ABSTRACT
One of the most active fields in cancer immunotherapy is the study of bispecific antibodies, which engage immune cells to kill cancer cells. However, a variety of issues are associated with most of current bispecific antibody formats. In this study, we present a novel bispecific antibody, BiSS (Bispecific antibody with Single domain, Single domain antibodies), which was constructed by linking 2 single domain antibodies, anti-CEA and anti-CD16, in tandem. Unlike most other bispecific antibodies, the BiSS antibody can be expressed and purified from E.coli in large quantities. By recruiting natural killer cells (NK cells) to CEA-positive cancer cells, BiSS led to cancer cell death in vitro. In xenograft models, the BiSS protein blocked cancer progression. The data suggested that the single domain-based bispecific antibody BiSS was functional and can be potentially applied to a broad range of immunotherapies.
Introduction
Bispecific antibody is a powerful immunotherapy approach that functions by directly engaging immune cells to cancer cells and results in cancer cell death. Over the past several years, a variety of bispecific antibody formats have been proposed.Citation1-3 One of the most advanced bispecific antibody formats is the BiTE (bispecific T cell Engager), which consists of 2 tandem single chain Fv (scFv) regions fused to the anti-CD3 scFv that directly engages T cells and the other scFv recognizing cancer cells.Citation4 BiTE can trigger potent cancer cell death by recruiting T cells to cancer cells.Citation5-10 Recently, Blinatumomab, a BiTE antibody that targets the B cell marker CD19, has been clinically approved for treating B cell leukemia.Citation11 Other BiTE antibodies in development include those that target the epithelial cell adhesion molecule (EpCAM; CD326),Citation12 prostate-specific membrane antigen (PMSA),Citation13 and carcinoembryonic antigen (CEA).Citation14
In this study, we used single domain antibodies to construct a bispecific antibody. Single domain antibodies were derived from the natural camel heavy-chain only antibodies (HCAbs),Citation15 which lack a light chain and the first constant domain (CH1) and are referred to as VHH. Compared with conventional scFV from human IgGs, a single domain antibody has several superior features, including a lower molecular weight, increased stability, and better solubility. Single domain antibodies have been explored for use as bispecific antibodies by 2 tandem single domain antibodies Citation16 or in a nanobody format.Citation17
To recognize cancer cells, we used an anti-CEA single domain antibody. CEA is a heavily glycosylated protein that is involved in cell adhesion and is normally produced by fetal gastrointestinal tissues. However, it is also overexpressed in approximately 95% of gastrointestinal and pancreatic cancers and in most lung and breast carcinomas.Citation18 As one of the most widely-studied cancer-associated antigens, the serum CEA level can be used as a disease recurrence or prognostic indicator in patients with certain cancers.Citation19 Because CEA is expressed at low levels at the luminal portion and not expressed at the basolateral surface of epithelial cells in normal tissues, however, localized at all sides of cancer cells in cancer tissue,Citation20,21 cancer CEA has also been used as a target for immunodiagnosis and/or immunotherapy.Citation21 The FDA has approved several radiolabeled anti-CEA antibodies or antibody fragments as imaging reagents, such as 99mTc-labled CEA-directed arcitumomab (CEA-Scan, Immunomedics, Inc.).Citation22 Recently, MEDI-565 (also known as MT111 or AMG211), which is a BiTE antibody that redirects T cells to cancer cells expressing CEA,Citation9 was shown to kill cancer cells with CEA expression, independent of the mutation status in these cells.Citation14 MEDI-565 is now in Phase I clinical trials in patients with advanced gastrointestinal adenocarcinomas (ClinicalTrials.gov, NCT02291614), thus supporting CEA as a valid target for immunotherapy.
BiSS antibody was constructed by linking an anti-CD16 to engage natural killer cells (NK cells). Autologous or allogeneic NK cell transplants have been used for cancer therapy as NK cells can recognize and kill malignant cells or cells sickened by infection.Citation23 NK cells are also able to function as effector cells in the bispecific antibody format using anti-CD16 antibodies.Citation24,25CD16 is an Fc receptor, which is present on most NK cells. Upon CD16 binding to Fc or anti-CD16 antibody, NK cells are activated, release cytokines, and kill target cells.Citation26,27
Different from the BiTE antibody or other conventional ScFv based bispecific antibodies, the BiSS antibody can be expressed and produced in E. coli in large quantities. The purified antibody recruited NK cells, and the recruited NK cells were able to kill CEA-positive cancer cells in vitro with high potency. In xenograft models, the BiSS antibody significantly suppressed cancer progression. Taken together, these findings indicate that BiSS presents another potential immunotherapy for CEA-positive cancers with potential advantages over other bispecific antibodies.
Materials and methods
Expression vector design and generation of bispecific antibodies
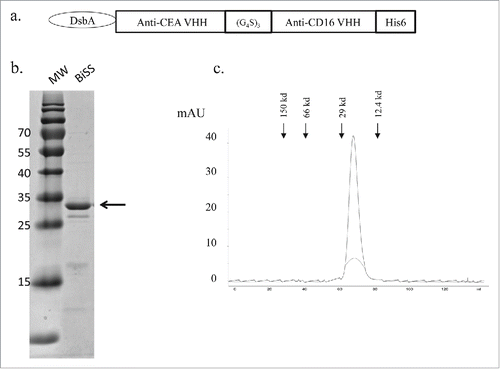
The BiSS antibody was constructed by linking 2 single domain antibodies, anti-CEA and anti-CD16, with a (GGGGS)3 linker (). Anti-CEA single domain antibody (GenBank: ABS29544.1) and anti-CD16 single domain antibody (GenBank: ABQ52436.1) were synthesized based on sequences published previously.Citation28,29 A histidine tag was added to the carboxyl terminus for detection and purification. A DsbA signal peptide was added to the N-terminus to promote periplasmic localization in E. coli. The construct was cloned into pET21a(Novogen) for protein expression and purification.
Figure 1. Expression and purification of the BiSS in E. coli. (a) The bacterial BiSS expression construct contains a DsbA signal sequence for periplasmic expression and an anti-CEA and anti-CD16 single domains with a (GGGGS)3 linker. To facilitate protein detection and purification, a his6 tag was added to the c-terminal end; (b) Coomassie blue–staining of purified proteins after Ni-NTA affinity chromatography and Q-chromatography; (c), Gel filtration chromatography of BiSS identifies a protein at approximately 28 kD.
Protein expression and purification
Periplasmic protein purification of BiSS was performed as described previously.Citation30 Briefly, BL21 cells transformed with BiSS expression plasmid were inoculated in LB medium containing 100 µg/mL Amp and grown at 37°C until A600 reached 1.0. Expression of the BiSS was induced with 0.05 mM isopropyl β-1-ithiogalactopyranoside(IPTG) at 16°C for 16 hours. The periplasmic protein fraction was extracted by osmotic shock in the presence of protease inhibitors. The BiSS protein was then purified by immobilized metal affinity chromatography (Qiagen), followed by Q-sepharose HP (GE pharmacia). The flow through of Q-sepharose HP was collected as it contains the purified BiSS protein.
Gel filtration analysis
Gel filtration was performed using HiPrep 16/60 sephacryl S-200 HR (GE health,Cat# 17-0584-10) with a flow rate of 0.5 ml/min. Fractions (1 ml per fraction) were collected, and then subjected to SDS-PAGE analysis under reducing conditions. Protein was visualized by coomassie blue staining. Protein markers (Sigma Aldrich, Cat# MWGF200) were loaded as standard controls for gel filtration analysis.
Cell lines and human NK cell purification
The human ovarian cancer cell line, SKOV-3, and the human colon cancer cell lines, LS174T and HT-29, were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. SKOV3 was cultured in 1640 medium (Gibco, Life Technologies, China) with 10% HI fetal bovine serum (Gibco, Life Technologies, USA) and 1% Penicillin/Streptomycin (Hyclone), and HT-29 and LS174T were cultured in DMEM medium (Gibco, Life Technologies, China) with 10% HI fetal bovine serum (Gibco, Life Technologies, USA) and 1% Penicillin/Streptomycin (Hyclone).
To isolate human NK cells, fresh peripheral blood mononuclear cells (PBMCs) were isolated from the blood that had been collected from healthy donors by buoyant density centrifugation on Ficoll-Plaque Plus (GE health) at 400 g for 30 min at 20°C. NK cells were then isolated from PBMCs using an EasySep™ Human NK Cell Enrichment Kit according to the manufacturer's instructions (Stem cell Co. Ltd, Cat# 19055).
Flow cytometry assays
Single cell suspensions were incubated with the BiSS antibody or control anti-CEA antibody (R&D, Cat# MAB41281). After washing, the cells were then incubated with FITC-conjugated anti-His IgG (Abcam, Cat# Ab1206) or Alexa 488-conjugated goat-anti-mouse IgG (Lifetechnologies, A11J01) respectively. Flow cytometry analysis was then performed on a FC500 (Beckman Coulter).
Western blot analysis
For western blot analysis of CEA expression, 30 µg of total cell lysate was used for SDS-PAGE. After electrophoresis and membrane transfer, protein gel blot membranes were probed with the BiSS antibody and then anti-His HRP IgG(Abcam, Cat# ab1187). The signal was detected by using Immobilon Western chemiluminescent HRP substrate (Merck Millipore, cat# WBKLS#500).
In vitro cytotoxicity assay
In vitro cytotoxicity assays were performed as described previously.Citation8,31 Briefly, cancer cells were plated in 96-well plates at approximately 5,000 cells/well and were incubated overnight at 37°C, 5% CO2. Then, 50,000 NK cells and different concentrations of proteins (ranging from 0.0001 to 100 nM) were mixed in growth medium and added to each well. After 48 hrs the Cell Counting Kit-8 reagent (Dojindo) was applied. After 1–4 hour incubation, OD450nm was measured using a TECAN microplate reader.
In the soluble CEA competition assays, LS174T and SKOV3 cells were incubated with NK cells and 0.01 nM of BiSS. Different concentrations of recombinant CEA protein (Abcam, Cat. # ab81699) were then applied. The cytotoxic assays were then performed as described above.
The cell survival rate was calculated as: [(As-Ab)/(A0-Ab)]*100%, where As is the absorbent value of the measurement group, Ab is the absorbent value of medium and A0 is the absorbent value of measurement group at 0 nM.
In vivo cancer growth inhibition
Briefly, human colon cancer cells, LS174T cells, were cultured, harvested, and resuspended in PBS. As control group, the cells were then injected subcutaneously into the right flank of NOD/SCID mouse (1 × 106 LS174T cells per mouse, volume at 0.4 ml). To test the efficacy of BiSS, LS174T were mixed with peripheral blood mononuclear cells (PBMC) that were freshly isolated from healthy donors. The mixed cell suspension was then injected subcutaneously into the right flank of NOD/SCID mouse (1 × 16 LS174T cells and 5 × 106 human PBMCs per mouse, volume at 0.4 ml). One hour after cell engraftment, vehicle control (PBS) or BiSS (20 μg per mouse) were administered intraperitoneally (i.p.) into different treatment group (n = 5 each group). The animals were treated daily (PBS or 20 μg BiSS per mouse) over the following 7 d The cancer volumes were measured using calipers in 2 perpendicular dimensions and calculated using the formula (width2 × length)/2.Citation33
Results
BiSS protein expression and purification
The BiSS antibody was constructed by fusing an anti-CEA VHH and anti-CD16 VHH with a his-tag at the c-terminal to facilitate Ni-NTA purification (). The construct was led by a signal peptide DsbA for periplasmic expression. The BiSS protein purification was conducted by Ni-NTA affinity purification followed by Q-ion exchange chromatography (), with a final yield of 0.5 mg/L.
To further characterize the purified BiSS protein, gel filtration was performed to analyze the molecular weight of the BiSS protein. The BiSS protein ran as a single peak with a molecular size of approximately 28 kd, which was the expected size of BiSS monomer, suggesting that the majority of the protein is in the form of monomer, and no protein aggregation was observed ().
BiSS antibody recognizes CEA antigen
To confirm whether the purified BiSS antibody recognizes CEA-positive cells, flow cytometry analysis was conducted using the CEA-positive cell lines LS174T and HT29 and the CEA-negative cell line SKOV3. The BiSS antibody can bind to LS174T cells, though with weaker binding on HT29 cells. It demonstrated no binding to CEA-negative SKOV3 cells (a). These results are consistent with previous results using other anti-CEA antibodies.Citation14
Figure 2. BiSS recognizes the CEA antigen. Flow cytometry analysis of cells treated with BiSS (red line) or a control anti-CEA antibody (blue line) in the CEA-negative cancer cell line SKOV3 (left), CEA-positive cell line HT29 (middle) or LS174T (right).Citation38 Black line: no antibody (negative control); green line: only Anti-His-FITC (control); red line: BiSS as primary antibody and then Anti-His-FITC as secondary antibody; purple line: anti-CEA antibody (positive control). (b), Western blot analysis of CEA expression in different cell lines using BiSS protein. SKOV3/CEA indicates SKOV3 cells that were transiently transfected with plasmid encoding CEA.
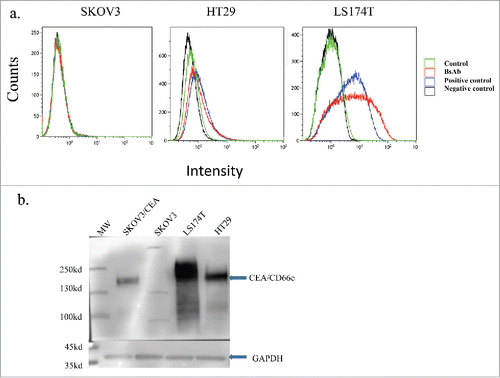
Western blot analysis further confirmed that the BiSS antibody successfully recognized CEA on CEA-positive cell lines LS174T and HT29, but not SKOV3 cells (). Consistent with the higher binding of BiSS on LS174T cells (), western blot analysis showed much higher CEA expression on LS174T cells than on HT29 cells (). These data confirmed that BiSS can specifically bind to CEA on CEA-positive cancer cells.
BiSS kills CEA-expressing cancer cells
To evaluate whether BiSS can trigger cancer cell killing, cytotoxicity assays were performed for different cancer cells. No cytotoxicity was observed for the CEA-negative cell, SKOV3 (). For the CEA-positive cell HT29, BiSS induced cytotoxicity, which increased when the BiSS concentration increased (). Potent cytotoxicity was induced for the CEA-positive cell lines, LS174, even at 10−3 nM BiSS (). No cytotoxicity was observed when BiSS was not present (data not shown) or when NK cells were not present.
Figure 3. BiSS promotes cell death of CEA-positive cancer cells. (a), Human NK cells were mixed with 3 different cancer cells in the presence of the indicated concentrations of BiSS antibody (0 nM, 10−1 nM, 10−2 nM, and 10−3 nM). The mixtures were then incubated for 48 h before cytotoxicity was measured as described in the Materials and Methods; (0 nM vs. 10−1 nM, 10−2 nM, or 10−3 nM,* indicates P < 0.01 by t test). (b), The dose response of BiSS was determined in HT29 and LS174T cells; (c), A soluble CEA competition assay was performed as described in the Materials and Methods. The data represents measurements normalized against cancer cells with NK cells only. All data are the means of quadruplicates with error bars representing the standard deviation. (* indicates P < 0.01 by t test).
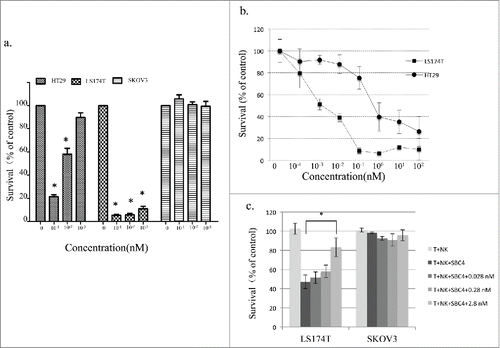
The potency of BiSS on cancer cells was further evaluated by using different dosages of BiSS protein. Corresponding to the higher expression of CEA on LS174 cells than HT29 cells, BiSS induced cytotoxicity in LS174 cells at lower concentration ().
Because CEA can be released from cancer cells in cancer patients, the presence of soluble CEA in blood may potentially affect the efficacy of BiSS in patients by competing for available binding sites on the cancer cell surface. The effect of soluble CEA on the cytotoxic activity of BiSS was thus examined. Because the typical CEA found in the serum of colon cancer patients was in the range of 4.2–102 ng/ml (˜0.023 to 0.56nM),Citation34 soluble CEA at final concentrations of 0.028 nM, 0.28 nM and 2.8 nM was incubated with 0.01 nM of BiSS protein. With only 0.01 nM BiSS protein, no significant effect of soluble CEA was observed until the concentration reached 2.8 nM, which was well above the highest CEA concentration in patients. These data suggested that soluble CEA can affect BiSS cytotoxic activity but requires very high concentration, similar to the previous results obtained with anti-CEA BiTE bispecific antibody.Citation14
BiSS inhibits tumor growth in vivo
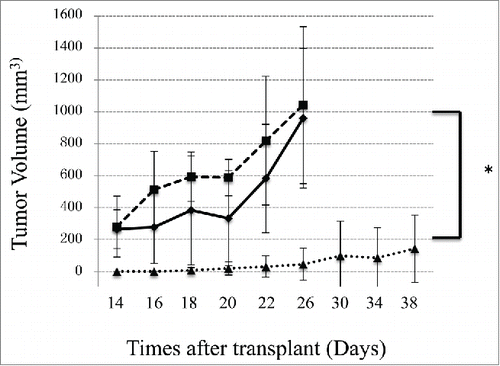
To investigate whether BiSS can suppress cancer growth in vivo, LS174T cells were mixed with isolated human PBMCs and then engrafted subcutaneously on NOD-SCID mice. For mice transplanted with either LS174T alone or LS174T with PBMCs, rapid tumor growth was observed. Significant cancer growth inhibition was observed when mice were treated with the BiSS protein in the presence of PBMC (). In mice that were treated with BiSS protein, cancers developed in only one of the 5 mice. No cancer growth was observed in the other 4 mice. No body weight loss was observed, and no remarkable toxicity was observed in these mice. These results demonstrated that BiSS can inhibit cancer growth in xenograft mouse models.
Figure 4. The BiSS antibody inhibits cancer growth in vivo. NOD/SCID mice (n = 5/group) were engrafted subcutaneously with LS174T cells with or without human PBMCs as described in the Methods and Materials. Three groups of mice were then treated. Two control groups, mice transplanted with LS174T only, no PBMC (solid square), or mice transplanted with both LS174T and PBMC (open triangle) were treated with PBS only. One group of mice transplanted with both LS174T and PBMC were treated with BiSS (20 μg per mouse daily) (solid diamond). The data represent the average cancer volume of 5 mice with the error bars representing the standard deviation (*P < 0.01, t test, BiSS vs the other 2 groups).
Discussion
Cancer immunotherapy has generated great interest because of its potent therapeutic effects on cancer. Among the different approaches for cancer immunotherapy, bispecific antibodies are being intensively investigated. In contrast to traditional antibodies, bispecific antibodies can engage immune cells directly to cancer cells by having 2 different antigen binding sites, with one recognizing cancer cells and the other recognizing immune cells. Recently, blinatumomab, which is a BiTE bispecific antibody against CD19, has resulted in excellent responses in leukemia therapy by engaging T cells.Citation35 However, single-chain Fv fusions, which are used in BiTE and other similar technologies, are generally less stable Citation36 and have the tendency to aggregate.Citation37 Single-chain Fv fusions are also difficult to be expressed in bacteria,Citation37 similar to other IgG or Fab structure-based bispecific antibodies.
In this study, we constructed BiSS by linking 2 single domain antibodies. Differing from single-chain Fv fusions, the BiSS antibody can be expressed and purified from E. coli with good solubility and stability, which likely reflects the properties of single domain antibodies, including their lower molecular weight and generally more stable than conventional scFv.Citation37 Studies have shown that single domain antibodies can be expressed in bacterial in large quantities and with excellent solubility and stability. Indeed, our study showed that the BiSS protein can also be expressed in E. coli without compromising its bispecific antibody properties.
The BiSS bispecific antibody in this study targets the carcinoma marker CEA on cancer cells and CD16 on NK cells. In vitro experiments demonstrated that BiSS has potent cytotoxicity on CEA-positive cancer cells, HT29 and LS174T, at very low concentrations, similar to the high potencies observed when using other bispecific antibodies.
Bispecific antibodies using anti-CD16 have been studied previously by constructing fab-like bi-specific molecules.Citation17 Potent cancer cell cytotoxicity was observed for the Fab-like bispecific antibodies,Citation17 which suggests the flexibility of single domain antibodies for use as building blocks of bispecific antibodies. Compared with the Fab molecules, the BiSS protein has a lower molecular weight, which may have the disadvantage of more rapid clearance in vivo. However, the lower molecular weight of BiSS may penetrate tumor tissue better. Although our data showed that the BiSS protein is capable of inducing NK cells to kill cancer cells, the detailed molecular mechanism is not well understood. The capacity of bispecific antibodies to induce potent cytotoxicity indicates that similar to T cells, the close proximity of NK cells to cancer cells can trigger cytotoxicity. Further studies are needed to understand this process better.
In summary, a novel bispecific antibody BiSS was developed via the fusion of 2 single domain antibodies. Similar to other bispecific antibodies, BiSS can trigger potent cancer cell cytotoxicity, which suggests that single domain antibody holds great flexibility and promise for use as building blocks of bispecific or multi-specific antibodies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors' contributions
BD, CZ, PH, JL, SC and JM carried out the experiments. BD, QL and ZW conceived of the study, participated in its design and coordination, and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to Dr. Mengfeng Li and Dr. Jiang Li at Sun Yat-Sen University School of Medicine, and to Dr. Wei Xie at Sun Yat-Sen University School of Life Sciences for their technical support and valuable discussion.
Funding
This work was financially supported by the Introduced Innovative R&D Team Leadership of Guangdong Province (PR China) (2011Y038).
References
- May C, Sapra P, Gerber HP. Advances in bispecific biotherapeutics for the treatment of cancer. Biochem Pharmacol 2012; 84:1105-12; PMID:22858161; http://dx.doi.org/10.1016/j.bcp.2012.07.011
- Muller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs : clinical immunotherapeutics, Biopharmaceut Gen Ther 2010; 24:89-98; http://dx.doi.org/10.2165/11530960-000000000-00000
- Kontermann R. Dual targeting strategies with bispecific antibodies. mAbs 2012; 4:182-97; PMID:22453100; http://dx.doi.org/10.4161/mabs.4.2.19000
- Nazarian AA, Archibeque IL, Nguyen YH, Wang P, Sinclair AM, Powers DA. Characterization of Bispecific T-cell Engager (BiTE®) Antibodies with a high-capacity T-cell Dependent Cellular Cytotoxicity (TDCC) assay. J Biomol Screen 2015; 20:519-27; PMID:25477202; http://dx.doi.org/10.1177/1087057114561405
- Holliger P, Manzke O, Span M, Hawkins R, Fleischmann B, Qinghua L, Wolf J, Diehl V, Cochet O, Winter G, et al. Carcinoembryonic antigen (CEA)-specific T-cell activation in colon carcinoma induced by anti-CD3 x anti-CEA bispecific diabodies and B7 x anti-CEA bispecific fusion proteins. Cancer Res 1999; 59:2909-16; PMID:10383154
- Peng L, Oberst MD, Huang J, Brohawn P, Morehouse C, Lekstrom K, Baeuerle PA, Wu H, Yao Y, Coats SR, et al. The CEA/CD3-bispecific antibody MEDI-565 (MT111) binds a nonlinear epitope in the full-length but not a short splice variant of CEA. PloS one 2012; 7:e36412; PMID:22574157; http://dx.doi.org/10.1371/journal.pone.0036412
- Osada T, Patel SP, Hammond SA, Osada K, Morse MA, Lyerly HK. CEA/CD3-bispecific T cell-engaging (BiTE) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both PD1 and PD-L1. Cancer Immunol Immunother 2015; 64:677-88; PMID:25742933; http://dx.doi.org/10.1007/s00262-015-1671-y
- Schlereth B, Fichtner I, Lorenczewski G, Kleindienst P, Brischwein K, da Silva A, Kufer P, Lutterbuese R, Junghahn I, Kasimir-Bauer S, et al. Eradication of cancers from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res 2005; 65:2882-9; PMID:15805290; http://dx.doi.org/10.1158/0008-5472.CAN-04-2637
- Osada T, Hsu D, Hammond S, Hobeika A, Devi G, Clay TM, Lyerly HK, Morse MA. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br J Cancer 2010; 102:124-33; PMID:19953093; http://dx.doi.org/10.1038/sj.bjc.6605364
- Lutterbuese R, Raum T, Kischel R, Lutterbuese P, Schlereth B, Schaller E, Mangold S, Rau D, Meier P, Kiener PA, et al. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J Immunother 2009; 32:341-52; PMID:19342971; http://dx.doi.org/10.1097/CJI.0b013e31819b7c70
- Oak E BN. Blinatumomab for the treatment of B-cell lymphoma. Expert Opin Investig drugs 2015; 24:715-24; PMID:25739952; http://dx.doi.org/10.1517/3543784.2015.1021415
- Amann M, Friedrich M, Lutterbuese P, Vieser E, Lorenczewski G, Petersen L, Brischwein K, Kufer P, Kischel R, Baeuerle PA, et al. Therapeutic window of an EpCAM/CD3-specific BiTE antibody in mice is determined by a subpopulation of EpCAM-expressing lymphocytes that is absent in humans. Cancer Immunol Immunother 2009; 58:95-109; PMID:18594818; http://dx.doi.org/10.1007/s00262-008-0529-y
- Buhler P, Molnar E, Dopfer EP, Wolf P, Gierschner D, Wetterauer U, Schamel WW, Elsässer-Beile U. Target-dependent T-cell activation by coligation with a PSMA x CD3 diabody induces lysis of prostate cancer cells. J Immunother 2009; 32:565-73; PMID:19483653; http://dx.doi.org/10.1097/CJI.0b013e3181a697eb
- Oberst MD, Fuhrmann S, Mulgrew K, Amann M, Cheng L, Lutterbuese P, Richman L, Coats S, Baeuerle PA, Hammond SA. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. mAbs 2014; 6:1571-84; PMID:25484061; http://dx.doi.org/10.4161/19420862.2014.975660
- Pain C, Dumont J, Dumoulin M. Camelid single-domain antibody fragments: Uses and prospects to investigate protein misfolding and aggregation, and to treat diseases associated with these phenomena. Biochimie 2015; 111:82-106; PMID:25656912; http://dx.doi.org/10.1016/j.biochi.2015.01.012
- Els Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem 2001; 276:7346-50; PMID:11053416; http://dx.doi.org/10.1074/jbc.M007734200
- Rozan C, Cornillon A, Petiard C, Chartier M, Behar G, Boix C, Kerfelec B, Robert B, Pèlegrin A, Chames P, et al. Single-domain antibody-based and linker-free bispecific antibodies targeting FcgammaRIII induce potent antitumor activity without recruiting regulatory T cells. Mol Cancer Therapeut 2013; 12:1481-91; PMID:23757164; http://dx.doi.org/10.1158/1535-7163.MCT-12-1012
- Kuroki M, Hachimine K, Huang J, Shibaguchi H, Kinugasa T, Maekawa S, Kuroki M. Re-targeting of cytotoxic T lymphocytes and/or natural killer cells to CEA-expressing tumor cells with anti-CEA antibody activity. Anticancer Res 2005; 25:3725-32; PMID:16302732
- Kuroki M, Ueno A, Matsumoto H, Abe H, Li T, Imakiire T, Yamauchi Y, Uno K, Shirota K, Shibaguchi H, et al. Significance of tumor -associated antigens in the diagnosis and therapy of cancer: an overview. Anticancer Res 2002; 22:4255-64; PMID:12553066
- Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 1999; 9:67-91; PMID:10202129; http://dx.doi.org/10.1006/scbi.1998.0119
- Khare PD, Shao-Xi L, Kuroki M, Hirose Y, Arakawa F, Nakamura K, Tomita Y, Kuroki M. Specifically targeted killing of carcinoembryonic antigen (CEA)-expressing cells by a retroviral vector displaying single-chain variable fragmented antibody to CEA and carrying the gene for inducible nitric oxide synthase. Cancer Res 2001; 61:370-5; PMID:11196189
- Hong H, Sun J, Cai W. Radionuclide-Based Cancer Imaging Targeting the Carcinoembryonic Antigen. Biomark Insights 2008; 3:435-51; PMID:19578524
- Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncog 2014; 19:133-41; PMID:24941379; http://dx.doi.org/10.1615/CritRevOncog.2014011091
- Turini M, Chames P, Bruhns P, Baty D, Kerfelec B. A FcgammaRIII-engaging bispecific antibody expands the range of HER2-expressing breast tumor s eligible to antibody therapy. Oncotarget 2014; 5:5304-19; PMID:24979648; http://dx.doi.org/10.18632/oncotarget.2093
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329-39; PMID:17438573; http://dx.doi.org/10.1038/nri2073
- Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res 2013; 19:3844-55; PMID:23690482; http://dx.doi.org/10.1158/1078-0432.CCR-13-0505
- Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther 2012; 11:2674-84; PMID:23075808; http://dx.doi.org/10.1158/1535-7163.MCT-12-0692
- Behar G, Chames P, Teulon I, Cornillon A, Alshoukr F, Roquet F, Pugnière M, Teillaud JL, Gruaz-Guyon A, Pèlegrin A, et al. Llama single-domain antibodies directed against nonconventional epitopes of tumor-associated carcinoembryonic antigen absent from nonspecific cross-reacting antigen. FEBS J 2009; 276:3881-93; PMID:19531051; http://dx.doi.org/10.1111/j.1742-4658.2009.07101.x
- Behar G, Siberil S, Groulet A, Chames P, Pugniere M, Boix C, Sautès-Fridman C, Teillaud JL, Baty D. Isolation and characterization of anti-FcgammaRIII (CD16) llama single-domain antibodies that activate natural killer cells. Protein Eng, Des Selec 2008; 21:1-10; PMID:18073223; http://dx.doi.org/10.1093/protein/gzm064
- Kwong KY, Rader C. E. coli expression and purification of Fab antibody fragments. Curr Protoc Protein sci 2009; 55:6.10:6.10.1-6.10.14. PMID:19235139; http://dx.doi.org/10.1002/0471140864.ps0610s55
- Loffler A, Kufer P, Lutterbuse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dörken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000; 95:2098-103; PMID:10706880
- Schoonjans R, Willems A, Schoonooghe S, Fiers W, Grooten J, Mertens N. Fab chains as an efficient heterodimerization scaffold for the production of recombinant bispecific and trispecific antibody derivatives. J Immunol 2000; 165:7050-7; PMID:11120833; http://dx.doi.org/10.4049/jimmunol.165.12.7050
- Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014; 123:3016-26; PMID:24652987; http://dx.doi.org/10.1182/blood-2013-10-533398
- Bhatnagar J1 TH, Bhatnagar M, Austin GE. Comparison of carcinoembryonic antigen in tissue and serum with grade and stage of colon cancer. Anticancer Res 1999; 19:2181-7; PMID:10472328
- Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29:2493-8; PMID:21576633; http://dx.doi.org/10.1200/JCO.2010.32.7270
- Dall'Acqua W, Carter P. Antibody engineering. Curr Opin Struct Biol 1998; 8:443-50; PMID:9729735; http://dx.doi.org/10.1016/S0959-440X(98)80121-8
- Zhang Z, Li ZH, Wang F, Fang M, Yin CC, Zhou ZY, Lin Q, Huang HL. Overexpression of DsbC and DsbG markedly improves soluble and functional expression of single-chain Fv antibodies in Escherichia coli. Protein Exp Purificat 2002; 26:218-28; PMID:12406675; http://dx.doi.org/10.1016/S1046-5928(02)00502-8
- Lefterova P, Marten A, Buttgereit P, Weineck S, Scheffold C, Huhn D, Schmidt-Wolf IG. Targeting of natural killer-like T immunologic effector cells against leukemia and lymphoma cells by reverse antibody-dependent cellular cytotoxicity. J Immunother 2000; 23:304-10; PMID:10838659; http://dx.doi.org/10.1097/00002371-200005000-00003
