ABSTRACT
The consumption of dietary fibers has been implicated with a lowered risk of human colorectal cancer. Proposed mechanisms involve alterations in the stool consistency, transit time, and formation of short-chain fatty acid by dietary fiber fermentation, and the reorganization of gut microbiota. Here we show that Fibersol-2, a digest-resistant maltodextrin, not only inhibits proliferation of colorectal SW480 cancer cell lines by increasing reactive oxygen species (ROS), but decreases the numbers of the adenoma count in Multiple Intestinal Neoplasia (MIN) mice carrying a mutation in the Adenomatous Polyposis Coli gene by 84 d of age. These observations provide direct evidence that Fibersol-2 intrinsically contains anti-cancer activity, independent of the intestinal metabolism and any potential interactions with the microbiota.
Introduction
Human colorectal cancer (CRC) is the second cause of cancer mortality in the USA.Citation1 The incidence of CRC is typically associated with the accumulation of mutations in oncogenes and tumor suppressor genes.Citation2,3 In CRC specifically, one of the commonly affected tumor suppressor genes is Adenomatous Polyposis Coli (Apc) occurring during the early stages of CRC.Citation4 Apc plays an essential role in the Wnt/β-catenin signaling pathway Citation5,6 which regulates the stabilization and accumulation of β-CATENIN protein in the nucleus and the cytoplasm. Nuclear β-CATENIN is the hallmark of an active canonical Wnt pathway. The majority of all cases of CRC have a mutation of the canonical Wnt pathway. Without the presence of Wnt signaling, cells regulate β-Catenin activity via a multiprotein complex, which can phosphorylate β-CATENIN, targeting it for ubiquitination and subsequent degradation.Citation7 The β-CATENIN complex is formed by APC, AXIN and GSK3β. AXIN and APC form a structural scaffold that allows GSK3β to phosphorylate β-CATENIN, as well as APC and AXIN. When Wnt ligand binds to its receptor, FRIZZLED (Fz), it triggers a signaling cascade destabilizing the degradation complex now allowing unphosphorylated β-CATENIN to accumulate and translocate into the nucleus, where it acts as a cofactor for T-cell-/lymphoid enhancing factor (TCF/LEF).Citation8 This triggers transcriptional activation of various genes related to cell fate and cell proliferation.Citation5,10-13 80-90% of patients with sporadic CRC do show a mutation in the Apc gene.Citation14,15 APC is a large protein (312 kDa) that has multiple and diverse functions in cell cycle regulation, chromosome stability, cell migrations and adhesion.Citation9,11-13
In the current study we investigated the effects of Fibersol-2 (FS2) on cells with mutated Apc in cell culture and the pathophysiology in Apc mutant mice.Citation16 Dietary fiber supplements have long been associated with improved intestinal function and reduced risk for CRC. Fibersol-2 (FS2), a digestion resistant maltodextrin, is a soluble corn fiber which is not digested or absorbed in the human small intestine, passing to the large intestine, it is fermented producing short-chain fatty acids, lower pH levels, gaseous byproducts and effecting the microbiota. Citation17 Clinical studies have shown that it helps by decreasing the postprandial blood glucose spikes and insulin levels and retaining healthy serum triglycerides levels.Citation18
Here, an Apc mutant colon cancer cell line (SW480)Citation19,20 and C57BL/6J mice spontaneously developing multiple intestinal neoplasia (MIN) caused by an ENU induced mutation in the Apc gene Citation21,22 were used for analyzing the effects of FS2 in cell culture and in vivo. We found that FS2 treatment increases apoptosis and ROS of SW480 cells. In vivo MIN mice studies demonstrated that FS2 treatment significantly decreases the formation of adenomas in the small intestine and reduces the number of polyps in the colon of the animals.
Material and methods
Cells and reagents
SW480 (human colorectal adenocarcinoma) cells were purchased from ATCC (CCL-228). The cells were grown in Leibovitz's L-15 Medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), penicillin/streptomycin (100 units/ml, Invitrogen) and 10 mM sodium pyruvate (Sigma, St. Louis, MO).
Fibersol-2 was provided by Matsutani America (Itasca, IL), and solubilized in autoclave sterile water for mouse feeding.
ROS detection and apoptosis
SW480 cells were treated with or without Fibersol-2 (5% final concentration). After 24 hours culture ROS and apoptosis were examined.
MitoSOX (mitochondrial superoxide indicator), and CM-H2DCFDA (general oxidative stress indicator) were obtained from Invitrogen. For measurement of mitochondrial and cellular ROS cells were cultured in complete DMEM containing 5 μM Mitosox or CM-H2DCFDA for 10 min at 37°C protected from light. Cells were detached by 0.1 trypsin/EDTA solution after incubation and cell suspensions were analyzed by flow cytometry.
Apoptosis was determined by annexinV/ Propidium Iodide (PI) double staining kit (BD Biosciences) according to manufacturer's instruction by a flow cytometer (LSR II, BD Biosciences, Franklin Lakes, NK). The data were analyzed with FCS Express 4 program (De Novo software Los Angeles, CA).
Apc mutant mice
Breeder pairs of C57BL/6J ApcMIN/+ mice were obtained from Dr. William Dove (UW-Madison, Madison, WI).Citation21 The mice used were bred and maintained at University of Wisconsin Parkside (Kenosha, WI) under a constant LD (light/dark) 12hours/12hours cycle and the experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC). New litters were weaned at 21 d of age, separated by sex and grouped housed no more than 4 per cage under the same light/dark cycle. Males were housed in a separate room from females. Mice were fed a diet containing 10% fat (Lab Diet 5 K20) recommended by Dr. Dove to support the MIN phenotype.
Water bottles were filled with either regular tap water (control group) or Fibersol-2 (1% final concentration) (treated group). All efforts were made to achieve a 50/50 ratio of treated vs. control offspring produced from the same male MIN breeder. At weaning, Fibersol-2 treatment was initiated by supplementing the drinking water. The 1% concentration of Fiberol-2 was established as optimal for water supplementation in a feasibility study (data not shown) and is lower than the optimal dosage used in the cell culture studies.
All mice were euthanized at 84 d of age using a flow of CO2 gas to effect. The intestinal tract, cecum and colon were removed. Cecums were stored at −20°C. Intestinal tract and colon were placed in a petri dish containing 1X PBS solution. The intestinal tract was divided in 4 equal parts and placed on bibulous paper saturated with PBS. Tracts and colons were split open longitudinally with micro-scissors and spread open. Debris within was washed out with a flow of PBS interchanged with 70% ethanol (a pre-fixation of tissue). Papers were stacked, rolled up and stored in a 50 ml conical tube containing 10% formalin for at least 24 hours. Formalin solution was replaced with 70% ethanol for indefinite storage.
Blind scoring of the intestinal adenomas occurred any time after this point. One animal's tissue was removed from the conical tube; the tissue was removed from the paper and placed in a Petri dish containing 70% ethanol. The tissue was viewed using a dissecting microscope under a 10X magnification. The number of intestinal tumors in each segment of the small intestine and the colon were determined and recorded. The tissue was returned to the conical tube and stored.
Results
Fibersol-2 increases ROS generation in SW480
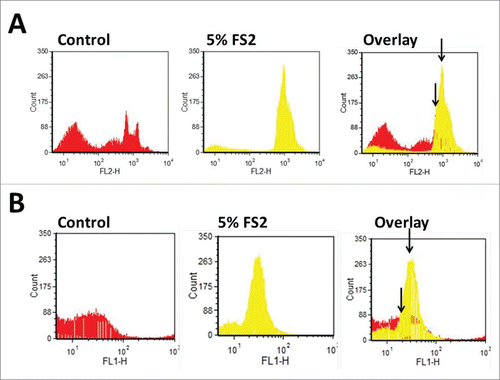
ROS are byproducts of normal cell metabolism. Mitochondria generate ATP by oxidizing lipids, amino acids and glucose, and also generate O2 by transferring electrons to the electron transport chain (ETC). But, in this process, free radicals are also generated. Excessive ROS generation, or failure of oxidant scavenging systems, can disrupt cellular function by causing oxidation of proteins, DNA and lipids. Lower levels of oxidants act as signal transduction messengers in redox signaling pathways, which have important roles in the regulation of cell function, including proliferation. Citation23,24 Cancer-inducing mutations in mitochondrial enzymes can augment the generation of ROS from multiple sites. Several studies suggest a direct relationship between ROS and cancer development; free radicals can induce DNA mutation resulting in genomic instability and carcinogenesis.Citation25 Cancer cells have higher levels of ROS resulting from their increased metabolic activity, peroxisome activity, mitochondrial dysfunction, oncogene activity, increased activity of oxidases, etc.Citation26-28 Previous studies of our group have demonstrated that FS2 can reduce the mitochondrial membrane potential causing mitochondrial malfunction and increased mitochondrial ROS production Citation29 in HCT116 and HCT116 p53(-) cell lines.Citation30 In the current study the effects of FS2 on the SW480 cell line was investigated. SW480 cells carry mutations in both the p53 and the Apc genes.Citation31 Basal levels of cellular and mitochondrial ROS were determined by CM-H2DCFDA and MitoSox respectively. CM-H2DCFDA has been used extensively to measure cellular H2O2 and other ROS, but it does not measure superoxide directly,Citation32 on the other hand, MitoSOX red is a selective indicator of mitochondrially-generated superoxide production.Citation30 The SW480 cells were either treated with FS2 (final concentration 5%) or with water (control) for 24 hours. An increase of 15% in the mitochondria ROS production (using MitoSox) after FS2 treatment was observed in FS2 treated SW480 cells. In order to determine if the cause of the observed increase in ROS was due to mitochondria or other organelles responding to FS2 treatment, CM-H2DCFDA ROS production was determined to measure the total cellular ROS formation. An increase in total ROS was observed after FS2 treatment and that increase is 10% higher that the observed superoxide production (25% more than untreated cells). These results strongly suggest that ROS is generated not only in mitochondria, and that some other organelles are producing ROS when treated with FS2 ().
Fibersol-2 increases apoptosis in SW480 cells
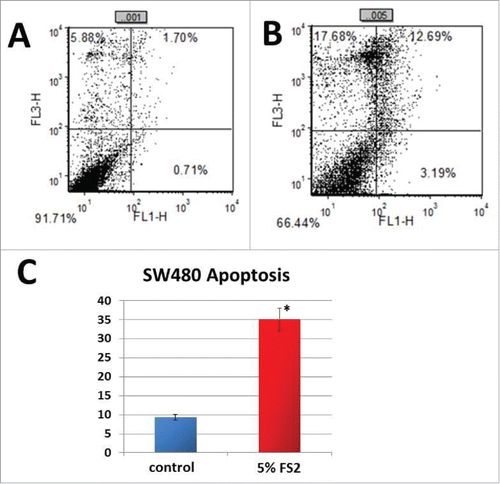
It has been well demonstrated that many of the therapeutic reagents increase cellular ROS levels, causing DNA damage and subsequent apoptosis of cancer cells. Previous results of our group have shown that FS2 induce activation of caspase 3 and 9 in HCT116 human colorectal cancer cells and this activation is p53/Bax dependent.Citation29 In the present study we looked at whether the increased levels of ROS induced by FS2 treatment subsequently can cause apoptosis of SW480 colorectal cancer cells. Cells were treated with FS2 (final concentration 5%) or water (as a control) for 48 h. Apoptosis activity was analyzed by Annexin V/PtdIns double staining and flow cytometry detection. As shown in apoptosis is 3.75 fold higher in SW480 treated with FS2 than in untreated cells.
Fibersol-2 decreases the number of adenomas in the small intestine of ApcMIN/+ mutant mice
In order to examine if FS2 exhibits inhibitory characteristics in vivo, drinking water supplemented with 1% FS2 was provided to mice carrying a heterozygous mutant set of the Apc gene (ApcMIN/+ ), which typically show high levels of adenomas in their digestive tract within 84 d of age.Citation21 ApcMIN/+ mice present pathological differences in the small-intestinal and colonic regions Citation33,34 as well as female and male specific variances.Citation35-37 Thus, FS2s anti-tumor activity was studied in both male and female ApcMIN/+ mice. Three week old mice were given 1% final concentration of FS2 in the drinking water for a total duration of 9 weeks before euthanasia. The intestinal tract from duodenum to colon was harvested. The duodenum, jejunum and ileum were analyzed under the dissecting microscope and the total small intestinal adenoma count for each animal was determined. An example of an adenoma in the small intestinal region is shown in . The group averages (control vs FS2, males vs females) are presented in . Female ApcMIN/+ mice typically show a higher intestinal tumor count compared to their male littermates.Citation35,37 In the present study a 2 factor ANOVA confirmed a significant prevalence for intestinal tumors in female mice (gender; F = 5.844, p = 0.017). At the same time, animals treated with 1% FS2 showed a significant reduction in total adenoma counts (FS2; F =5.985, p=0.015) compared to the water treated groups, but we found no interaction between gender and treatment (F=0.029, p=0.865) (see ).
Figure 3. 10x magnification of histological samples of a small intestine and colon from an 84 d old ApcMIN/+ mouse. The small intestinal tract was divided into 4 equal parts and placed on bibulous paper saturated with PBS. Tracts and colons were split open longitudinally with micro-scissors and spread open. Debris within was washed out with a flow of PBS interchanged with 70% ethanol (a pre-fixation of tissue). Samples were scored blind and are reported on . (A) The arrow point toward 2 examples of adenomas. (B) Two polyps can be clearly identified as dark spots on the tissue (arrows). Polyps were more numerous in male mice and are absent in C57BL/6J wildtype animals.
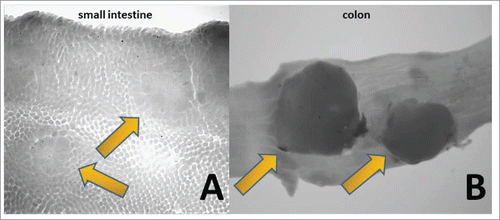
Figure 4. Total average counts in the small intestine for each group are shown. The error bars indicate the Standard Error from the Mean (SEM). The dotted and the solid lines indicated male and female animals respectively. Females displayed a significantly higher number of tumors over males, and a significant reduction of counts was observed between the control (H2O) and the 1%FS2 treated animals.
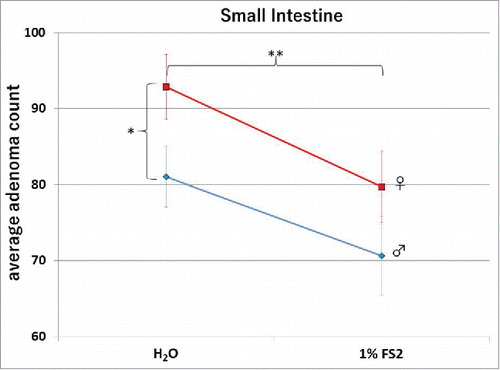
Table 1. The 4 tested groups; males, females with and without the administration of 1% fibersol 2 are shown in this table. The n for each group and the average total tumor counts and the Standard deviations from the mean are indicated.
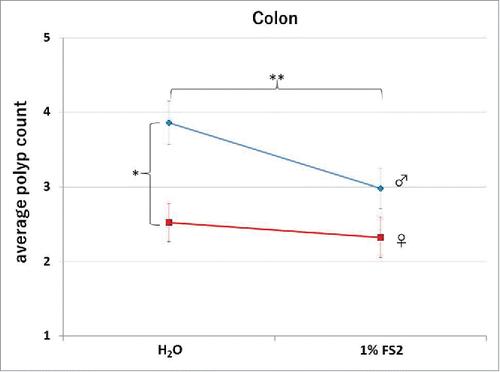
Fibersol-2 decreases the number of polyps in the colon in male and female ApcMIN/+ mutant mice
In the colon the Apc mutation leads to an increased development of polyps. In humans, mutations of Apc have been associated with familial adenomatous polyposis (FAP) or Gardner's syndrome.Citation38,39 While the development of adenomas in the small intestine in affected mice is rapid, we investigated if the development of colonic adenomatous polyposis is also affected by FS2. We compared ApcMIN/+ colon tissues of FS2 treated versus untreated mice independently and the results are summarized in , an image of a polyp is shown in and the group averages are presented in . In the large intestine, the gender bios for polyposis is reversed Citation35,37 and in the tested groups the average count of polyps in males is significantly higher than the one in females (2 factor ANOVA gender; F = 13.230, p < 0.001). More importantly, a significant reduction in the number of polyps after the administration of 1%FS2 (2 factor ANOVA FS2; F = 3.920,p = 0.049), yet no interaction between gender and FS2 (F = 1.566, p = 0.212) was observed. This is consistent with the effects of FS2 seen in the small intestine and suggests Apc signal regulation of FS2.
Figure 5. Total average counts in the colon for each group are shown. The error bars indicate the Standard Error from the Mean (SEM). The dotted and the solid lines indicated male and female animals respectively. Males displayed a significantly higher number of tumors over females, and a significant reduction of counts was observed between the control (H2O) and the 1%FS2 treated animals.
Discussion
Dietary fiber consumption has been associated with a lower risk of human colorectal cancer.Citation40,41 Different mechanisms have been proposed to explain how fiber can protect colorectal cells from carcinogenesis, those including increased stool bulk and reduced stool transit time, formation of short-chain fatty acid by dietary fiber fermentation, and the reduction of secondary bile acid production.Citation42 Fermentation production of short-chain fatty acids are suggested to promote a healthier gut microbiota Citation43 and induce differentiation, arrest growth, and apoptosis of cells in the gastrointestinal tract .Citation44
Development of colorectal cancers is closely associated with mutations in the Apc gene, leading to increased transcription of genes involved in cell fate, proliferation and carcinogenesis.Citation5,45 ApcMIN/+ mice are highly susceptible to spontaneous formation of intestinal adenomas and adenomatous polyposis.Citation21
In the current study we demonstrated that FS2 has anticancer effects by increasing apoptosis and ROS generation in SW480. In Multiple Intestinal Neoplasia mice (ApcMIN/+), treatment with FS2 did significantly reduce the number of adenomas developing in the small intestine and decreased the number of polyps formed in the colon in the animals. The finding that FS2 affected Apc-mutant cell line as well as ApcMIN/+ mice's pathophysiology suggests an anti-tumor mechanism independent of the microbiota and fermentation products.
Previous studies of our group demonstrated that the apoptotic activity of FS2 is, at least in part, p53/Bax dependent, leading to increased cleavage of caspase 3 and 9.Citation29 In the present study we demonstrate that a significant increase in apoptosis caused by FS2 was observed in SW480 cells in which p53 are mutant.Citation46 Furthermore an increase in ROS formation was observed in SW480 cells treated with FS2. These results suggest that ROS could mediate p53-independent apoptosis in these cells. Similarly, previous studies demonstrated that FS2 reduces mitochondrial membrane potential, increasing ROS production in HCT116 cells.Citation29
In conclusion, these data strongly suggest that direct exposure of cancer cells to components of FS2 inhibit cancer cell growth and proliferation, presumably independent of metabolic products formed from FS2 fermentation in the large intestine. Future studies are required to elucidate the mechanism of a microflora independent FS2 activity in more detail.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We want to thank Dr. William Dove at the University of Wisconsin Madison, WI for supplying the ApcMin/+ mice and his laboratory staff for providing assistance establishing the colony.
Funding
This works was supported by Matsutani Research Fund, R01CA90631 and 5P01CA16056 (T.O.).
References
- Parkin MFB, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74-108; PMID:15761078; http://dx.doi.org/10.3322/canjclin.55.2.74
- Fearon ER VB. A genetic model for colorectal tumorigenesis. Cell 1990; 61:759-67; PMID:2188735; http://dx.doi.org/10.1016/0092-8674(90)90186-I
- Kinzler KW VB. Lessons from hereditary colorectal cancer. Cell 1996; 87:159-70; PMID:8861899; http://dx.doi.org/10.1016/S0092-8674(00)81333-1
- Powell SMZN, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature 1992; 359:235-37; PMID:1528264; http://dx.doi.org/10.1038/359235a0
- Giles RH vEJ, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et biophysica acta 2003; 1653(1):1-24; PMID:12781368; http://dx/doi.org/10.1016/S0304-419X(03)00005-2
- Polakis P. Wnt signaling and cancer. Gen Dev 2000; 14(15):1837-51; PMID:10921899; http://dx.doi.org/10.1101/cshperspect.a008052
- Orford KCC, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of β- catenin. J Biol Chem 1997; 272(40):24735-8; PMID:9312064; http;//dx.doi.org/10.1074/jbc.272.40.24735
- Satoh SDY, Furukawa Y,Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 2000; 24(3):245-50; PMID:10700176; http://dx.doi.org/10.1038/73448
- Piefer MPP. Wnt signaling in oncogenesis and embryogenesis - A look outside the nucleus. Science 2000; 287(5458):1606-9; PMID:10733430; http://dx.doi.org/10.1126/science.287.5458.1606
- Fodde R. The APC gene in colorectal cancer. Eur J Cancer 2002; 38(7):867-71; PMID:11978510; http://dx.doi.org/10.1016/S0959-8049(02)00040-0
- Neufeld KL ZF, Cullen BR, White RL. APC-mediated downregulation of β-catenin activity involves nuclear sequestration and nuclear export. EMBO Rep 2000; 1(6):519-23; PMID:11263497; http://dx.doi.org/10.1093/embo-reports/kvd117
- Rosin-Arbesfeld RTF, Blenz M. The APC tumour suppressor has a nuclear export function. Nature 2000; 406(6799):1009-12; PMID:10984057; http://dx.doi.org/10.1038/35023016
- Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat Cell Biol 2000; 2(9):653-60; PMID:10980707; http://dx.doi.org/10.1038/35023605
- Abdelmaksoud-Damak R, Miladi-Abdennadher I, Triki M, Khabir A, Charfi S, Ayadi L, Frikha M, Sellami-Boudawara T, Mokdad-Gargouri R. Expression and mutation pattern of β-catenin and adenomatous polyposis coli in colorectal cancer patients. Arch Med Res 2015 Jan; 46(1):54-62; PMID:25660336; http://dx.doi.org/10.1016/j.arcmed.2015.01.001
- Allen JI. Molecular biology of colon polyps and colon cancer. Semin Surg Oncol 1995 Nov-Dec; 11(6):399-405. Review; http://dx.doi.org/10.1002/ssu.2980110606
- Li G, Tamura K, Yamamoto Y, Sashio H, Utsunomiya J, Yamamura T, Shimoyama T, Furuyama J. Molecular and clinical study of familial adenomatous polyposis for genetic testing and management. J Exp Clin Cancer Res 1999; 18:519-29; PMID:10746979
- ToKunaga KMA. Effects of a food for specified health use (FOSHU) which contains indigestible dextrin as an effective ingredient on glucose and lipid metabolism. Journal of the Japan Diabetes Society 1999; 42:61-5
- Asp NG. Dietary fibre–definition, chemistry and analytical determination. Mol Aspects Med 1987; 9(1):17-29; PMID:3031413; http://dx.doi.org/10.1016/0098-2997(87)90014-8
- Xu MH, Zang GY. Effect of indomethacin on cell cycle proteins in colon cancer cell lines. World J Gastroenterol 2005; 11:1693-96;PMID:15786552; http://dx.doi.org/10.3748/wjg.v11.i11.1693
- Zhao LLL, Wang S, Zhang YF, Yu L, Ding YQ. Differential proteomic analysis of human colorectal carcinoma cell lines metastasis-associated proteins. J Cancer Res Clin Oncol 2007; 133:771-82; PMID:17503081; http://dx.doi.org/10.1007/s00432-007-0222-0
- Moser AR, WF Dove, Roth, KA, Gordon JI. The Min Mutation: its effect on gut epithelial cell differentiation and interation with a modifier system. J Cell Biol 1992 Mar; 116(6):1517-26; PMID:1541640; http://dx.doi.org/10.1083/jcb.116.6.1517
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 1992 May 1; 256(5057):668-70; PMID:1350108; http://dx.doi.org/10.1126/science.1350108
- Murphy MP HA, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nyström T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab 2011; 13(4):361-6; PMID:21459321; http://dx.doi.org/10.1016/j.cmet.2011.03.010
- Chance B SHaBA. Hydroperoxide metabolism in mammalian organs. Physiol Rev 1979; 59:527-605; PMID:37532
- Martinez-Outschoorn UE BR, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. . Cell Cycle 2010; 9(16):3256-76; PMID:20814239; http://dx.doi.org/10.4161/cc.9.16.12553
- Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005; 10:1881-96; PMID:15769673; http://dx.doi.org/10.2741/1667
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 1991; 51(3):794-8; PMID:1846317
- Babior BM. NADPH oxidase: an update. Blood 1999; 93(5):1464-76; PMID:10029572
- Eui Young So MO, Sara Cuesta-Sancho, Susan Losee Olson, Dirk Reif, Kazuhiro Shimomura, Toru Ouchi. Tumor suppression by resistant maltodextrin, Fibersol-2. Cancer Biol Ther 2015; 16(3):460-5; PMID:25692338; http://dx.doi.org/10.1080/15384047.2015.1009269
- Robinson KM JM, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nature protocols 2008; 3:941-7; PMID:18536642; http://dx.doi.org/10.1038/nprot.2008.56
- Goyette MC, Cho K, Fasching CL, Levy DB, Kinzler KW, Paraskeva C, Vogelstein B, Stanbridge EJ. Progression of colorectal cancer is associated with multiple tumor suppressor gene defects but inhibition of tumorigenicity is accomplished by correction of any single defect via chromosome transfer. Mol Cell Biol 1992 Mar; 12(3):1387-95; PMID:1347643; http://dx.doi.org/10.1128/MCB.12.3.1387
- LeBel CP IH, Bondy SC. Evaluation of the probe 2',7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical Res Toxicol 1992; 5:227-31; PMID:1322737; http://dx.doi.org/10.1021/tx00026a012
- Giroux V, Lemay F, Bernatchez G, Robitaille Y, Carrier JC. Estrogen receptor beta deficiency enhances small intestinal tumorigenesis in ApcMIN/+ mice. Int J Cancer 2008 Jul 15; 123(2):303-11; PMID:18464259; http://dx.doi.org/10.1002/ijc.23532
- Ritchie KJ, Walsh S, Sansom OJ, Henderson CJ, Wolf CR. Markedly enhanced colon tumorigenesis in Apc(Min) mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci U S A 2009 Dec 8; 106(49):20859-64; PMID:19915149; http://dx.doi.org/10.1073/pnas.0911351106
- Herukuri DP, Ishikawa TO, Chun P, Catapang A, Elashoff D, Grogan TR, Bugni J, Herschman HR. Targeted Cox2 gene deletion in intestinal epithelial cells decreases tumorigenesis in female, but not male, ApcMIN/+ mice. Mol Oncol 2014 Mar; 8(2):169-77; PMID:24268915; http://dx.doi.org/10.1016/j.molonc.2013.10.009
- Leo VI, Tan SH, Bergmann H, Cheah PY, Chew MH, Lim KH, Ruland J, Reilly PT. CARD9 Promotes Sex-Biased Colon Tumors in the APCmin Mouse Model. Cancer Immunol Res 2015 Jul; 3(7):721-6; PMID:25941350; http://dx.doi.org/10.1158/2326-6066.CIR-14-0148
- Amos-Landgraf JM, Heijmans J, Wielenga MC, Dunkin E, Krentz KJ, Clipson L, Ederveen AG, Groothuis PG, Mosselman S, Muncan V, Hommes DW, Shedlovsky A, Dove WF, van den Brink GR. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc Natl Acad Sci U S A 2014 Nov 18; 111(46):16514-9; PMID:25368192; http://dx.doi.org/10.1073/pnas.1323064111
- Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 2000 Sep; 157(3):747-54; PMID:10980114; http://dx.doi.org/10.1016/S0002-9440(10)64588-9
- Aceto GM, Fantini F, De Iure S, Di Nicola M, Palka G, Valanzano R, Di Gregorio P, Stigliano V, Genuardi M, Battista P, Cama A, Curia MC. Correlation between mutations and mRNA expression of APC and MUTYH genes: new insight into hereditary colorectal polyposis predisposition. J Exp Clin Cancer Res 2015 Oct 28; 34:131; PMID:26511139; http://dx.doi.org/10.1186/s13046-015-0244-4
- Bingham SA DN, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, Tjønneland A, Overvad K, Martinez C, Dorronsoro M, Gonzalez CA, Key TJ, Trichopoulou A, Naska A, Vineis P, Tumino R, Krogh V, Bueno-de-Mesquita HB, Peeters PH, Berglund G, Hallmans G, Lund E, Skeie G, Kaaks R, Riboli E. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet 2003; 361(9368):1496-501; PMID:12737858; http://dx.doi.org/10.1016/S0140-6736(03)13174-1
- Ben QSY, Chai R, Qian A, Xu B, Yuan Y. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology 2014; 146(3):689-99; PMID:24216326; http://dx.doi.org/10.1053/j.gastro.2013.11.003
- Young GP HY, Le Leu RK, Nyskohus L. Dietary fibre and colorectal cancer: a model for environment–gene interactions. Mol Nutrition Food Res 2005; 49(6):571-84; PMID:15864783; http://dx.doi.org/10.1002/mnfr.200500026
- Chen HM YY, Wang JL, Lin YW, Kong X, Yang CQ, Yang L, Liu ZJ, Yuan YZ, Liu F, Wu JX, Zhong L, Fang DC, Zou W, Fang JY. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutrition 2013; 97(5):1044-52; PMID:23553152; http://dx.doi.org/10.3945/ajcn.112.046607
- Augenlicht LH AG, Church TL, Edelmann W, Kucherlapati R, Yang K, Lipkin M, Heerdt BG. Short-chain fatty acid metabolism, apoptosis, and Apc-initiated tumorigenesis in the mouse gastrointestinal mucosa. Cancer Res 1999; 59(23):6005-9; PMID:10606249
- Jun Yang WZ, Paul M. Evans, Xi Chen, Xi He and Chunming Liu. Adenomatous Polyposis Coli (APC) Differentially Regulates β-Catenin Phosphorylation and Ubiquitination in Colon Cancer Cells. J Biol Chem 2006; 26:17751-7; PMID:16798748; http://dx.doi.org/10.1074/jbc.M600831200
- Çoker-Gürkan A1, Arisan ED, Obakan P, Palavan-Unsal N. Lack of functional p53 renders DENSpm-induced autophagy and apoptosis in time dependent manner in colon cancer cells. Amino Acids 2015 Jan; 47(1):87-100; PMID:25311224; http://dx.doi.org/10.1007/s00726-014-1851-7