ABSTRACT
Purpose: Large and consistent evidence supports the role of body mass index (BMI) as a prognostic and predictive indicator in breast cancer. However, there is paucity of data specifically referred to women diagnosed at a young age across the different disease settings. We investigated the impact of BMI on treatment outcomes in 86 breast cancer patients aged 45 y or less treated with neoadjuvant chemotherapy (CT) followed by surgery.
Methods: Pathologic complete response (pCR) was defined as the eradication of cancer from both breast and lymph nodes. Overall survival (OS) and disease-free survival (DFS) were calculated using the Kaplan-Meier product-limit method. Curves were compared by long rank test for significance. Potential predictors of survival were tested in Cox models.
Results: We observed a pCR in 19 patients (22%). Lower values of BMI were more commonly associated with pCR (p = 0.05). Results from univariate, but not multivariate, models were somewhat supportive of higher pCR rates in leaner women (p = 0.06). None of the variables impacted DFS. OS was longer in leaner patients (medians and 95%CI: 74.6 months, 66.2–82.9 and 58.5 months, 49.6–67.4, p = 0.009). Longer OS was also related to lower T-stage, adjuvant radiotherapy (RT), and non triple negative (TN) subtype (p = 0.046, p = 0.024, and p = 0.015, respectively). Cox models confirmed the protective role of lower BMI (Hazard Ratios: 0.30, 95%CI: 0.12–0.71, p = 0.007), non TN subtype and adjuvant RT (p = 0.008 and p = 0.024).
Conclusions: In young breast cancer patients treated with neoadjuvant CT followed by surgery, lower values of BMI are associated with longer OS. Our data also showed longer OS in association with a non TN molecular subtype and adjuvant RT. The modifiable nature of BMI and aggressive biologic behavior of the disease diagnosed at a young age encourage further studies to corroborate our findings.
Background
Body mass index (BMI) is an anthropometric parameter widely used for medical purposes. Its applicability both in clinical routine and research spans across several and heterogeneous areas including major chronic diseases and common causes of death.Citation1-5 BMI is by nature an easily assessable indicator and, if standardized operative procedures are applied at the time of data collection, a highly reproducible measure.Citation6 Medical oncologists have first relied on BMI to fulfill dose definition needs in patients treated with chemotherapeutic agents. Since then, the potential value of this anthropometric factor has significantly evolved until its role as a prognostic and/or predictive determinant has consistently emerged in breast and other cancers.Citation7-8 The growing interest of the cancer research community toward BMI is further enhanced by its modifiable nature and encouraging results from trials of life-style modifications and/or administration of drugs acting on energy metabolism in breast cancer patients.Citation9-13
Breast cancer tends to assume a particularly aggressive behavior in younger patients.Citation14-15 Given the rarity of these events in the overall population, young patients do not benefit from specifically tailored screening initiatives, neither have they been stably placed within pre-existing breast cancer screening programs mostly conceived for women aged 50 and older. This translates into worse outcomes which are comparable to those of older women not participating in the screening programs.Citation16 The barely sustainable physical, social and lifestyle challenges faced by young women diagnosed with breast cancer makes it particularly meaningful investigating factors with a prognostic and/or predictive role.Citation17
The impact of BMI on treatment outcomes in breast cancer has been recently addressed in the pooled analysis of data from 8 neoadjuvant trials performed by Fontanella and colleagues. According to their results, a higher BMI was associated with a lower rate of complete pathologic response (pCR) and had a detrimental impact on survival outcomes.Citation18 Subsequently, in a further combined individual patient data analysis, women aged less than 40 y appeared significantly more likely to achieve pCR following neoadjuvant chemotherapy (CT) compared to their older counterpart, particularly if diagnosed with hormone receptor (HR) positive, human epidermal growth factor receptor 2 negative (HR+/HER2-), or triple negative (TN) breast cancers.Citation19
Based on these findings, we investigated whether BMI impacts treatment outcomes following neoadjuvant chemotherapy (CT) in a historic cohort of women aged ≤45 seeking clinical and instrumental breast examination.
Patients and methods
Data included in the present analysis are from 86 patients aged ≤45 years who received pre-operative CT followed by surgery between October 2007 and August 2013. Most of these women were part of a larger cohort participating in the initiative called “Underforty Women Breast Cancer Care,” which was supported by the Campania Region jointly with the Italian Cancer League (LILT from the Italian “Lega Italiana per la Lotta Tumori”).Citation20 The overall initiative was approved by the Institutional Ethical Board of the institutions involved. All patients released a written consent for participating in the study and allowing the collection of data and their anonymized use for scientific purposes. Records concerning demographics, anthropometrics, pathological and clinical features, therapy administered and treatment outcomes were gathered, filed and analyzed by qualified research assistants working in strict collaboration with the surgeons, oncologists and pathologists from our team. For all the study patients, medical therapy was established and administered in full agreement with the current indications and recommendations in both the neoadjuvant and adjuvant phase of treatment. Decisions on breast surgery were informed by the most updated evidence and oriented by the disease characteristics and patient individual needs. Adjuvant radiotherapy was administered to patients having undergone breast conserving surgery (BCS) and, eventually, to women treated with mastectomy based on an individual patient evaluation including axillary lymph node involvement, primary tumor size, margins invasion, grade and age.Citation21-24
Statistical methods
Descriptive statistics were computed for all the variables of interest. Means and ranges were used for continuous data while frequencies and percentage values for categorical variables. Existing differences between medians were evaluated using the T- Student or One Way Anova test according to the number (2 or more) of groups compared. Similarly, depending upon the number and size of groups compared, we used the Pearson's Chi-squared test of independence or Fisher's exact test (2-tailed) to assess the relationship between categorical variables.
BMI was computed as weight in kilograms divided by the square of height in meters (kg/m2) and considered as a categorical variable whose modalities were defined according to a cut off value of 26, namely, the mean BMI computed at this study population level. pCR was defined as no invasive residual tumor in breast and lymph nodes (ypT0/is ypN0) Citation25 and analyzed for the entire study population and in subgroups defined upon BMI, mean age, molecular subtypes,Citation26 type of neoadjuvant regimen, and surgical approach. Given the study focus, results from analysis testing age, molecular subtype, and surgery were further stratified by BMI. We also tested these variables for association with pCR using univariate logistic regression analysis. Multivariate models were then built by including those factors testing significant at the univariate analysis and/or for which evidence of a putative role on the association of interest was supported by the available literature.
Overall survival (OS) and disease-free survival (DFS) were calculated using the Kaplan-Meier product-limit method and curves were compared by long rank test for significance. DFS was defined as the time from the starting date of pre-surgical chemotherapy to the date of disease progression or last follow-up evaluation. OS was calculated as the time from the commencement of pre-operative CT to the date of death from any cause or last contact. Cox proportional hazards models were used to test the impact of features related to the patients, disease and administered treatment on survival outcomes in multivariate analyses. The variable choice was oriented by the evidence emerged from univariate analysis and previous studies. The level of significance was set at p<0.05. SPSS software was used for all statistical evaluations (SPSS version 21.0, SPSS Inc., Chicago, Illinois, USA).
Results
Main descriptive characteristics of the study participants are reported in . Mean age was 38 y and ranged between 20 and 45 y. A BMI value equal to or greater than (≥) 26 was slightly more common and observed in 46 patients (53.5%). Luminal B breast cancers were the most commonly represented subtype [39(45.3)]. At cancer diagnosis, our patients most frequently exhibited a T3-4 and/or N0-1stage [58(67.4) and 48(55.8), respectively]. The greatest majority of our study participants underwent a pre-surgical neoadjuvant therapy based on an anthracycline- and/or taxane-including regimen [79(91,9)]. In about 49%(42 patients) of our study population breast surgery was performed according to a conservative approach. Adjuvant RT and HT were administered in about 65.0% (56) and 62.8%(54) of patients. Nineteen (22.0%) patients received adjuvant trastuzumab. The median follow up was 45.0 months (range:8.0–90.0).
Table 1. Descriptive characteristics of the study participants (N:86).
In , pCR is described for the overall study population and in groups differing by disease- and patient-related features including age, BMI, intrinsic molecular subtypes (IMS) and treatment administered. Overall, we recorded a pCR in 19 patients over 86, namely, in about 22% of our study population. Lower BMI values were more often associated with pCR compared to what observed for the heavier counterpart, though at a not fully significant extent (p = 0.05). Stratification by BMI did not significantly affect results from descriptive analyses (Supplementary ). Results from univariate models were somewhat supportive of higher pCR rates in women with lower BMI (p = 0.06). However, this result was not confirmed in multivariate models (Supplementary ).
Table 2. Pathologic complete response(pCR) in the overall study population and in subgroups defined by participant- and disease-related features (N:19 pCR out of 86 study participants).
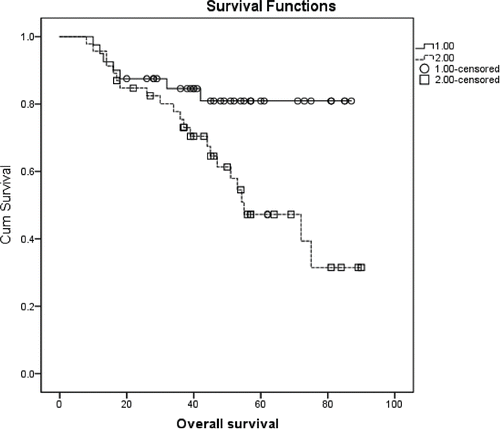
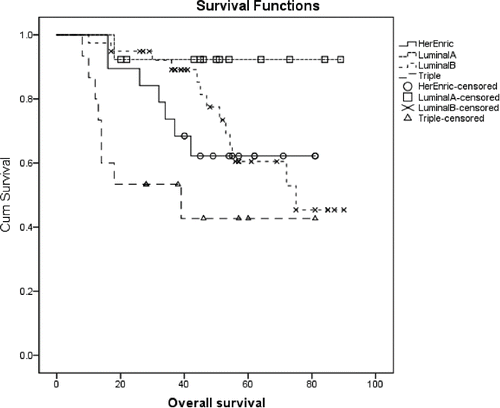
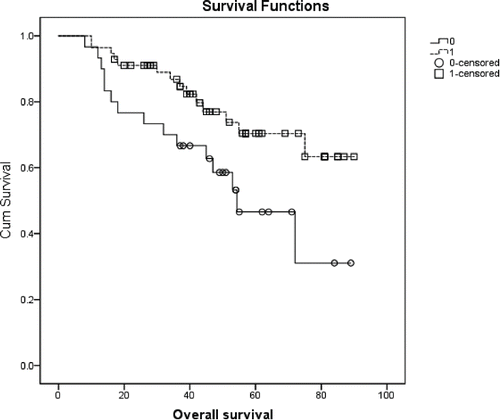
None of the variables tested impacted DFS significantly (available upon request). Conversely, OS was affected by BMI (p=0.009) (). A significantly longer OS was also observed in patients with lower stage at diagnosis (p = 0.046, available upon request), and in women who received adjuvant RT (72.4 months, 64.6–80.2 and 55.3 months, 43.6–67.0, (p = 0.024) (). A longer OS was also conditioned by IMS (p = 0.015, ) and, at some extent, by the chances of being treated with HT (71.2 months, 63.5–79.0 vs 54.3 months, 43.8–64.8, p = 0.05) (available upon request). Results from multivariate analysis are shown in . In Cox models, the protective role of lower BMI was confirmed (HR: 0.30, 95%CI: 0.12–0.71, p = 0.007). In addition, women lived significantly longer if diagnosed with a non TN breast cancer, that is, if the disease was molecularly characterized as luminal A, B or HER2+ (p = 0.008), and if having received adjuvant RT (p = 0.024).
Table 3. Cox proportional hazards models of factors associated with Overall Survival (N:86).
Discussion
In this observational study, we analyzed data from a moderately sized cohort including 86 women aged 45 y or less treated with neoadjvant CT followed by surgery. Our scope was to address an increasingly debated issue, that is, the impact of BMI on short- and long-term treatment outcomes. Our study cohort was essentially characterized by 2 distinctive features, namely, the young age and neoadjuvant setting. We observed a pCR in about 22% of our cohort, with a suggestion in support of the association between favorable outcomes and lower BMI. This was somewhat confirmed in univariate, but not in multivariate, analysis. The role of BMI emerged more clearly from survival analysis, with the inherent data showing a significantly prolonged OS in women whose BMI fell in the lowest category. The impact of BMI on OS was confirmed in multivariate analysis, along with the influence of the molecular characteristics and adjuvant RT.
To the best of our knowledge, no previous study has addressed the role of BMI in the neo-adjuvant setting with a focus on young age at diagnosis. Indeed, 2 groups of colleagues have separately worked on the impact of BMI on neoadjuvant treatment outcomes in breast cancer Citation18 and on the outcomes of neoadjuvant chemotherapy in young breast cancer patients.Citation19 The pCR rate observed in our cohort was about 22%. This is consistent with what reported by Fontanella and co-authors in their individual data pooled analysis on BMI and neoadjuvant treatment outcomes, wherein a pCR rate of 21.3% was observed, i.e., 1,890/8,872.Citation18 Our results are also consistent with those from the work of Loibl and colleagues, who focused on young breast cancer patients and found evidence of an inverse association between age at diagnosis and pCR rate. When compared across groups differing by age, the pCR rates were 20.9 (303/1,453), 17.7 (545/3,073) and 13.7% (608/4,423) for women aged less than 40 years, between 40 and 49 years, and at least 50 years, respectively.Citation19
Results from our analysis provide some support to the association between lower BMI and higher chances of pCR achievement. This is in key with what described by Fontanella and co-authors, who observed a higher pCR rate in normal weight patients, namely, in women whose BMI was between 18.5 and 25, compared with other BMI groups.Citation18 In our work, BMI groups were defined based on the mean value computed at the study population level, namely, 26. This latter cut off is slightly higher than that suggested by the world health organization (WHO) to distinguish between normal weight and overweight patients.Citation27 However, when BMI categories were re-defined according to this same cut off, i.e. Twenty-five rather than 26, the 57.9% (11/19) of pCR still fell in the lowest BMI categories (p = 0.14).
Overall survival was significantly longer in patients within the lowest BMI category. The detrimental effect of BMI on OS in the neoadjuvant setting was also reported by Fontanella and co-authors. In addition, large and consistent evidence links poorer survival outcomes to higher BMI values not only in the neoadjuvant setting but also in the metastatic and adjuvant settings (28-29-30). A number of mechanisms may actually concur to provide a biological rationale for the role of BMI on treatment outcomes in the neoadjuvant and other breast cancer settings. In regard to survival outcomes, several and heterogeneous determinants may potentially be involved. Hormones, adipocytokines, and meaditors of inflammatory processes such as cytokines are all related to key aspects of cell survival or apoptosis, migration, and proliferation. Though in premenopausal women estrogens are mainly of gonadic origin, adipose tissues represent an adjunctive source of these hormones via aromatization from androgens. Higher levels of insulin are commonly observed in overweight and obese women and related to poorer prognosis in breast cancer patients. Possible interactions have been described between leptin and insulin, and obesity-related markers of inflammation have also been described in association with breast cancer outcomes. Further mechanisms could include obesity-related co-morbidities which may act as contraindications to the most effective treatment at the individual patient level.Citation31-35
Several studies have shown that young breast cancer patients are more likely to exhibit a TN subtype (36–37). As shown by Anders and colleagues, in breast cancer arising at a young age, specific biologic differences may be subtype dependent.Citation37 In our cohort, OS was negatively associated with TN breast cancer. This finding replicates what observed by Chen and colleagues in 187 chinese breast cancer patients aged less than 40 y old. In Cox models including lymph node status and molecular subtype, young patients with TN breast cancer showed significantly worse OS compared to other molecular subgroups (p = 0.048).Citation38
In our case series, women who did not undergo adjuvant RT showed a significant disadvantage in terms of OS. According to the results from descriptive analysis, 56 women (65.1%) received RT. Among them, 42 (48.8%) had previously been treated with BCS, while only 14 (31.8%) of those having undergone mastectomy received adjuvant RT. Recently, the administration of postmastectomy radiotherapy (PMRT) has increasingly attracted the researchers' attention. However, we still lack data from randomized trials which may clearly orient toward the delivery or omission of PMRT in patients who had been treated with neoadjuvant chemotherapy, which is now an progressively more common approach. Neither are the available data specifically referred to women diagnosed with breast cancer at a young age.Citation39
Our study has limitations. In first place, the observational nature of the study design makes per sè our research more prone to confounding and bias compared to randomized clinical trials. However, thus far, the association of interest has not been addressed using data from trials. In addition, evidence from a real world population may fairly suit the clinicians' needs and integrate knowledge from future randomized studies. Indeed, due to the strict selection criteria applied at the time of enrollment, patients from trials may significantly differ from patients from the clinical practice. This fuels doubts concerning the external validity of the results from randomized trials and their applicability to a definable group of patients in routine practice.Citation40
Our study also has some important strengths. In first place, novelty of findings should be considered. We observed first time evidence on the role of BMI, an increasingly used anthropometric indicator of general obesity, on treatment outcomes in a well characterized cohort of women with an indication to neoadjuvant treatment due to breast cancer diagnosed at a young age. Similarly, to the best of our knowledge, this is the very first source of data concerning PMRT in young women having undergone neoadjuvant systemic treatment. In addition, when considering the rarity of the condition,Citation41 our series size appears only limitedly restricted and quite well characterized concerning patients' molecular and clinical features.
In summary, we carried out an observational study focused on the role of BMI on the short- and long-term outcomes of 86 women aged 45 or younger who received neoadjuvant CT followed by surgery. We found borderline evidence of higher pCR rates in women within the lowest BMI category compared to their heavier counterpart. The evidence emerged from survival analysis of OS was more robust, with lower values of BMI being associated with significantly longer OS. Results from the univariate were confirmed in Cox models. These latter were also confirmative for more favorable survival outcomes (OS) in patients with non TN breast cancer and in women who have received adjuvant RT. It is notable that every social group is vulnerable to the familial, health and economic burden of cancer. However, current evidence indicates that physical and emotional distress are significantly amplified in patients diagnosed at a young age.Citation42 In this view, further evidence collected throughout ad hoc, prospective studies is urgently needed to confirm our findings and eventually explore interventions targeting BMI for their impact on treatment outcomes in breast cancer patients diagnosed at a young age.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Data.zip
Download Zip (25.9 KB)Funding
This work was partly supported by the Campania Region and the Italian Cancer League (LILT from the Italian “Lega Italiana per la Lotta Tumori”).
References
- Hollander DD, Kampman E, van Herpen CM. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit Rev Oncol Hematol 2015 Jun 18; pii: S1040-8428(15)00120-1; 96(2)
- Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV, Norat T. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 2015 Aug;26(8):1635-48; http://dx.doi.org/10.1093/annonc/mdv142
- James FR, Wootton S, Jackson A, Wiseman M, Copson ER, Cutress RI. Obesity in breast cancer–what is the risk factor? Eur J Cancer 2015 Apr;51(6):705-20; http://dx.doi.org/10.1016/j.ejca.2015.01.057
- Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015 May 15; 115(10):1428-34; http://dx.doi.org/10.1016/j.amjcard.2015.02.024
- Yatsuya H, Li Y, Hilawe EH, Ota A, Wang C, Chiang C, Zhang Y, Uemura M, Osako A, Ozaki Y, Aoyama A. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ J 2014; 78(12):2807-18; PMID:25391910; http://dx.doi.org/10.1253/circj.CJ-14-0850
- De Miguel-Etayo P, Mesana MI, Cardon G, De Bourdeaudhuij I, Góźdź M, Socha P, Lateva M, Iotova V, Koletzko BV, Duvinage K, et al. ToyBox-study group. Reliability of anthropometric measurements in European preschool children: the ToyBox-study. Obes Rev 2014 Aug;15 Suppl 3:67-73; http://dx.doi.org/10.1111/obr.12181
- Chan DS, Norat T. Obesity and breast cancer: not only a risk factor of the disease. Curr Treat Options Oncol 2015 May; 16(5):22; http://dx.doi.org/10.1007/s11864-015-0341-9
- Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 2015 Mar 10; 107(2); http://dx.doi.org/10.1093/jnci/djv088
- Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012; 62:243-74; PMID:22539238; http://dx.doi.org/10.3322/caac.21142
- Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Crawford JJ, Mackey JR. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 2014; 46:1744-51; PMID:24633595; http://dx.doi.org/10.1249/MSS.0000000000000297
- Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev 2014; 15:749-68; PMID:24891269; http://dx.doi.org/10.1111/obr.12190
- Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, Blackburn GL, Findlay B, Gralow JR, Mukherjee S, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol 2014; 32:2231-9; PMID:24934783; http://dx.doi.org/10.1200/JCO.2013.53.1517
- Goodwin PJ, Parulekar WR, Gelmon KA, et al. Effect of metformin versus placebo on weight and metabolic factors in initial patients enrolled onto NCIC CTG MA.32, a multicenter adjuvant randomized controlled trial in early-stage breast cancer (BC). J Clin Oncol 2013; 31 (suppl; abstr 1033)
- Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008; 26:3324-30; PMID:18612148; http://dx.doi.org/10.1200/JCO.2007.14.2471
- Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol 2009 Jun;36(3):237-49; http://dx.doi.org/10.1053/j.seminoncol.2009.03.001
- Hofvind S, Lee CL, Elmore JG. Stage-specific breast cancer incidence rates among participants and non-participants of a population-based mammographic screening program. Breast Cancer Res Treat 2012; 135:291-9; PMID:22833199; http://dx.doi.org/10.1007/s10549-012-2162-x
- Burg MA, Adorno G, Lopez ED, Loerzel V, Stein K, Wallace C, Sharma DK. Current unmet needs of cancer survivors: analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II. Cancer 2015 Feb 15; 121(4):623-30; http://dx.doi.org/10.1002/cncr.28951
- Fontanella C, Lederer B, Gade S, Vanoppen M, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Gerber B, Hanusch C, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat 2015 Feb; 150(1):127-39. Epub 2015 Feb 13; http://dx.doi.org/10.1007/s10549-015-3287-5
- Loibl S, Jackisch C, Lederer B, Untch M, Paepke S, Kümmel S, Schneeweiss A, Huober J, Hilfrich J, Hanusch C, et al. Outcome after neoadjuvant chemotherapy in young breast cancer patients: a pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 2015 Jul;152(2):377-87; http://dx.doi.org/10.1007/s10549-015-3479-z
- http://www.underforty.it/, last accessed in August 2015.
- Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, Falkson G, Falkson HC, Taylor SG, 4th, Tormey DC. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol 1999; 17:1689-700; PMID:10561205
- Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, Hortobagyi G, Buzdar AU, Theriault R, Singletary SE, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol 2000; 18:2817-27; PMID:10920129; http://dx.doi.org/10.1002/jcp.25213
- Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Holmberg SB, Lindtner J, Thürlimann B, Fey M, Werner ID, et al International Breast Cancer Study Group Trials I through VII Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol 2003; 21:1205-13; PMID:12663706; http://dx.doi.org/10.1200/JCO.2003.03.130
- Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, Wolmark N. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol 2004; 22:4247-54; PMID:15452182; http://dx.doi.org/10.1200/JCO.2004.01.042
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and longterm clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (2014); 384:164-172; PMID:24529560; http://dx.doi.org/10.1016/S0140-6736(13)62422-8
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013 Sep; 24(9):2206-23; http://dx.doi.org/10.1093/annonc/mdt303
- http://apps.who.int/bmi/index.jsp, last accessed in October 2015.
- Barba M, Pizzuti L, Sperduti I, Natoli C, Gamucci T, Sergi D, Di Lauro L, Moscetti L, Izzo F, Rinaldi M, et al. Body Mass Index and Treatment Outcomes in Metastatic Breast Cancer Patients Treated with Eribulin. J Cell Physiol 2015 Oct 9
- Widschwendter P, Friedl TW, Schwentner L, DeGregorio N, Jaeger B, Schramm A, Bekes I, Deniz M, Lato K, Weissenbacher T, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res 2015 Sep 18; 17:129; http://dx.doi.org/10.1186/s13058-015-0639-3
- Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014 Oct;25(10):1901-14; http://dx.doi.org/10.1093/annonc/mdu042
- Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol 2010; 28:4058-4065; PMID:20697088; http://dx.doi.org/10.1200/JCO.2010.27.9935
- Lonning PE. Aromatase inhibition for breast cancer treatment. Acta Oncol 1996; 35(Suppl 5):38-43; PMID:9142963; http://dx.doi.org/10.3109/02841869609083966
- Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat 2009; 114:517-525; PMID:18437560; http://dx.doi.org/10.1007/s10549-008-0019-0
- Goodwin PJ, Ennis M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N. Is leptin a mediator of adverse prognostic effects of obesity in breast cancer? J Clin Oncol 2005; 23:6037-6042; PMID:16135472; http://dx.doi.org/10.1200/JCO.2005.02.048
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 2009; 27:3437-3444; PMID:19470939; http://dx.doi.org/10.1200/JCO.2008.18.9068
- Carvalho FM, Bacchi LM, Santos PP, Bacchi CE. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics (Sao Paulo) 2010; 65:1033-6; PMID:21120307; http://dx.doi.org/10.1590/S1807-59322010001000019
- Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, Perou CM. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 2011; 29:e18-20; PMID:21115855; http://dx.doi.org/10.1200/JCO.2010.28.9199
- Chen HL, Ding A, Wang FW. Prognostic effect analysis of molecular subtype on young breast cancer patients. Chin J Cancer Res 2015 Aug; 27(4):428-36; http://dx.doi.org/10.3978/j.issn.1000-9604
- Chapman CH, Jagsi R. Postmastectomy Radiotherapy After Neoadjuvant Chemotherapy: A Review of the Evidence. Oncology (Williston Park) 2015 Sep; 29(9). pii: 213357; PMID:26384802
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet 2005 Jan 1–7; 365(9453):82-93; http://dx.doi.org/10.1016/S0140-6736(04)17670-8
- American Cancer Society (2011) Breast cancer facts and figures 2011–2012. http://www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-and-figures-2011-2012. Accessed 4 Nov 2013.
- Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol 2004 May 15; 22(10):1849-56; http://dx.doi.org/10.1200/JCO.2004.04.173