Abstract
The recurrence of colorectal cancer after chemotherapy is the leading cause of its high mortality. We propose that elucidating the mechanisms of tumor regrowth after chemotherapy in tumor-bearing mice may provide new insights into tumor relapse in cancer patients. We firstly report the identification of a chemokine, CXCL4, that plays an important role in the molecular mechanism of cancer regrowth after chemotherapy. A syngenic transplantation tumor model was established with murine colon cancer CT26 cells and treated with 5-FU. Genome-wide gene expression analysis determined that CXCL4 was transiently upregulated in the tumor model. Systemic overexpression of CXCL4 accelerated cancer growth in vivo, but not in vitro. Conversely, the anti-CXCL4 monoclonal antibody (CXCL4-mab) retarded tumor-regrowth after 5-FU treatment in immune-competent mice, but not nude mice. The CXCL4-mab treatment increased the local expression levels of IFN-γ and Gran-b genes in the tumor-bed, and elevated the function of CTLs against CT26 cells. Thus, the colon cancer cells in responding to the cytotoxic stress of 5-FU produce a high level of CXCL4, which suppresses antitumor immunity to confer the residual cancer cells an advantage for regrowth after chemotherapy. Our findings provide a novel target for developing therapeutics aiming to increase antitumor immunity after chemotherapy.
Abbreviations
| CRC | = | colorectal cancer |
| 5-FU | = | 5-fluorouracil |
| L-OHP | = | oxaliplatin |
| CXCL4-mab | = | anti-CXCL4 monoclonal antibody |
| mAb | = | monoclonal antibody |
| IFN-γ | = | interferon-γ |
| Gran-b | = | granzyme-b |
| CTLs | = | cytotoxic T lymphocytes |
| rhCXCL4 | = | recombinant human CXCL4. |
Introduction
Colorectal cancer (CRC) is the third most frequent cancer and a major cause of cancer mortality worldwide.Citation1-2 With around one million new cases and more than half a million deaths every year globally, there is an urgent need for therapeutic progress to reduce the risk of recurrence and metastasis after conventional treatments for CRC. 5-FU either alone or combined with oxaliplatin (L-OHP) remains the current standard of care.Citation3 It is commonly accepted that chemotherapy will not eliminate all cancer cells without harm to normal cells. In addition to the direct cytotoxic effects, the indirect effects of chemotherapy drugs affecting malignant cells, as well as their environmental cells, have begun to be addressed. Importantly, the indirect effects have been identified to be responsible for tumor regrowth, in which the surviving cancer cells rapidly repopulate the badly damaged tumor by proliferating at a markedly accelerated pace.Citation4-6 We assert that the molecular mechanisms of tumor regrowth may account for the course of tumor relapse after cancer treatment. Identification of key players executing the mechanism may provide novel targets to prevent tumor relapse after chemotherapy.
We have taken a transcriptomics approach to systematically screen for the genes with homeostatic expression characteristics during the process of tumor regrowth after 5-FU treatment. The rationale of the screening strategy is that genes executing the process are proposed to be cell-autonomously controlled. The fluctuation of the genes’ expression is an active, not a passive, response to provide the stressed cells with a survival advantage. Once the process is completed, the elevated gene expression returns to the basal level, which completes the homeostatic expression cycle. Chemokine CXCL4 is one of the identified genes. We demonstrated a crucial role of CXCL4 in promoting colon cancer regrowth after 5-FU treatment. In addition, we made an unexpected discovery that tumor microenvironment cells mediated the role of CXCL4. Specifically, the 5-FU induced and tumor cell derived CXCL4 suppressed local antitumor immunity to create an environment favoring tumor regrowth at the most critical time after chemotherapy. We believe CXCL4-medicated tumor regrowth after chemotherapy may serve as a novel target for preventing tumor relapse.
Results
Expression of CXCL4 in colon cancer cells and tissues is elevated by 5-FU treatment
To elucidate the mechanism of colon cancer regrowth after chemotherapy, CT26 colon cancer bearing mice were treated with 5-FU. As expected, tumor volume was decreased after the treatment for the initial 4 d and rebounded thereafter (). We proposed that the homeostatically regulated transcriptome of the tumor tissues carried key information regarding cancer regrowth.
Figure 1. Expression of CXCL4 is homeostatically-regulated in 5-FU-treated colon cancer. BALB/c mice were inoculated with 1×106 CT26 cells, and when the tumor size was 400 – 500 mm3, 5-FU (200 mg/kg) was administrated. Mice were sacrificed at 2, 4, 6, and 8 d after 5-FU treatment. Controls were sacrificed at 0 d. CT26 tumors were measured and removed for pathology and gene expression analysis. (A) The HE stain of CT26 tumor tissues at different days after 5-FU treatment. (B) The volume change of CT26 tumor after 5-FU chemotherapy. (C) The signal intensity of CXCL4 mRNA in CT26 tumors revealed by gene expression array. (D) The expression of CXCL4 mRNA is presented relative to their baseline at 0 d by real-time PCR in CT26 tumor tissues at 4 d after 5-FU chemotherapy. (E) The CXCL4 protein expression in CT26 tumor tissues are presented by Western-blot as their relative induction over untreated mice at 0 d after normalization to their respective β-actin loading controls. CT26 mouse and HCT15 human colon cancer cells were treated with 5-FU (200 μg/ml) for 3 h and washed twice. After incubation with fresh medium for 4 h, 24 h and 48 h, the cells were subjected to mRNA expression test by real time PCR. (F) The expression of CXCL4 mRNA in CT26 cells, and (G) in HCT15 cells. Data are presented as mean ± SE (n = 3 mice per group, or 3 repeats for cells experiments). *P < 0.05, **P < 0.01 vs. control group.
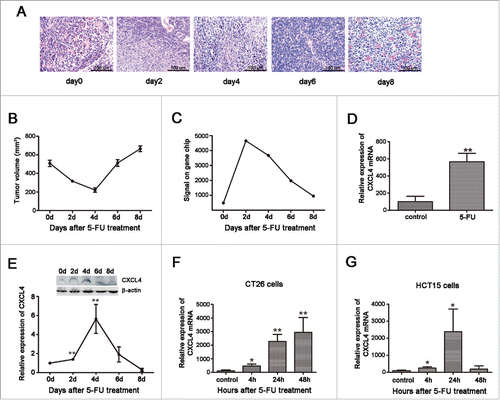
A genome-wide Affymetrix gene-expression array of the tumor samples revealed that CXCL4 mRNA expression was increased, accompanying the decline of tumor volume, and returned to the basal level thereafter as the tumor rebounded (). The CXCL4 expression pattern was further confirmed in the mRNA level by real-time PCR () and protein level by Western-blot (). Furthermore, colon cancer cell lines of mouse CT26 and human HCT15 were demonstrated to over-express CXCL4 after culture with 5-FU (), which demonstrates that the cancer cells are at least partially responsible for CXCL4 upregulation in tumor tissues. Another CRC first-line chemotherapy agent, oxaliplatin (L-OHP), was also shown to elevate CXCL4 expression in CT26 cells (Fig. S1).
Thus, the expression of CXCL4 mRNA in colon cancer cells and tissues after chemotherapy is homeostatically regulated. This suggests that CXCL4 may be involved in cancer regrowth after chemotherapy.
CXCL4 promotes colon cancer growth in vivo, but not in vitro
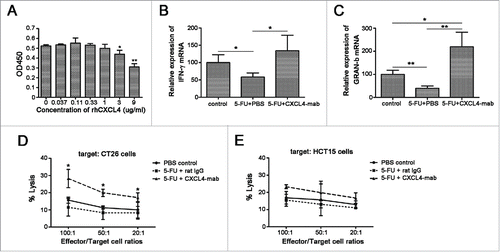
To test the role of CXCL4 in tumor regrowth, rhCXCL4 was added to the culture of the CT26 and HCT15 cells with or without 5-FU treatment. It is demonstrated that rhCXCL4 at the concentrations tested had no effect on the growth of CT26 cells () and HCT15 cells (). The protein was active in the cell-based biological assay as described.Citation7 5-FU at 200 μg/ml significantly suppressed the growth of both cancer cells. rhCXCL4 had no effect on the inhibitory roles of 5-FU to CT26 and HCT15 cells ().
Figure 2. The effects of CXCL4 on the growth of colon cancer cells in vitro and in vivo. (A) rhCXCL4 has no effect on the growth of CT26 cells in culture. (B) rhCXCL4 has no effect on 5-FU toxicity to CT26 cells in culture. **P < 0.01. (C) rhCXCL4 has no effect on the growth of HCT15 cells in culture. (D) rhCXCL4 has no effect on 5-FU toxicity to HCT15 cells in culture. **P < 0.01. (E) The over-expression of CXCL4 promotes CT26 tumor growth in vivo. Mice were electro-transfected with mCXCL4-pcDNA3.1 or blank pcDNA3.1 plasmid (50 μg/mouse). Mice were inoculated with 3×106 CT26 cells 3 d later, and the tumor volume was measured thereafter. n = 9 mice per group. **P < 0.01. (F) Mice were sacrificed on day 18 after inoculation of CT26 cells. Photographs of tumor tissue in each group are presented.
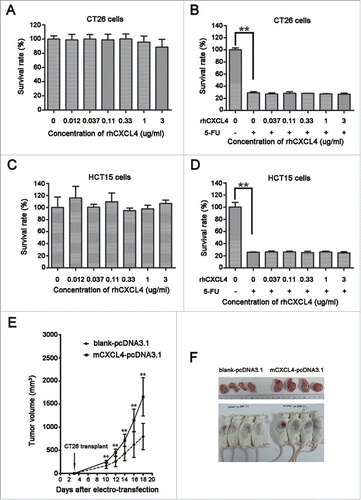
However, CXCL4 stimulated tumor growth in the CT26-bearing mice. The mCXCL4 expression plasmid mCXCL4-pcDNA3.1 was constructed to over-express CXCL4 (Fig. S2). The CXCL4 gene in the plasmid was delivered to secrete the chemokine in the thigh myocytes through electroporation. The tumor growth rate was significantly accelerated in the CT26-bearing mice that received the CXCL4 plasmid compared to the mice that received the control plasmid pcDNA3.1 (, P < 0.01).
Taken together, CXCL4 accelerates tumor growth in the colon cancer bearing mice, but not in the cell culture. This suggests that its role is indirect, which supports our proposal that the elevation of CXCL4 expression in the mice may account for tumor regrowth after chemotherapy.
CXCL4-mab suppresses colon cancer regrowth after 5-FU chemotherapy
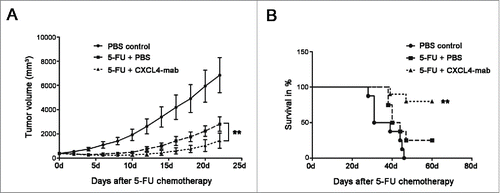
Previously, we have reported that injection of anti-CXCL4 monoclonal antibody (CXCL4-mab) alone to mice had no effect on tumor growth.Citation7 Here, we report that the antibody plus 5-FU therapy further suppressed tumor growth compared to the chemotherapy alone in mice. The CT26 tumor-bearing mice were treated with CXCL4-mab 4 h after the injection of 5-FU to neutralize the elevated levels of CXCL4. Tumor regrowth after chemotherapy was significantly suppressed (, P < 0.01). Importantly, the mice survival rate at day 60 was increased from 25% in the PBS to 80% in the antibody-treated group after 5-FU chemotherapy (, P < 0.01).
Figure 3. CXCL4-mab suppresses tumor regrowth after 5-FU chemotherapy. (A) CXCL4-mab further inhibited CT26 tumor growth in the 5-FU treated tumor-bearing mice. Mice were inoculated with 1×106 CT26 cells, and when the tumor size was about 400 mm3, CXCL4-mab (1 mg/kg) or equal volume saline was injected subcutaneously after 5-FU (150 mg/kg) administration. Tumor volume was measured. (B) CXCL4-mab further prolonged the lifespan of the CT26-bearing mice treated with 5-FU. Animal survival for 60 d after 5-FU plus CXCL4-mab treatment are presented as Kaplan-Meier survival curves and analyzed by a long-rank test. n = 8 mice for PBS control group or 5-FU + PBS control group, and n = 10 mice for 5-FU + CXCL4-mab group. **P < 0.01 vs. 5-FU + PBS control group.
Tumor shrinkage in the CXCL4-mab treated mice was further confirmed at the cellular levels. BrdU (100 mg/kg) was intraperitoneally injected 4 h before the tumor-bearing mice were sacrificed to label the proliferating cells. There was as much as 50% reduction at day 4 after chemotherapy in the number of BrdU-positive cells in the antibody-treated tumor samples than the controls. The proliferation reduction was already prominent from day 2 and continued to day 6 after chemotherapy (, P < 0.01).
Figure 4. CXCL4-mab inhibits proliferation and promotes apoptosis of colon-cancer cells after 5-FU chemotherapy. Mice were inoculated with 1×106 CT26 cells, and when the tumor size was about 400 mm3, CXCL4-mab (1 mg/kg) or rat IgG control (1 mg/kg) were injected after 5-FU (150 mg/kg) administration. 4 d later, mice were sacrificed, and tumor tissues were removed for proliferation and apoptosis immunohistochemical staining. (A) Representative photomicrographs of cells in the active cell cycle labeled by BrdU in CT26 tumor tissues. (B) Quantitative presentation of the BrdU-positive cells in CT26 tumor tissues. Five fields per mouse were counted for BrdU-positive cells. (C) Representative photomicrographs of scattered apoptotic cells in tumor tissues stained by the TUNEL method. (D) Quantitative presentation of the scattered TUNEL-positive cells in CT26 tumor tissue. Five fields per mouse were counted for TUNEL-positive cells. Six mice for each group. (E) Quantitative presentation of the percentage of apoptotic areas in the whole tumor tissue. Six mice for each group.*P < 0.05, **P < 0.01 vs. 5-FU treated control mice.
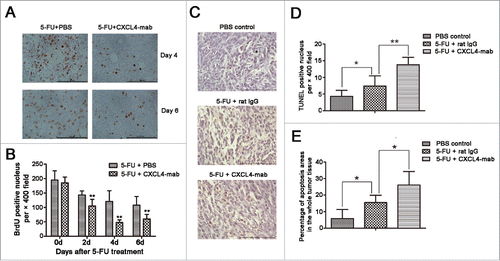
Consistent with the reduction of the tumor volume and proliferation rate, the apoptosis of the tumor tissues was also exacerbated in the CXCL4-mab treated mice after chemotherapy. The apoptotic cells in the tissue sections appeared as singular cells scattered across the sections and as areas filled with dead cells. The TUNEL-positive cells, as well as the areas, were significantly increased in the antibody-treated mice compared with the isotype control IgG-treated mice after chemotherapy (, P < 0.01. , P < 0.05).
Taken together, the blockade of the 5-FU elevated CXCL4 reduced the regrowth rate of the residual colon cancer cells treated by chemotherapy. The data strongly indicate that CXCL4 elevation in the tumor-bed by chemotherapy prevents tumor apoptosis and imparts tumor regrowth thereafter.
CXCL4-mab promotes antitumor immunity in tumor bearing mice treated with 5-FU
CXCL4 promotes the growth of CT26 in vivo, but has no effects in vitro (), which strongly indicates that its effect is indirect. We proposed that the elevated level of CXCL4 in the tumor-bed may suppress the local immunosurveillance to impart the advantage of the residual tumor cells to repopulate after a cytotoxic damage by chemotherapy. Firstly, rhCXCL4 was demonstrated to inhibit the proliferation of T cells in vitro in a dose-dependent manner (). Secondly, the expression levels of the anti-tumor mediators IFN-γ and Gran-b in the tumor-bed were also shown to be significantly increased in 5-FU plus CXCL4-mab-treated mice (). Thirdly, levels of the cytotoxicity of CTLs against CT26 cells were examined. The cytoxicities of the ex-vivo expanded CTLs to CT26 cells or unrelated HCT15 cells at different ratios demonstrated that CTLs from the CXCL4-mab-treated mice showed higher cytolytic capability to CT26 cells than to HCT15 cells (, P < 0.05).
Figure 5. CXCL4-mab promotes anti-tumor immunity in tumor-bearing mice treated with 5-FU. (A) CXCL4 inhibited the T lymphocytes proliferation in vitro. Lymphocytes were isolated from the mouse spleen and incubation with ConA (5 μg/ml) and various concentrations of rhCXCL4. *P < 0.05, **P < 0.01. (B, C) CXCL4-mab increased the local expression of IFN-γ and Gran-b in 5-FU-treated CT26-bearing mice. Mice were inoculated with 1×106 CT26 cells, and when the tumor size was about 400 mm3, CXCL4-mab (1 mg/kg) or rat IgG control (1 mg/kg) were injected after 5-FU (150 mg/kg) administration. 4 d later, mice were sacrificed, and tumor tissues were removed for gene expression analysis using RT-PCR. n = 3 mice per group. *P < 0.05, **P < 0.01. (D, E) CXCL4-mab promoted the tumor-specific cytotoxicity of CTLs in 5-FU-treated CT26-bearing mice. CTLs were prepared, and the cytotoxicity of LDH assay was performed against the specific target of CT26 cells (D) and the negative control target of HCT15 cells (Materials and Methods). All data are presented as the mean ± SEM from triplicate wells from a representative experiment that was repeated with similar results. *P < 0.05 vs. 5-FU treated control group.
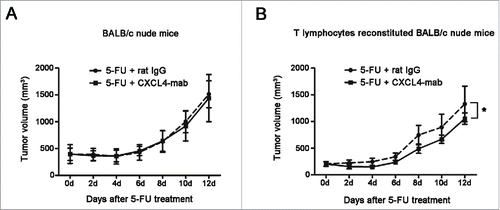
The importance of T cells was further tested in vivo. Syngeneic T cell reconstitution in nude mice was performed through 3 rounds of T cell transplantation. The effect of the T cells to the growth of CT26 tumor in the reconstituted nude mice was examined. There was not obvious growth change of the tumor related to the T cell reconstitution (Fig. S3). Subsequently, the contribution of T cells to the growth arrest of CT26 by CXCL4-mab was examined in the nude mice reconstituted with T cells. The results showed that CXCL4-mab regained its efficacy of antitumor regrowth in the CT26-bearing BALB/c nude mice only after the mice were transplanted with the T lymphocytes of the syngenic wild-type mice (). Taken together, the data demonstrate that the T cells mediated the antitumor effect of CXCL4-mab, and the suppression of T cell immunity by the elevated CXCL4 served as an important mechanism of tumor regrowth after chemotherapy.
Figure 6. Inhibition of tumor regrowth after chemotherapy by CXCL4-mab is T lymphocyte dependent. (A) Tumor growth is presented in the tumor-bearing BALB/c nude mice. BALB/c nude mice were inoculated with 1×106 CT26 cells, and when the tumor size was about 400 mm3, CXCL4-mab (1 mg/kg) or rat IgG control (1 mg/kg) were injected after 5-FU (150 mg/kg) administration. n = 6 mice for the 5-FU+ rat IgG control group, and n = 7 mice for the 5-FU + CXCL4-mab group. (B) Tumor volume is presented in the T lymphocyte reconstituted nude mice. BALB/c nude mice were injected through tail vein with 1×107 lymphocytes of the BALB/c mice, once a week for 3 weeks. 1×106 CT 26 cells were transplanted at day 3 after the last injection of lymphocytes. When the tumor volume reached 200 mm3, 5-FU (150 mg/kg) plus CXCL4mab (1 mg/kg) or rat IgG control (1 mg/kg) was given. A week later, the CXCL4-mab and the control (1 mg/kg) were given again. n = 8 mice per group. *P < 0.05 vs. 5-FU+ rat IgG group.
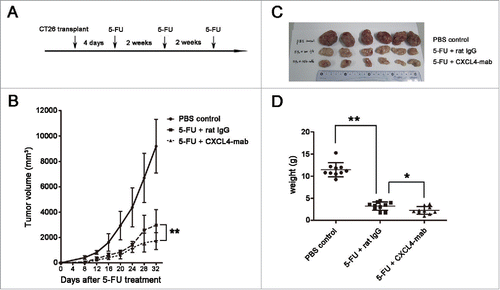
CXCL4-mab suppresses tumor regrowth after repeated 5-FU chemotherapy
Colon cancer patients usually undergo repeated 5-FU chemotherapy. In addition to attenuating tumor regrowth after a single injection of 5-FU by CXCL4-mab, its role in the repeated chemotherapy regimen was tested. The antibody was applied after each of the 3 cycles of 5-FU treatment (). Compared with the isotype IgG control, CXCL4-mab further retarded tumor growth in CT-26 bearing mice treated with multiple cycles of chemotherapy (, P < 0.01). On day 4 after the last 5-FU treatment, the tumor tissues were removed and weighed. The average tumor weight from the mice treated with the antibody was significantly lower than the control (). The data demonstrate that CXCL4-mab in combination with 5-FU is better than chemotherapy alone in the treatment of colon cancer in tumor-bearing wild-type mice.
Figure 7. CXCL4-mab suppresses tumor regrowth after repeated 5-FU chemotherapy. (A) Three-cycles of 5-FU chemotherapy are presented. (B) CXCL4-mab retarded the CT26 regrowth after the repeated 5-FU chemotherapy. Mice were inoculated with 1×106 CT26 cells, and 4 d later, CXCL4-mab (1 mg/kg) or rat IgG control (1 mg/kg) was injected after 5-FU (150 mg/kg) administration. n = 10 mice per group. (C) Mice were sacrificed on day 32 after inoculation of CT26 cells. Photographs of the tumor tissues in each group are presented. (D) The tumor weight of each group is presented. n = 10 mice per group. *P < 0.05, **P < 0.01 vs. 5-FU+ rat IgG group.
Discussion
The association of gene expression with 5-FU chemosensitivity in CRC has been reported.Citation8-9 Correlational studies of gene expression and tumor recurrence in colon cancer patients with surgery alone or surgery plus adjuvant 5-FU plus leucovorin have also identified gene groups for the estimation of cancer recurrence and benefits from chemotherapy.Citation10 However, CXCL4 was not reported in these studies, which we reasoned was due to that the approaches addressed the pharmacogenomics of the cancer chemotherapy. Here, we demonstrate that CXCL4 is temporarily induced by 5-FU in tumor cells in culture and in the tumor-bearing mice. The elevated expression lasts only a few days in vitro and a week in vivo (). The transient expression could not be identified by the design of the static association studies.
CXCL4 is firstly known as platelet factor-4 (PF4), which is often described as a platelet-associated factor. It is synthesized in megakaryocytes, stored in granules of platelets, and released upon stimulation by platelet-aggregating agents.Citation11 Both pro- and anti-coagulant properties of CXCL4 have been reported, but analysis of thrombus formation in the CXCL4 knock-out and transgenic mice provoked with vascular injury demonstrated that thrombus formation and stability were maximal at physiologic levels of PF4.Citation12 It is clear that the amount of PF4 released during the vascular injury by the platelets is critically regulated for its physiological role in the coagulation. Due to its high content and release upon the platelet aggregation, CXCL4 is widely present in experimental systems, namely blood, plasma, and serum, which may affect the interpretation of data and generate conflicting results.
The elevated levels of the platelet-associated CXCL4 either in the platelets or in serum after the platelet aggregation have been correlated with the occurrence of CRC,Citation13 pancreatic cancer,Citation14 in early growth of human liposarcoma, mammary adenocarcinoma, and osteosarcoma in xenograft mice.Citation15 Equally, decreased levels of CXCL4 were also reported in newly diagnosed metastatic diseases prior to treatment,Citation16 and in the sera of the metastatic carcinoma of prostate patients.Citation17 In addition, the serum CXCL4 was shown to be prognostic for survival in pancreatic ductal adenocarcinoma patients.Citation18 It is difficult to reach a consensus about CXCL4 in relationship to malignant development based on the measurement of the levels of platelet-born CXCL4.
We have reported the detection of elevated levels of CXCL4 in the tissue of intestine in mice after 5-FU chemotherapy.Citation7 Here, for the first time, we described the 5-FU-induced CXCL4 expression in colon cancer cells (). Production of CXCL4 other than megakaryocytes has been found in mast cells, monocytes, activated T cells, and microglia.Citation11 To the best of our knowledge, there are no reports in the literature describing induction of the transient expression of CXCL4 by chemotherapeutic drugs in cancer, as well as normal cells outside of blood.
The antitumor activity of the platelet-unrelated CXCL4 has been mostly assigned to its angiostatic effect on neo-vascularization.Citation11,19 As stated in the review, most if not all antitumor effect of CXCL4 was observed only after intra-tumoral injection of the recombinant protein due to its poor pharmacokinetics. The mechanisms of the angiostatic activity were indicated to involve the activation of its receptor CXCR3B, and the inactivation of angiogenic growth factors FGF, VEGF, and CXCL8. In addition, CXCL4 was recently reported to directly induce apoptosis of multiple myeloma by inhibition of STAT3 via upregulation of SOCS.Citation20 We have previously reported that CXCL4 induced by 5-FU triggered the apoptosis of intestinal epithelia by upregulation of p53 via activation of p38 MAPK.Citation7 Thus, both anti-proliferative and pro-apoptotic effects have been identified with the platelet-unrelated form of CXCL4.
However, we have failed to observe any of those direct effects of CXCL4 to colon cancer cells in vitro and in vivo (). Unexpectedly, accelerated tumor growth was observed in the mice overexpression of CXCL4 (). Blockade of CXCL4 after its induction by 5-FU in the tumor-bearing mice consistently blunted tumor regrowth (). Thus, a new role of platelet-unrelated CXCL4 supporting the development of colon cancer in vivo is firstly described. Importantly, the newly identified pro-tumor effect of CXCL4 is in the context of 5-FU chemotherapy. Therefore, it has potential clinical significance in oncology.
CXCL4 was reported to inhibit proliferation and cytokine (IL-2 and IFN-γ) release of activated T cells in culture.Citation21 Importantly, it affected the CD4+CD25+ T regulatory cells (Treg). The proliferation of Treg was enhanced, while the function of Treg was impaired, by CXCL4.Citation22
To provide clues of mechanisms of the pro-tumor effect of CXCL4, T cell assays were carried out. The lost anti-tumor effect of CXCL4-mab in the nude mice was recovered after the T cell reconstitution (). Furthermore, the anti-tumor molecules of IFN- γ and Gran-b in the tumor bed were significantly elevated in the antibody-treated mice (). CTLs recovered from the antibody-treated tumor-bearing mice showed the enhanced specific anti-CT26 cytotoxicity ().
The expression of CXCL4 in CT26 tumor tissues was induced by 5-FU for a period of 8 days in mice (). Its expression after 8 days was not detected (data not shown). Although its induced expression is transient, its immunosuppressive role has imparted the tumor cells to grow faster beyond 8 days. The antibody neutralization of initial short-term expression of CXCL4 after chemotherapy would therefore generate long-term benefit of tumor suppression by protecting the anti-cancer immunity from the damage of CXCL4.
Taken together, our data indicate that CXCL4 released from the tumor cells after 5-FU exposure reduced anti-tumor immunity, which may facilitate regrowth of the escaped colon cancer cells and lead to tumor relapse and metastasis. Studies are underway to identify the target cells and molecule mechanisms of CXCL4 in the promotion of tumor regrowth after chemotherapy.
Materials and Methods
Mice and reagents
The BALB/c and BALB/c nude mice (SPF-grade, SLACCAS, Shanghai, China) were 8-10 weeks old and maintained in air-filtered units at 22 ± 2°C with 50 ± 15% relative humidity, and were provided with sterile water and rodent chow with day light only. Animal experiments were authorized by the Animal Ethics Committee of Shanghai Jiaotong University. 5-FU was from Shanghai Xudong Haipu Pharmaceutical (Shanghai, China). Antibodies against CXCL4 (sc-50300) and β-actin (sc-47778) were from Santa Cruz Biotechnology (USA). CXCL4 monoclonal antibody (CXCL4-mab) and recombinant human CXCL4 (rhCXCL4) were produced internally as described.Citation7
Cell culture
CT26 cells, a murine Balb/c derived colon-cancer cell line, and HCT15 cells, a human colon-cancer cell line, were from ATCC (CRL-2638 and CCL-225, respectively). They were cultured in 1640 medium (Gibco, USA) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were used at the 17th to 22nd passage for all experiments.
Tumor-bearing mice experiments
1×106 CT26 cells were transplanted in the right oxter of BALB/c mice. When the tumor volume reached measurable size, 5-FU was intraperitoneally administrated for a single time (150 mg/kg), or 3 times (150 mg/kg, once per 2 weeks), as illustrated in the Results section. For the antibody treatment, 4 h after 5-FU injection, rat IgG (1 mg/kg, Sigma, USA) or CXCL4-mab (1 mg/kg) were administered subcutaneously. For the BALB/c nude mice experiments, the nude mice were injected once a week for 3 weeks through tail-vein with 1×107 lymphocytes isolated from the spleen of BALB/c mice using mouse percoll (Dakewe Biotech, Shenzhen, China). 1×106 CT26 cells were transplanted at day 3 after the last injection of the lymphocytes. When the tumor volume reached about 200 mm3, 5-FU plus CXCL4-mab or rat IgG control was given, and the antibodies were injected once again a week later.
Overexpression of CXCL4 in CT26-bearing mice
The plasmid construction and gene overexpression in vivo were previously described.Citation23 Briefly, mouse cxcl4 was amplified from the liver cDNA using primers (NheI: 5′– CTAGCTAGCATGAGCGTCGCTGCG, XhoI: 5′-CCGCTCGAGCTAACTCTCCAGGAT) and cloned into pcDNA3.1 plasmid between NheI and XhoI sites. The plasmid was sequenced and named mCXCL4-pcDNA3.1. The plasmid was transiently transfected into 293T cells by lipofectamin 2000, and the CXCL4 expression was confirmed by Western-blot. For the in vivo overexpression, mice were electro-transfected with mCXCL4-pcDNA3.1 or blank pcDNA3.1 plasmid (50 μg/mouse) in the thigh myocytes. 3 d later, mice were inoculated with 3×106 CT26 cells, and the tumor volume was measured thereafter.
Cell proliferation assays
The effect of rhCXCL4 on the proliferation of tumor cells was determined by MTT assay. First, CT26 cells were seeded on 96-well plates at 3,000 cells/well. After attachment, the cells were incubated with rhCXCL4 for 48 h. MTT solution (0.5 mg/well) was added to produce formazan, which was solubilized with dimethyl sulfoxide. The absorbance at 570 nm was determined by a microplate reader (TECAN, Switzerland). The viability of cells after 5-FU incubation was determined by MTT assay. Briefly, cells were seeded on 96-well plates at 5,000 cells/well. After attachment, the cells were treated with 200 μg/ml 5-FU for 3 h, and washed twice with PBS. The cells were incubated with rhCXCL4 for 48 h and subjected to MTT assay. The effect of rhCXCL4 on the proliferation of T lymphocytes was determined by CCK-8 assay. Briefly, lymphocytes were isolated from the mouse spleen using percoll solution (Dakewe Biotech, Shenzhen, China). Lymphocytes were seeded on 96-well plates at 106 cells/well and incubated with ConA (5 μg/ml, Sigma, USA) and rhCXCL4 for 72 h. CCK-8 solution (10 μl/well, Dojindo, Japan) was added and the absorbance at 450 nm was determined.
Real-time PCR experiments
Total RNA was extracted from the tumor tissues or cells using the Trizol reagent (Invitrogen, USA), and reverse transcription (RT) reactions were performed with the total RNA (0.5 μg) according to the ExScript RT reagent kit (Takara Bio., Japan). The product was amplified in a reaction volume of 20 μl containing 9.2 μg RT product, 10 μl SYBR Premix ExTaqII (2×), and 20 pmol of each primer. PCRs were performed for 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s in a 7900HT Fast Real Time RT-PCR System (ABI, USA). All data were normalized to mouse β-actin or human GAPDH mRNA levels, and the fold change for each mRNA was calculated using the delta Ct method. The primer sequences are shown as follows: mouse β-actin (sense 5′-AGCCTTCCTTCTTGGGTATG and antisense 5′-GTGTTGGCATAGAGGTCTTTAC), mouse CXCL4 (sense 5′-TCCAGTGGCACCCTCTTGAC and antisense 5′-ATCTCCATCGCTTTCTTCGG), mouse IFN-γ ( sense 5′-ACTCAAGTGGCATAGATGTGGAA and antisense 5′-ATGACGCTTATGTTGTTGCTGAT), mouse gran-b (sense 5′-AACAGGAGAAGACCCAGCAAGT and antisense 5′-CCCAACCAGCCACATAGCAC), human CXCL4 (sense 5′-TTCTGCGCCTCACGCCCC and antisense 5′-TGGGACGGACCTGGGAG), and human GAPDH (sense 5′-TTCTGCGCCTCACGCCCC and antisense 5′-TGGGA-CGGACCTGGGAG).
Immunoblotting
For the preparation of the Western-blot samples, the tumor tissues were rapidly homogenized in liquid nitrogen. The tissue powder was reconstituted in ice-cold RIPA buffer with protease inhibitors. Supernatants were recovered after centrifugation, and determined for their protein content using the BCA method (Beyotime Biotechnology, Haimen, China). The sample of 50 μg proteins was subjected to sodium dodecyl sulfate-PAGE (SDS-PAGE), and blotted following standard methods with the first and the corresponding second antibodies. The membranes were washed and developed using the ECL detection system (Beyotime Biotechnology, Haimen, China).
Immunohistochemistry
Cellular apoptosis and proliferation were quantified for the CT26 tumor tissues using the transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) assay and 5-Bromo-2-deoxyUridine (BrdU) staining, respectively. Briefly, tissues were fixed in 4% buffered formaldehyde, embedded in paraffin, and 4 μm thick sections were prepared and stained using the TUNEL or BrdU agents according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The TUNEL-positive or BrdU-positive cells were counted under microscope at a magnification of 400 times (400×). Five fields per mouse were counted for the positive cells.
LDH assay for CTLs experiments
BALB/c mice were inoculated with 1×106 CT26 cells, and when the tumor size reached about 400 mm3, CXCL4-mab (1 mg/kg) or rat IgG control (1 mg/kg) were injected after 5-FU (150 mg/kg) administration. 4 d later, lymphocytes were isolated sterilely from the mouse spleen using the percoll kit (Dakewe Biotech, Shenzhen, China). The CT26 cells were treated with 50 μg/ml Mitomycin C (Sigma, USA) for 1 h, washed twice with PBS, and cultured with the isolated lymphocytes at a ratio of 1:10, in the presence of mIL-2 (50 U/ml, PeproTech, USA). After incubation for 5 d, the activated T lymphocytes were co-cultured for 4 h with CT26 or HCT15 cells as target cells at a ratio of 100:1, 50:1, and 20:1, respectively. The cellular lysis at each ratio was determined using the lactate dehydrogenase (LDH) cytotoxicity assay kit (Beyotime Biotechnology, Haimen, China) according to the manufacturer’s instructions.
Statistical analysis
The data are presented as mean ± SD. The parametric and non-parametric data from the 2 groups are analyzed for significance using the 2-tailed Student t test and the Mann–Whitney U test, respectively. A one-way analysis of variance with the Kruskal–Wallis H test is used to compare multiple groups. Results from the survival experiments are analyzed using a log-rank test and are expressed as Kaplan–Meier survival curves. P values less than 0.05 are considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Author’s Contributions
W.H. and Y.Y. developed the concept for the studies; W.H., Y.Y., and Y.Z. designed the experiments; J.G., Y.Z., and H.Y. participated in the production of recombinant human CXCL4 protein and the CXCL4 antibody; X.W. and SD performed in vitro analysis of CXCL4 function; S.Z. and M.W. performed in vivo assays; J.G., W.G., and Y.Z. performed the tumor experiments of CXCL4-mab; and W.H., Y.Y., and Y.Z. wrote the manuscript.
KCBT_A_1095404.zip
Download Zip (5.2 MB)Funding
This work was supported by the National Natural Science Foundation of China (grant 81373467/H3108, 81173113/H3109, and 81273573/H3108).
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9-29; PMID:24399786; http://dx.doi.org/10.3322/caac.21208
- Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol 2014; 20(14):3738-50; PMID:24833840; http://dx.doi.org/10.3748/wjg.v20.i14.3738
- Price TJ, Segelov E, Burge M, Haller DG, Ackland SP, Tebbutt NC, Karapetis CS, Pavlakis N, Sobrero AF, Cunningham D, et al. Current opinion on optimal treatment for colorectal cancer. Expert Rev Anticancer Ther 2013; 13(5):597-611; PMID:23617351; http://dx.doi.org/10.1586/era.13.37
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, O'Sullivan B, He Z, Peng Y, Tan AC, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 2011; 17:860-6; PMID:21725296; http://dx.doi.org/10.1038/nm.2385
- Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19:57-64; PMID:23202296; http://dx.doi.org/10.1038/nm.2999
- Luo Y, Chihara Y, Fujimoto K, Sasahira T, Kuwada M, Fujiwara R, Fujii K, Ohmori H, Kuniyasu H. High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. Eur J Cancer 2013; 49:741-51; PMID:23040637; http://dx.doi.org/10.1016/j.ejca.2012.09.016
- Gao J, Gao J, Qian L, Wang X, Wu M, Zhang Y, Ye H, Zhu S, Yu Y, Han W. Activation of p38-MAPK by CXCL4/CXCR3 axis contributes to p53-dependent intestinal apoptosis initiated by 5-fluorouracil. Cancer Biol Ther 2014; 15:982-91; PMID:24800927; http://dx.doi.org/10.4161/cbt.29114
- Matsuyama R, Togo S, Shimizu D, Momiyama N, Ishikawa T, Ichikawa Y, Endo I, Kunisaki C, Suzuki H, Hayasizaki Y, et al. Predicting 5-fluorouracil chemosensitivity of liver metastases from colorectal cancer using primary tumor specimens: Three-gene expression model predicts clinical response. Int J Cancer 2006; 119:406-13; PMID:16477629; http://dx.doi.org/10.1002/ijc.21843
- Pezo RC, Gandhi SJ, Shirley LA, Pestell RG, Augenlicht LH, Singer RH. Single-cell transcription site activation predicts chemotherapy response in human colorectal tumors. Cancer Res 2008; 68:4977-82; PMID:18593893; http://dx.doi.org/10.1158/0008-5472.CAN-07-6770
- O'Connell MJ, Lavery I, Yothers G, Paik S, Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J, et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 2010; 28:3937-44; PMID:20679606; http://dx.doi.org/10.1200/JCO.2010.28.9538
- Vandercappellen J, Van Damme J, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev 2011; 22:1-18; http://dx.doi.org/10.1016/j.cytogfr.2010.10.011
- Eslin DE, Zhang C, Samuels KJ, Rauova L, Zhai L, Niewiarowski S, Cines DB, Poncz M, Kowalska MA, et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis:dissociation between anticoagulant and antithrombotic effect of heparin. Blood 2004; 104(10):3173-80; PMID:14764524; http://dx.doi.org/10.1182/blood-2003-11-3994
- Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Connors S, Oenick M, D'Amato RJ, Klement GL, Folkman J. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis 2012; 15:265-73; PMID:22402885; http://dx.doi.org/10.1007/s10456-012-9259-z
- Fiedler GM, Leichtle AB, Kase J, Baumann S, Ceglarek U, Felix K, Conrad T, Witzigmann H, Weimann A, Schütte C, et al. Serum peptidome profiling revealed platelet factor 4 as a potential discriminating peptide associated with pancreatic cancer. Clin Cancer Res 2009; 15:3812-9; PMID:19470732; http://dx.doi.org/10.1158/1078-0432.CCR-08-2701
- Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE Jr, et al. Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 2008; 111(3):1201-7; PMID:17914028; http://dx.doi.org/10.1182/blood-2007-04-084798
- Wiesner T, Bugl S, Mayer F, Hartmann JT, Kopp HG. Differential changes in platelet VEGF, Tsp, CXCL12, and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis 2010; 27:141-9; PMID:20182908; http://dx.doi.org/10.1007/s10585-010-9311-6
- Lam YW, Mobley JA, Evans JE, Carmody JF, Ho SM. Mass profiling-directed isolation and identification of a stage-specific serologic protein biomarker of advanced prostate cancer. Proteomics 2005; 5:2927-38; PMID:15952230; http://dx.doi.org/10.1002/pmic.200401165
- Poruk KE, Firpo MA, Huerter LM, Scaife CL, Emerson LL, Boucher KM, Jones KA, Mulvihill SJ. Serum platelet factor 4 is an independent predictor of survival and venous thromboembolism in patients with pancreatic adenocarcinoma. Cancer Epidem Biomar Prev 2010; 19:2605-10; http://dx.doi.org/10.1158/1055-9965.EPI-10-0178
- Wang Z, Huang H. Platelet factor-4 (CXCL4/PF-4): an angiostatic chemokine for cancer therapy. Cancer Lett 2013; 331(2):147-53; PMID:23337289; http://dx.doi.org/10.1016/j.canlet.2013.01.006
- Liang P, Cheng SH, Cheng CK, Lau KM, Lin SY, Chow EY, Chan NP, Ip RK, Wong RS, Ng MH. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica 2013; 98:288-95; PMID:22929979; http://dx.doi.org/10.3324/haematol.2012.065607
- Fleischer J, Grage-Griebenow E, Kasper B, Heine H, Ernst M, Brandt E, Flad HD, Petersen F. Platelet factor 4 inhibits proliferation and cytokine release of activated human T cells. J Immunol 2002; 169(2):770-7; PMID:12097379; http://dx.doi.org/10.4049/jimmunol.169.2.770
- Liu CY, Battaglia M, Lee SH, Sun QH, Aster RH, Visentin GP. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25− (nonregulatory) T cells. J Immunol 2005; 174:2680-6; PMID:15728475; http://dx.doi.org/10.4049/jimmunol.174.5.2680
- Li JJ, Gao J, Yan D, Yuan Y, Sah S, Satyal U, Liu M, Han W, Yu Y. Neutralization of chemokine CXCL14 (BRAK) expression reduces CCl4 induced liver injury and steatosis in mice. Eur J Pharmacol 2011; 671:120-7; PMID:21978833; http://dx.doi.org/10.1016/j.ejphar.2011.09.174
