ABSTRACT
Metformin has been reported to inhibit the growth of various types of cancers, including prostate cancer. Yet the mode of anti-cancer action of metformin and the underlying mechanisms remain not fully elucidated. We hypothesized that the antitumorigenic effects of metformin are mediated through upregulation of pigment epithelium-derived factor (PEDF) expression in prostate cancer cells. In this report, metformin treatment significantly inhibited the proliferation and colony formation of prostate cancer cells, in a dose- and time-dependent manner. Meanwhile, Metformin markedly suppressed migration and invasion and induced apoptosis of both LNCaP and PC3 cancer cells. Metformin also reduced PC3 tumor growth in BALB/c nude mice in vivo. Furthermore, metformin treatment was associated with higher PEDF expression in both prostate cancer cells and tumor tissue. Taken together, metformin inhibits prostate cancer cell proliferation, migration, invasion and tumor growth, and these activities are mediated by upregulation of PEDF expression. These findings provide a novel insight into the molecular functions of metformin as an anticancer agent.
Abbreviations
| PEDF | = | pigment epithelium-derived factor |
| AMPK | = | 5′-AMP-activated protein kinase |
| mTOR | = | mammalian target of rapamycin |
| IL8 | = | interleukin8 |
| MAPK | = | mitogen-activated protein kinase |
| Met | = | metformin |
| qPCR | = | quantitative polymerase chain reaction |
| PBS | = | fetal bovine serum |
| CCK8 | = | Cell Counter Kit-8 |
| PBS | = | phosphate-buffered saline solution |
| PtdIns | = | propidium iodide |
| SD | = | standard deviation |
Introduction
Prostate cancer is the most frequently diagnosed cancer of all new cancer cases each year and the second leading cause of death by cancer in American men. Citation1 In most cases, prostate cancer initially grows slowly and depends on androgen for growth, and thus early stage androgen-sensitive prostate cancer is responsive to androgen-depletion therapy. Unfortunately, the disease eventually becomes refractory and progresses from androgen dependence to androgen independence (i.e., castration resistant), which brings great challenges to its treatment. Citation2,3 Identifying a new and effective therapeutic approach that prevents this progression has become the focus in the fight against prostate cancer.
Metformin, a biguanide derivative, is the favored initial drug for monotherapy in type 2 diabetes. Citation4 The antihyperglycemic action of metformin is mainly a consequence of reduced gluconeogenesis Citation5 and enhancement of glucose uptake in peripheral tissue. Citation6 Moreover, metformin enters the mitochondria and decreases cellular ATP via transient inhibition of complex I of the mitochondrial electron transport chain, leading to activation of 5′-AMP-activated protein kinase (AMPK). Citation7 In addition to its efficacy in lowering glucose levels, metformin has the clinical advantage of reducing the risk of cancer, including prostate cancer. Citation8-10 Several mechanisms by which metformin inhibits cancer migration and growth have been reported. Besides its main molecular target, AMPK, metformin also directly inhibits the mTOR pathway, Citation11 lipogenesis, Citation12 and the androgen receptor signaling pathway. Citation13 Nevertheless, the molecular mechanisms underlying these beneficial effects in prostate cancer remain unclear.
Pigment epithelium-derived factor (PEDF), encoded by SERPINF1, is a serpin that has multiple biological actions. PEDF is expressed and secreted in many tissues. Citation14 In the prostate gland, PEDF is expressed by epithelial and stromal cells, including smooth muscle cells. Downregulation of PEDF expression in prostate cancer has been linked to poor prognosis. Citation15 Recently, PEDF has been identified as a major antitumorigenic or antimetastatic factor. Citation16 Studies have shown that PEDF limited proliferation of prostate cancer cells through the PPARγ–nuclear factor-κB–IL8 pathway Citation17 and induced apoptosis mediated through the PPARγ/p53 cascade. Citation18 Moreover, Konson et al. reported that the proapoptotic and antimigratory activities of PEDF were independently regulated by 2 different MAPK pathways, namely JUN N-terminal kinase and p38, respectively. Citation19
Interestingly, it has been reported that patients with newly diagnosed type 2 diabetes who are taking metformin present higher serum levels of PEDF than those not on metformin. Citation20 Thus, we hypothesized that the antitumorigenic effects of metformin are mediated through upregulation of PEDF expression in prostate cancer cells. To test this hypothesis, we investigated the effects of metformin on prostate cancer cells and their underlying mechanisms, particularly the involvement of PEDF, both in vitro and in vivo.
Results
Metformin reduces prostate cancer cell proliferation, colony formation and induces apoptosis
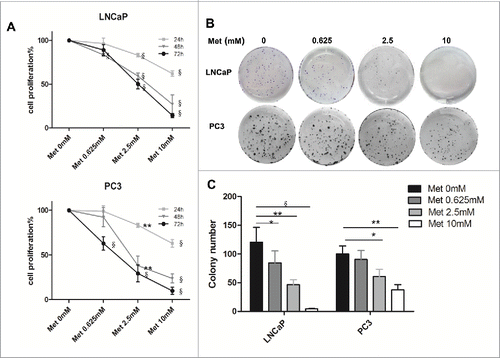
Prostate cancer cell proliferation was reduced after treatment with metformin in a time- and dose-dependent manner (). Cell proliferation was significantly inhibited 24 h after treatment with 2.5 mM or 10 mM metformin compared to the control (p < 0.001 for LNCaP, p < 0.01–0.001 for PC3). Even at a lower metformin concentration of 0.625 mM, LNCaP cell proliferation was inhibited 48 h after treatment (p < 0.001). In addition, colony formation of both cell lines were inhibited by metformin in a dose-dependent manner compared with the control (p < 0.05–0.001 for LNCaP, p < 0.05–0.01 for PC3; ).
Figure 1. Metformin inhibits the proliferation and colony formation of prostate cancer cells. LNCaP and PC3 cells were treated with metformin (Met) at various concentrations (0, 0.625, 2.5, and 10 mM). (A) Cell proliferation was determined at 24, 48, or 72 h by the CCK-8 kit. (B-C) Colony formation was quantified 14 d after treatment with metformin by counting the colonies formed. *p < 0.05; **p < 0.01; §p < 0.001 vs the control.
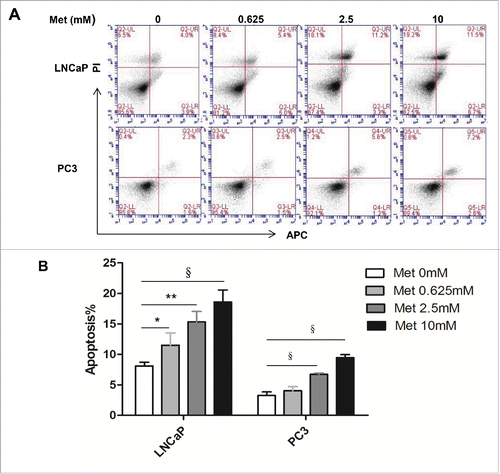
Metformin treatment for 24 h increased both early and late apoptotic cell populations in a dose-dependent manner compared with the control (p < 0.05-0.001 for LNCaP, p < 0.001 for PC3; ). However, metformin did not significantly alter the cell cycle progression of either prostate cancer cell line (Fig. S1).
Figure 2. Metformin induces apoptosis of prostate cancer cells. LNCaP and PC3 cells were treated with metformin (Met) at various concentrations (0, 0.625, 2.5, and 10 mM). After 24 h, apoptosis was quantified by Annexin V-APC assay and PI staining. (A) Early apoptotic cells (lower right, LR) and late apoptotic cells (upper right, UR) are shown. (B) Histograms represent quantification of the rate of apoptosis. *p < 0.05; **p < 0.01; §p < 0.001 vs the control.
Metformin inhibits the migration and invasion of prostate cancer cells
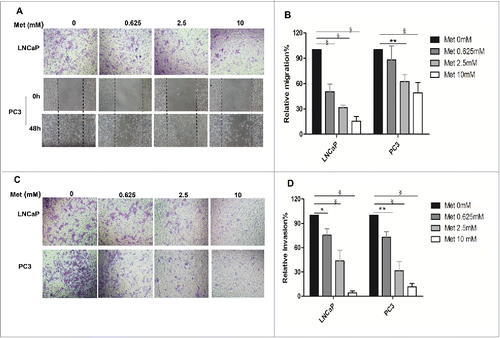
Prostate cancer cells were treated with 0 mM, 0.625 mM, 2.5 mM, or 10 mM metformin for 48 h. Significant inhibitory effects on the migration of LNCaP and PC3 cells were observed (p < 0.001 for LNCaP, p < 0.01-0.001 for PC3; ). Similarly, metformin treatment resulted in strong inhibitory effects on the invasion of LNCaP and PC3 cells (p < 0.05-0.001 for LNCaP, p < 0.01-0.001 for PC3; ).
Figure 3. Metformin inhibits prostate cancer cell migration and invasion. (A-B) Cell migration was determined by a Boyden chamber assay (LNCaP) or a wound closure assay (PC3) following treatment with metformin (Met) for 48 h at the indicated concentrations (0, 0.625, 2.5, and 10 mM). The images are representative counted fields for LNCaP and wound closures for PC3. Data (mean ± SD) are expressed graphically as percentage of cells migrating. (C-D) The invasion assay was performed in Boyden chambers after 48 h in the presence of various concentrations of metformin (0, 0.625, 2.5, and 10 mM). Cells that invaded into the lower chamber were counted, and the images are representative counted fields. Data (mean ± SD) are expressed graphically as percentage of cells invading. *p < 0.05,**p < 0.01, §p < 0.001 vs the control.
Metformin inhibits PC3 tumor growth in vivo
Metformin administration inhibited tumor growth in vivo throughout the course of treatment, resulting in smaller tumors and lower weights in the xenograft tumor model (all panels, p < 0.01; ). Growth of tumors in the metformin groups slowed compared to those in the control group 6 d after beginning metformin treatment (21 d after implantation; all panels, p < 0.01; ). At day 12 of metformin treatment (27 d after implantation), the difference in tumor size between the Met 125 group and the Met 250 group became significant (p < 0.001). During the course of treatment, metformin did not cause visible side effects or changes in mouse body weight (Fig. S2).
Figure 4. Metformin inhibits xenograft tumor growth in nude mice. Xenograft tumors were generated by subcutaneous implantation of PC3 cells (5 mice/group). Metformin treatment (125 mg/kg [Met 125] or 250 mg/kg [Met 250] daily) was initiated 15 d later, and the treatment continued 20 d. (A) PC3 xenograft tumors were measured every 3 d during treatment; mean tumor volumes over time are shown. (B-C) Photographs show the xenograft tumor-bearing mice and tumors by treatment group. (D) Average weights of excised tumors by treatment group are shown. *p < 0.05; **p < 0.01; §p < 0.001 vs the control. #p < 0.001, Met 125 vs Met 250.
![Figure 4. Metformin inhibits xenograft tumor growth in nude mice. Xenograft tumors were generated by subcutaneous implantation of PC3 cells (5 mice/group). Metformin treatment (125 mg/kg [Met 125] or 250 mg/kg [Met 250] daily) was initiated 15 d later, and the treatment continued 20 d. (A) PC3 xenograft tumors were measured every 3 d during treatment; mean tumor volumes over time are shown. (B-C) Photographs show the xenograft tumor-bearing mice and tumors by treatment group. (D) Average weights of excised tumors by treatment group are shown. *p < 0.05; **p < 0.01; §p < 0.001 vs the control. #p < 0.001, Met 125 vs Met 250.](/cms/asset/51d5c49f-d7bd-40fc-b506-af27b58325fc/kcbt_a_1156273_f0004_oc.gif)
Metformin increases PEDF levels in prostate cancer cells
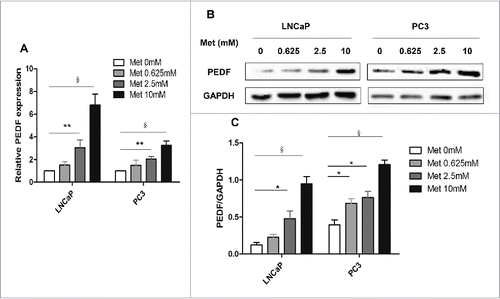
The results from the qPCR assays revealed that PEDF mRNA levels were significantly upregulated in both LNCaP and PC3 cells treated with metformin (all panels, p < 0.01-0.001; ). Metformin had a similar effect on PEDF protein expression (all panels, p < 0.05-0.001; ).
Figure 5. Metformin increases PEDF expression in prostate cancer cells. LNCaP and PC3 cells were treated with metformin (Met) at different concentrations (0, 0.625, 2.5, and 10 mM) for 24 h. (A) Total RNA was isolated and reverse-transcribed for qPCR to estimate the levels of PEDF mRNA in LNCaP and PC3 cells. (B-C) The whole-cell proteins were analyzed by protein gel blot. Data represent mean ± SD. *p < 0.05; **p < 0.01; §p < 0.001 vs the control.
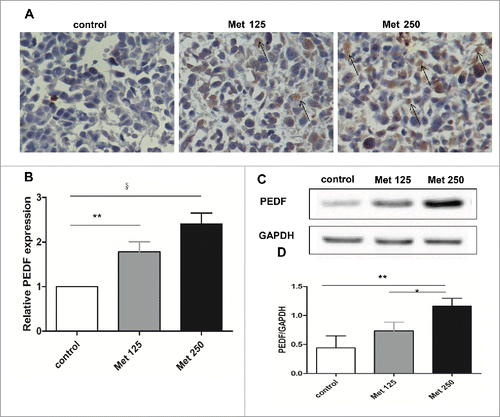
Metformin increases PEDF expression in vivo
Immunohistochemical analysis revealed substantially greater numbers of PEDF-positive cells in tumor tissue sections from the metformin treatment groups than from the control (). Determination of PEDF mRNA and protein expression in tissue extracts by qPCR and western blot analysis yielded findings consistent with the immunohistochemical results: PEDF mRNA and protein levels were upregulated after metformin treatment ().
Figure 6. Metformin increases PEDF expression in vivo. (A) Paraffin sections of tumors excised from mice treated with vehicle (control), metformin 125 mg/kg (Met 125), or metformin 250 mg/kg (Met 250) were assessed for PEDF expression by immunohistochemical staining. PEDF-positive cells are highlighted with arrows (400× magnification). (B-D) PEDF levels were quantified by qPCR (B) and western blot (C&D). *p < 0.05; **p < 0.01; §p < 0.001 vs the control.
Discussion
The findings reported here demonstrate that metformin treatment significantly inhibited the proliferation, colony formation, migration, and invasion of LNCaP and PC3 prostate cancer cells. Metformin treatment also induced apoptosis of these cancer cells. Moreover, metformin reduced prostate tumor growth in BALB/c nude mice in vivo. Metformin-induced increases in PEDF expression were observed in both prostate cancer cells and tumor tissue.
Population-based studies show that metformin, widely used for the treatment of type 2 diabetes mellitus, Citation8-10 is associated with a dose-dependent reduction in cancer risk. Citation21 Previous studies demonstrated that metformin restrained prostate cancer cell proliferation, migration, and invasion and enhanced apoptosis. Citation13,22 These findings are consistent with our results. In contrast, Miyoshi et al. suggested that metformin inhibited the growth of hepatoma cells by inducing G1 cell cycle arrest. Citation23 We observed no notable change in cell cycle distribution and thus conclude that metformin-induced growth inhibition might be partially attributed to increased apoptosis. These different effects of metformin may be dose related. Yi et al. demonstrated that a low concentration of metformin induced p53-dependent senescence in hepatoma cells, whereas higher doses induced apoptotic cell death in these cells. Citation24 Our observation that metformin administration restrained the growth of xenograft tumors in BALB/c nude mice is in line with a previous report that metformin treatment decreased tumorigenic potential. Citation25
Further investigation of the mechanisms underlying metformin's antitumor effect in prostate cancer revealed that metformin increased PEDF expression in both prostate cancer cells and tumor tissue. This is supported by a previous study showing that metformin treatment of patients with newly diagnosed diabetes is associated with a significant increase in serum PEDF level. Citation20 A great deal of evidence links PEDF to tumor suppression. Besides inhibiting tumor angiogenesis, exogenous or naturally secreted PEDF also induces the differentiation of cancer cells to a less-malignant phenotype, Citation26,27 which provided the first suggestion that PEDF could reduce malignant phenotype and act directly on tumors. Moreover, PEDF reduction in prostate cancer has been linked to poor prognosis, Citation28,29 while increased PEDF may act directly on prostate tumors to promote apoptosis, inhibit proliferation, and strongly suppress metastasis. Citation16
Our data suggest that the altered expression of PEDF induced by metformin might be involved in its antitumor activity. However, the exact mechanisms of this association and the regulation between metformin and PEDF need further in-depth investigation. A further study of the effect of metformin on survival in prostate cancer in this animal model is warranted.
Conclusions
Our study demonstrates that metformin treatment reduces prostate cancer cell proliferation, migration, and invasion and induces cell apoptosis in vitro. Metformin also restrains tumor growth in vivo. These effects are mediated at least partially through upregulation of PEDF expression. This result is of particular importance since it is the first time that metformin has been shown to induce PEDF expression in both tumor cells and mouse xenograft tumors. These findings suggest that metformin may have potential therapeutic implications for patients with prostate cancer.
Materials and methods
Cell culture and reagents
The human prostate cancer cell lines LNCaP (androgen dependent) and PC3 (androgen independent) were purchased from the Type Culture Collection of the Chinese Academy of Sciences. Both cell lines were cultured in F12 medium (Gibco #11765054) supplemented with 10% fetal bovine serum (FBS; Bioind #040011A) and 1% 100 U/mL penicillin-streptomycin (Solarbio P1400) at 37°C in an incubator with humidified air and 5% carbon dioxide. The culture medium for LNCaP cells also was supplemented with 1% glutamine.
Metformin (1,1-dimethylbiguanide hydrochloride), purchased from Sigma-Aldrich (D150959), was dissolved in culture medium or physiological saline solution to make a 1 M stock solution and stored at −20°C.
Cell proliferation assay
Cell proliferation was assessed by the Cell Counter Kit-8 (CCK-8; Dojindo CK04).Citation3 In brief, cells were plated in 96-well plates (8×103 cells per well, LNCaP; or 1×104 cells per well, PC3) and allowed to adhere overnight. The cells were then treated with metformin at various concentrations (0 mM, 0.625 mM, 2.5 mM, or 10 mM) for 24, 48, or 72 h before CCK-8 assay reagent (10 µL) was added to each well. After incubation at 37°C for 2 h, optical density units of absorbance at 450 nm were measured by an electroluminescence immunosorbent assay reader (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's instructions as an indicator of cell density.
Colony formation assay
The plate colony formation assay was performed to assess cell growth. Citation30 Cells were seeded into 6-well plates (1×103 cells per well) and treated with metformin at various concentrations (0 mM, 0.625 mM, 2.5 mM, or 10 mM). After 14 days, the resulting colonies were fixed with 4% cold paraformaldehyde for 15 min and stained with crystal violet (Beyotime C0121) for 15 min at room temperature. Only clearly visible colonies (diameter >50 μm) were counted.
Flow cytometry analysis of cell cycle phase and apoptosis
Cells (1×106) treated with metformin for 24 h were harvested and washed twice with cold phosphate-buffered saline solution (PBS) to remove floating cells before fixation in 70% ethanol overnight or analysis by the Annexin V-APC Apoptosis Detection Kit (KeyGEN Biotech KGA1030). For cell cycle analysis, cells were treated with ribonuclease for 30 min at 37°C and stained with propidium iodide (PI; KeyGEN Biotech KGA511) for 30 min at room temperature. Apoptosis and cell cycle phase were evaluated with a flow cytometry analyzer (BD Biosciences, San Jose, CA). Data were analyzed by BD Accuri C6 software.
Wound healing assay and Boyden chamber assay
Migration of PC3 cells was assessed by the wound healing assay. Cells were seeded in 12-well plates (1×105 cells per well). When grown to 80% confluence, the cells in each well were scratched with wound lines vertically to the bottom of the well with a 200-µL pipette tip. After being washed with PBS 3 times, cells were incubated in growth medium containing 2% FBS with metformin at indicated concentrations. The wound width was determined 48 h later under a microscope (Nikon, Tokyo, Japan). The percentage of wound closure was calculated as follows: percentage of wound closure= 1−(widtht/width0)×100%.
Migration of LNCaP cells and invasiveness of both cell lines were quantified by transwell chamber assays. Boyden chambers with 8-µm pore membrane inserts (Corning, Corning, NY) were used. To promote response to the chemoattractant, cells were serum-starved overnight. Cells were then seeded in the upper chambers (1×105 cells per chamber) in serum-free F12 medium in the presence or absence of metformin, and complete F12 medium with 10% FBS was placed in the lower chambers. For invasion experiments, the inserts were coated with 40 µL matrigel solution (matrigel:serum-free medium ratio 1:10). After incubation with metformin for 48 h, cells on the upper surface of the chamber were removed with a cotton swab and the inserts were fixed and stained with crystal violet. Migrating or invading cells attached to the lower surface of the filter were counted in 5 randomly selected areas under a microscope at ×100 magnification.
Tumor xenograft model in mice
Four-week-old male BALB/c athymic nude mice were purchased from the Slack Laboratory Animal Co.. Each mouse was implanted subcutaneously into the right flank with PC3 cells harvested from culture in logarithmic phase (7×106 cells per mouse). Fifteen days later, the animals were randomly allocated to the control or 2 experimental groups (5 mice per group); the two experimental groups received different concentrations of metformin. The metformin was dissolved in physiological saline solution and administered via intraperitoneal injection once daily at a dose of 125 mg/kg (Met 125) or 250 mg/kg (Met 250). The control group received physiological saline solution only. Tumor growth was monitored by caliper measurements. After 20 d of treatment, the difference between control group and experimental group became dramaticlly, the mice were sacrificed by cervical vertebra dislocation and the tumors excised; the tumors were weighed, and portions were frozen in liquid nitrogen or fixed in 4% paraformaldehyde for further study. Tumor volume was calculated as V=length× width2/2.
All murine studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animal Ethics Committee of Wenzhou Medical University.
Real-time quantitative polymerase chain reaction analysis
Total RNA was extracted from cells or prostate tumor tissue treated with metformin by using TRIzol (Invitrogen #15596-018) according to the manufacturer's instructions. Reverse-transcribed cDNA was measured by real-time quantitative polymerase chain reaction (qPCR) using an Applied Biosystems 7500 Fast Sequence Detection System and SYBR Green PCR Kit (Takara RR086A) under the following conditions: predenaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 sec and annealing and extension at 60°C for 30 sec. Primers (Sangon Biotech) for SERPINF1 were 5′-ATCCATCATTCACCGGGCTC-3′ (forward) and 5′-GGGAGGCACTCTTGAGGTTC-3′ (reverse). Primers for the internal control GAPDH were 5′-AAGGTGAAGGTCGGAGTCAAC-3′ (forward) and 5′-GGGGTCATTGATGGCAACAATA-3′ (reverse).
Western blot analysis
Tumor tissues or cells were subjected to lysis in the presence of a protease and phosphatase inhibitor mixture (3 µL) and then homogenized. Protein concentration was measured by using a bicinchoninic acid protein assay kit (Beyotime P0010). Electrophoretic transfer onto nitrocellulose membranes was followed by blocking with 5% nonfat milk and incubation of membranes at 4°C overnight with anti-PEDF antibody (1:1000 dilution; Abcam ab157207) and anti-GAPDH antibody (Goodhere Biochemical). The membranes then were incubated with the secondary antibody, rabbit horseradish peroxidase-conjugated anti-goat IgG (1:2000 dilution; Beyotime A0208) for 1 h at room temperature. Final signal was detected by using the ChemiDoc XRST and processed by Image Lab Software (both, Bio-Rad, Inc., Hercules, CA, USA). The gray value of each band in the imaging data was analyzed by Quantity One software (Bio-Rad, Inc.).
Immunohistochemistry
PEDF expression in mouse prostate tumors was determined by immunohistochemical analysis. In brief, formalin-fixed, paraffin-embedded tumor sections were deparaffinized and rehydrated via a series of ethanol washes. For antigen retrieval, slides were steamed for 30 min in 1× citrate buffer solution. Tumor sections were then probed with anti-PEDF antibody (1:100 dilution in goat serum) and incubated at 4°C overnight. The sections were then incubated with secondary antibody at 37°C for 1 h. The slides were treated with 3,3′-diaminobenzidine to visualize staining and counterstained with hematoxylin. Data reported are the means of 4 independent counts per section.
Statistical analyses
Results were obtained from at least 3 independent experiments. All results are reported as means ± standard deviation (SD). Statistical analyses were performed with SPSS 17.0 (Chicago, Illinois) and GraphPad Prism 5 (La Jolla, CA) software packages. Comparisons between groups were analyzed by analysis of variance or 2-tailed Student t-test. A p-value <0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Data.zip
Download Zip (253.6 KB)Acknowledgments
The authors thank Danli Xie and Jinshuang Bo for assistance with experiments.
Funding
This research was supported in part by grants from the National Natural Science Foundation of China (81170257) and the MD Anderson Cancer Center Startup Fund.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9-29; PMID:24399786; http://dx.doi.org/10.3322/caac.21208
- Wozney JL, Antonarakis ES. Growth factor and signaling pathways and their relevance to prostate cancer therapeutics. Cancer and Metastasis Reviews 2014; 33:581-94; PMID:24402967; http://dx.doi.org/10.1007/s10555-013-9475-z
- Yang X, Yang Y, Gan R, Zhao L, Li W, Zhou H, Wang X, Lu J, Meng QH. Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PloS One 2014; 9:e98833; PMID:24892674; http://dx.doi.org/10.1371/journal.pone.0098833
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38:140-9; PMID:25538310; http://dx.doi.org/10.2337/dc14-2441
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310:1642-6; PMID:16308421; http://dx.doi.org/10.1126/science.1120781
- Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab 2003; 88:1323-32; PMID:12629126; http://dx.doi.org/10.1210/jc.2002-021394
- Pollak M. Potential applications for biguanides in oncology. J Clin Invest 2013; 123:3693-700; PMID:23999444; http://dx.doi.org/10.1172/JCI67232
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330:1304-5; PMID:15849206; http://dx.doi.org/10.1136/bmj.38415.708634.F7
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012; 35:299-304; PMID:22266734; http://dx.doi.org/10.2337/dc11-1313
- Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, Mucci LA, Adami HO, Sorensen HT. Metformin use and prostate cancer risk. Eur Urol 2014; 66:1012-20; PMID:24857538; http://dx.doi.org/10.1016/j.eururo.2014.04.027
- Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011; 71:4366-72; PMID:21540236; http://dx.doi.org/10.1158/0008-5472.CAN-10-1769
- Loubiere C, Goiran T, Laurent K, Djabari Z, Tanti JF, Bost F. Metformin-induced energy deficiency leads to the inhibition of lipogenesis in prostate cancer cells. Oncotarget 2015; 6:15652-61; PMID:26002551; http://dx.doi.org/10.18632/oncotarget.3404
- Wang Y, Liu G, Tong D, Parmar H, Hasenmayer D, Yuan W, Zhang D, Jiang J. Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor. Prostate 2015; 75:1187-96; PMID:25894097; http://dx.doi.org/10.1002/pros.23000
- Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J Cell Biochem 2009; 106:769-75; PMID:19180572; http://dx.doi.org/10.1002/jcb.22072
- Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J, Crawford SE. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med 2003; 9:774-80; PMID:12740569; http://dx.doi.org/10.1038/nm870
- Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer 2013; 13:258-71; PMID:23486238; http://dx.doi.org/10.1038/nrc3484
- Hirsch J, Johnson CL, Nelius T, Kennedy R, Riese W, Filleur S. PEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/phospholipase A2 and regulation of NFkappaB and PPARgamma. Cytokine 2011; 55:202-10; PMID:21570865; http://dx.doi.org/10.1016/j.cyto.2011.04.010
- Li L, Yao YC, Fang SH, Ma CQ, Cen Y, Xu ZM, Dai ZY, Li C, Li S, Zhang T, et al. Pigment epithelial-derived factor (PEDF)-triggered lung cancer cell apoptosis relies on p53 protein-driven Fas ligand (Fas-L) up-regulation and Fas protein cell surface translocation. J Biol Chem 2014; 289:30785-99; PMID:25225287; http://dx.doi.org/10.1074/jbc.M114.590000
- Konson A, Pradeep S, D'Acunto CW, Seger R. Pigment epithelium-derived factor and its phosphomimetic mutant induce JNK-dependent apoptosis and p38-mediated migration arrest. J Biol Chem 2011; 286:3540-51; PMID:21059648; http://dx.doi.org/10.1074/jbc.M110.151548
- Akin S, Aksoy DY, Cinar N, Aydin K, Karaagaoglu E, Ariyurek M, Gulcelik NE, Usman A, Gurlek A. Pigment epithelium-derived factor increases in type 2 diabetes after treatment with metformin. Clin Endocrinol (Oxf) 2012; 77:852-6; PMID:22248012; http://dx.doi.org/10.1111/j.1365-2265.2012.04341.x
- Lin HC, Kachingwe BH, Lin HL, Cheng HW, Uang YS, Wang LH. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: a 6-year follow-up study. Pharmacotherapy 2014; 34:36-45; PMID:23864581; http://dx.doi.org/10.1002/phar.1334
- Dirat B, Ader I, Golzio M, Massa F, Mettouchi A, Laurent K, Larbret F, Malavaud B, Cormont M, Lemichez E, et al. Inhibition of the GTPase Rac1 mediates the antimigratory effects of metformin in prostate cancer cells. Mol Cancer Ther 2015; 14:586-96; PMID:25527635; http://dx.doi.org/10.1158/1535-7163.MCT-14-0102
- Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, et al. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol 2014; 45:322-32; PMID:24806290; http://dx.doi.org/10.3892/ijo.2014.2419
- Yi G, He Z, Zhou X, Xian L, Yuan T, Jia X, Hong J, He L, Liu J. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int J Oncol 2013; 43:1503-10; PMID:23982736; http://dx.doi.org/10.3892/ijo.2013.2077
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008; 27:3576-86; PMID:18212742; http://dx.doi.org/10.1038/sj.onc.1211024
- Filleur S, Volz K, Nelius T, Mirochnik Y, Huang H, Zaichuk TA, Aymerich MS, Becerra SP, Yap R, Veliceasa D, et al. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res 2005; 65:5144-52; PMID:15958558; http://dx.doi.org/10.1158/0008-5472.CAN-04-3744
- Seigel GM, Tombran-Tink J, Becerra SP, Chader GJ, Diloreto DA, Jr., del Cerro C, Lazar ES, del Cerro M. Differentiation of Y79 retinoblastoma cells with pigment epithelial-derived factor and interphotoreceptor matrix wash: effects on tumorigenicity. Growth Factors 1994; 10:289-97; PMID:7803045; http://dx.doi.org/10.3109/08977199409010995
- Nelius T, Samathanam C, Martinez-Marin D, Gaines N, Stevens J, Hickson J, de Riese W, Filleur S. Positive correlation between PEDF expression levels and macrophage density in the human prostate. Prostate 2013; 73:549-61; PMID:23038613; http://dx.doi.org/10.1002/pros.22595
- Halin S, Wikstrom P, Rudolfsson SH, Stattin P, Doll JA, Crawford SE, Bergh A. Decreased pigment epithelium-derived factor is associated with metastatic phenotype in human and rat prostate tumors. Cancer Res 2004; 64:5664-71; PMID:15313905; http://dx.doi.org/10.1158/0008-5472.CAN-04-0835
- Ma J, Guo Y, Chen S, Zhong C, Xue Y, Zhang Y, Lai X, Wei Y, Yu S, Zhang J, et al. Metformin enhances tamoxifen-mediated tumor growth inhibition in ER-positive breast carcinoma. BMC Cancer 2014; 14:172; PMID:24612549; http://dx.doi.org/10.1186/1471-2407-14-172
