ipilimumab was compared with ipilimumab monotherapy; the trial also included a nivolumab monotherapy arm.Citation65 Overall survival data are not yet available, but PFS with nivolumab plus ipilimumab was significantly longer than with ipilimumab monotherapy (median 11.5 vs 2.9 months; HR for death or disease progression 0.42; 99.5% CI, 0.31 to 0.57; P< .001). Although the study was not designed for formal comparison of the nivolumab group and the nivolumab plus ipilimumab group, median PFS with nivolumab was 6.9 months and the HR for the comparison was 0.74 (95% CI, 0.60 to 0.92). Subgroup analysis showed consistently longer PFS with nivolumab or with nivolumab plus ipilimumab than with ipilimumab, with subgroups including those defined by PD-L1 status and BRAF mutation status. Combination therapy was, however, associated with greater toxicity; the rate of grade 3/4 AEs was 55%, 16% and 27% for the combination, nivolumab alone, and ipilimumab alone, respectively. Given that, in a phase I trial using a concurrent regimen with various doses of nivolumab (1 or 3 mg/kg) plus ipilimumab (0.3, 1, or 3 mg/kg) 2-year OS was 79%,Citation103 it will be interesting to see whether the phase III efficacy results translate into an OS benefit with the combination.
Immune checkpoint inhibitors seem likely to form a key part the management of patients with advanced melanoma for many years to come. However, other types of immunotherapy are also being developed which in the future may play a role in treating this tumor, either alone or in combination with currently approved agents. For example, two ongoing phase I trials are investigating the activity of ipilimumab combined with, respectively, varlilumab (CDX-1127), an anti-CD27 monoclonal antibody that induces activation and proliferation of human T-cells when combined with T-cell receptor stimulation (NCT02413827), or MGA271, an anti-B7-H3 monoclonal antibody that mediates antibody-dependent cellular cytotoxicity and which has shown antitumor activity in preclinical studies (NCT02381314).Citation104 Finally, since adenosine is known to play a key role in regulating melanoma progression, immune checkpoint inhibition may be enhanced by blockade of the adenosine A2a receptor (A2aR), which plays a critical role in the regulation of T-cell function.Citation105,Citation106 Several A2aR antagonists have shown encouraging antitumor activity in preclinical development in combination with either anti-PD-1 or anti-CTLA-4 agents,Citation107 with a number in clinical development for non-cancer indications; a phase I study of the adenosine A2aR antagonist, PBF-509, is also ongoing in patients with non-small cell lung cancer (NCT02403193).
Conclusion
The decision of which immunotherapy to begin treatment with in eligible patients – IL-2, ipilimumab, pembrolizumab, or nivolumab – should be made based on expected benefit versus risk. All four therapies offer the possibility of extended survival in some patients. IL-2 is the only immunotherapy known to be associated with a “cure”, albeit in a small number of patients, yet some patients treated with ipilimumab experience long-term survival up to 10 years. Pembrolizumab and nivolumab are currently the only agents approved by the FDA for use after progression on or intolerance to ipilimumab and a BRAF inhibitor (for patients with mutated BRAF). However, this guidance may change rapidly, as phase III studies have demonstrated that treatment with a PD-1 checkpoint inhibitor is superior to ipilimumab in the first or second-line setting and that the combination of ipilimumab and nivolumab is superior to either agent alone in the first-line setting. Indeed, nivolumab monotherapy was approved in 2015 in the EU for the treatment of advanced melanoma in both the first- and second-line settings.
To enable individual patients to achieve maximal benefit from these immunotherapy agents, prompt identification and management of AEs (including irAEs) are critical. Most AEs are reversible with appropriate management and do not prevent continuation of immunotherapy for advanced melanoma.
As more checkpoint inhibitors and other immunotherapies are developed, the opportunities for combination or sequential therapy will increase rapidly. Consequently, the dynamic and evolving field of immunotherapy for melanoma will continue to offer challenges in terms of optimal patient management for the foreseeable future.
Abbreviations
Acknowledgments
Drs Hughes and Klairmont have no financial disclosures to report. The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoint and expertise. Professional medical writing and editorial assistance were provided by Rebecca Turner, PhD, and Ward A. Pedersen, PhD, at StemScientific, an Ashfield Company, and were funded by Bristol-Myers Squibb. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript. The authors did not receive financial compensation for authoring the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi:https://doi.org/10.1038/nature05661.
- Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruit CN, Gibson LE. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clinic Proc. 2012;87:328–334.
- American Cancer Society. Cancer facts & figures 2014 [Internet]. Atlanta: American Cancer Society Inc.; 2013 [ cited 2014 Oct 23]. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, et al. SEER cancer statistics review, 1975-2009 (Vintage 2009 Populations) [Internet]. Bethesda (MD): National Cancer Institute; April 2012 [cited 2014 Oct 23]. Available from: http://seer.cancer.gov/csr/1975_2009_pops09/.
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, et al. SEER cancer statistics review, 1975-2004 [Internet]. Bethesda (MD): National Cancer Institute; 2007 [cited 2014 Oct 24]. Available from: http://seer.cancer.gov/csr/1975_2004.
- Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836.
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516.
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365.
- Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18:717–725.
- Kaufman HL, Kirkwood JM, Hodi FS, Agarwala S, Amatruda T, Bines SD, Clark JI, Curti B, Ernstoff MS, Gajewski T, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol. 2013;(2013(10):588–598. doi:https://doi.org/10.1038/nrclinonc.2013.153.
- Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–319.
- McDermott D, Haanen J, Tt C, Lorigan P, O’Day S. Investigators MDX. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol. 2013;24:2694–2698.
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. In J Clin Oncol. 2015; Feb 9. [Epub ahead of print]
- Hodi FS, Sznol M, Kluger HM, McDermott DF, Carvajal RD, Lawrence DP, Topalian SL, Atkins MB, Powderly JD, Sharfman WH, et al.Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial,J Clin Oncol:32, abstract,9002.2014.
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015a;372:320–330.
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015b;372:2521–2532.
- Js W, Sp D, Minor D, Fs H, Gutzmer R, Neyns B, Hoeller C, Ni K, Wh M, Cd L, et al. Nivolumab compared with chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 therapy. Lancet Oncol. 2015;16:375–384.
- Fs H, Sj O, Df M, Rw W, Ja S, Jb H, Gonzalez R, Robert C, Schadendorf D, Jc H, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723.
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Jf B, Testori A, Jj G, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev Cancer. 2012;2:252–264.
- Keytruda (pembrolizumab) prescribing information [Internet] Whitehouse Station (NJ): Merck & Co., Inc; 2014 [cited 2014 Oct 23]. 12p. Available from: http://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf.
- Opdivo (nivolumab) prescribing information [Internet]. Princeton (NJ): Bristol-Myers Squibb Company; 2015 [ cited 2015 Jan 27]. 8p. Available from: http://packageinserts.bms.com/pi/pi_opdivo.pdf.
- NCCN Clinical Practice Guidelines in Oncology. Melanoma. Version 3, 2015. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed Jul 7
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570.
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271.
- Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–3130.
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008.
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492.
- Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987; 316:889–897.
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116.
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190.
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479.
- Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177.
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499.
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182.
- McDermott DF, Atkins MBPD-1. as a potential target in cancer therapy. Cancer Med. 2013;2:662–673.
- Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165.
- Fong L, Ej S. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283.
- Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339.
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074.
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe A. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029.
- Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856.
- Patnaik A, Kang SP, Tolcher AW, Rasco DW, Papadopoulos KP, Beeram M, Drengler R, Chen C, Smith L, Perez C, et al.Phase I study of MK-3475 (anti-PD-1 monoclonal antibody) in patients with advanced solid tumors,J Clin Oncol:31, abstract,2512.2012.
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6:S11–4.
- Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618.
- Lindsey KR, Rosenberg SA, Sherry RM. Impact of the number of treatment courses on the clinical response of patients who receive high-dose bolus interleukin-2. J Clin Oncol. 2000;18:1954–1959.
- Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191–1196.
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420.
- Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1.
- Yervoy (ipilimumab) prescribing information. Princeton (NJ): Bristol-Myers Squibb Company; 2013 [cited 2014 Apr 16]. Available from: http://packageinserts.bms.com/pi/pi_yervoy.pdf.
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TC, et al. 2014. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 384(9948):1109–1117.
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030.
- Konrad MW, Hemstreet G, Hersh EM, Mansell PW, Mertelsmann R, Kolitz JE, Bradley EC. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50:2009–2017.
- Ribas A, Hodi FS, Kefford R, Hamid O, Daud A, Wolchok JD, Hwu W-J, Gangadhar TC, Patnaik A, Joshua AM, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). J Clin Oncol 2014; 32: abstractLBA9000.
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132.
- Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805.
- Krouse RS, Royal RE, Heywood G, Weintraub BD, White DE, Steinberg SM, Rosenberg SA, Schwartzentruber DJ. Thyroid dysfunction in 281 patients with metastatic melanoma or renal carcinoma treated with interleukin-2 alone. J Immunother Emphasis Tumor Immunol. 1995;18:272–278.
- Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24:287–293.
- Fisher B, Keenan AM, Garra BS, Steinberg SM, White DE, DiBisceglie AM, Hoofnagle JH, Yolles P, Rosenberg SA, Lotze MT. Interleukin-2 induces profound reversible cholestasis: a detailed analysis in treated cancer patients. J Clin Oncol. 1989;7:1852–1862.
- Guleria AS, Yang JC, Topalian SL, Weber JS, Parkinson DR, MacFarlane MP, White RL, Steinberg SM, White DE, Einhorn JH, et al. Renal dysfunction associated with the administration of high-dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal carcinoma. J Clin Oncol. 1994;12:2714–2722.
- Denicoff KD, Rubinow DR, Papa MZ, Simpson C, Seipp CA, Lotze MT, Chang AE, Rosenstein D, Rosenberg SA. The neuropsychiatric effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1987;107:293–300.
- Lee SH, Baig M, Rusciano V, Dutcher JP. Novel management of pruritus in patients treated with IL-2 for metastatic renal cell carcinoma and malignant melanoma. J Immunother. 2010;33:1010–1013.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34.
- Bryce J, Passoni C. Nursing management of patients with metastatic melanoma receiving ipilimumab. Oncol Nurs Forum. 2013;40:215–218.
- Della Vittoria Scarpati G, Fusciello C, Perri F, Sabbatino F, Ferrone S, Carlomagno C, Pepe S. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. Onco Targets Ther. 2014;7:203–209.
- Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–743.
- Ledezma B, Heng A. Real-world impact of education: treating patients with ipilimumab in a community practice setting. Cancer Manag Res. 2013;6:5–14.
- Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697.
- Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–1682.
- Squibb B-M. Yervoy (ipilimumab): immune-mediated adverse reaction management guide [Internet]. Princeton (NJ): Bristol-Myers Squibb; 2011 [cited 2014 Jun]. 18 p. Available from: https://www.hcp.yervoy.com/pdf/rems-management-guide.pdf.
- Merck. A guide to monitoring patients during treatment with Keytuda(pembrolizumab). Merck Sharp & Dohme Corp 2014. Available from: https://www.keytruda.com/static/pdf/adverse-reaction-management-tool.pdf.
- Squibb B-M. Opdivo (nivolumab) immune-mediated adverse reactions management guide. Princeton NJ: Bristol-Myers Squibb; 2015. Available from: http://www.opdivohcp.bmscustomerconnect.com/servlet/servlet.FileDownload?file=00Pi000000GL6RoEAL.
- Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48:806–810.
- Slangen RM, van Den Eertwegh AJ, van Bodegraven AA, de Boer N. Diarrhoea in a patient with metastatic melanoma: ipilimumab ileocolitis treated with infliximab. World J Gastrointest Pharmacol Ther. 2013;4:80–82.
- Pagès C, Gornet JM, Monsel G, Allez M, Bertheau P, Bagot M, Lebbé C, Viguier M. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23:227–230.
- Mavropoulos JC, Wang TS. Managing the skin toxicities from new melanoma drugs. Curr Treat Options Oncol. 2014;15:281–301.
- Bot I, Blank CU, Boogerd W, Brandsma D. Neurological immune-related adverse events of ipilimumab. Pract Neurol. 2013;13:278–280.
- Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16:589–593.
- Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381.
- Lammert A, Schneider HJ, Bergmann T, Benck U, Krämer BK, Gärtner R, Metzner C, Schöfl C, Berking C. Hypophysitis caused by ipilimumab in cancer patients: hormone replacement or immunosuppressive therapy. Exp Clin Endocrinol Diabetes. 2013;121:581–587.
- Chen TW, Razak AR, Bedard PL, Siu LL, Hansen AR. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol. 2015; Apr 17. [ Epub ahead of print.
- Smith FO, Goff SL, Klapper JA, Levy C, Allen T, Mavroukakis SA, Rosenberg SA. Risk of bowel perforation in patients receiving interleukin-2 after therapy with anti-CTLA 4 monoclonal antibody. J Immunother. 2007;30:130.
- Kaufman H, Lutzky J, Clark J, Margolin KA, Lawson DH, Amin A, Collichio FA, Pecora A, Urba WA, Bennett KL, et al. 2013. Safety and efficacy of ipilimumab in melanoma patients who received prior immunotherapy on phase III study MDX010-020. J Clin Oncol. 31(abstract):9050.
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688.
- Joseph RW, Eckel-Passow JE, Sharma R, Liu P, Parker A, Jakob J, Buchbinder E, Bassett RL, Davies MA, Hwu P, et al. Characterizing the clinical benefit of ipilimumab in patients who progressed on high-dose IL-2. J Immunother. 2012;35:711–715.
- Maio M, Danielli R, Chiarion-Sileni V, Pigozzo J, Parmiani G, Ridolfi R, De Rosa F, Del Vecchio M, Di Guardo L, Queirolo P, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol. 2013;24:2911–2915.
- Luke JJ, Callahan MK, Postow MA, Romano E, Ramaiya N, Bluth M, Giobbie-Hurder A, Lawrence DP, Ibrahim N, Ott PA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119:3687–3695.
- Deo M, Van Maanen A, Cornélis F, De Potter P, Baurain J-F. Long-term survival benefit from ipilimumab treatment in metastatic uveal melanoma patients. J Clin Oncol. 2014;32(abstract):3060.
- Piulats Rodriguez JM, Ochoa de Olza M, Codes M, Lopez-Martin JA, Berrocal A, Garcia M, Gurpide A, Homet B, Martin-Algarra S. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): the GEM-1 trial. J Clin Oncol. 2014;32:9033. abstract.
- Zimmer L, Eigentler TK, Vaubel J-M, Mohr P, Jradi Z, Kiecker F, Utikal J, Berking C, Kampgen E, Hauschild A, et al. Open-label, multicenter, single-arm phase II study (DeCOG-Trial) to further evaluate the efficacy and safety of ipilimumab in patients with cutaneous melanoma and rare subgroups. J Clin Oncol. 2014; 32(5s): abstract903
- Del Vecchio M, Di Guardo L, Ascierto PA, Grimaldi AM, Sileni VC, Pigozzo J, Ferraresi V, Nuzzo C, Rinaldi G, Testori A, et al. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50:121–127.
- Postow MA, Luke JJ, Bluth MJ, Ramaiya N, Panageas KS, Lawrence DP, Ibrahim N, Flaherty KT, Sullivan RJ, Ott PA, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist. 2013;18:726–732.
- Kottschade LA, McWilliams RR, Markovic S, Block MS, Bisneto JV, Pham AQ, Dronca RS. The use of pembrolizumab for the treatment of metastatic uveal melanoma. J Clin Oncol. 2015;33:9010. abstract.
- Min L, Hodi FS. Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2:15–18.
- Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012;6:242–250.
- Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016.
- Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi:https://doi.org/10.1056/NEJMoa1302369.
- Curran MA, Montalvo W, Yagita H, Allison JPPD-1. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–4280. doi:https://doi.org/10.1073/pnas.0915174107.
- Selby M, Engelhardt J, Lu L-S, Quigley M, Wang C, Chen B, Korman AJ. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol. 2013;31(15_suppl):3061. doi:https://doi.org/10.1200/jco.2013.31.15_suppl.3061.
- Sznol M, Kluger HM, Callahan MK, Postow MA, Gordon RA, Segal NH, Rizvi NA, Lesokhin AM, Atkins MB, Kirkwood JM, et al. 2014. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol. 32(18_suppl):LBA9003–LBA9003. abstractLBA9003 https://doi.org/10.1200/jco.2014.32.18_suppl.lba9003.
- Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y, et al. Development of an Fc-Enhanced Anti–B7-H3 Monoclonal Antibody with Potent Antitumor Activity. Clin Cancer Res. 2012 Jul 15;18(14):3834–3845. doi:https://doi.org/10.1158/1078-0432.CCR-12-0715.
- Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am J Cancer Res. 2014;4(2):172–181.
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti–PD-1 through Enhanced Antitumor T-cell Responses. Cancer Immunol Res. 2015;3(5):506–517. doi:https://doi.org/10.1158/2326-6066.CIR-14-0211.
- Leone RD, Lo Y-C, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265–272. doi:https://doi.org/10.1016/j.csbj.2015.03.008.
