ABSTRACT
The 8p11 myeloproliferative syndrome (EMS), also known as 8p11 myeloproliferative neoplasm (8p11 MPN), is a collection of rare hematologic malignancies that are associated with fusion genes involving the tyrosine kinase receptor gene FGFR1 in chromosome 8p11. The entity is an aggressive disease with a high rate of transformation to acute myeloid leukemia (AML) and pathologically characterized by its associated eosinophilia. In this study, we reported a distinctive EMS case featuring an in-frame ZMYM2-FGFR1 fusion gene identified by next-generation sequencing technology (NGS). This patient exhibited not only typical EMS signs including elevated white blood cells in peripheral blood and hypercellular bone marrow with marked leukocytosis, but also exceptional characteristics including erythrocytosis in blood and bone marrow basophilia. Moreover, we detected 2 novel genomic mutations in 2 known leukemogenic genes, IKZF1 and ASXL1. Whether these 2 mutations play a part in EMS pathogenesis or contribute to its specific presentations clinically remain to be determined. In summary, we present a unique EMS case involving a ZMYM2-FGFR1 fusion with distinctive hematologic characteristics.
Abbreviations
| EMS | = | 8p11 myeloproliferative syndrome |
| MPN | = | myeloproliferative neoplasm |
| AML | = | acute myeloid leukemia |
| NGS | = | next-generation sequencing |
| WGS | = | whole-genome sequencing |
| TKI | = | tyrosine kinase inhibitor |
| CT | = | Computed Tomography |
| MDS | = | myelodysplastic syndrome |
Introduction
8p11 myeloproliferative syndrome (EMS), also known as 8p11 myeloproliferative neoplasm (8p11 MPN), is a rare disorder molecularly associated with fusion genes between the tyrosine kinase receptor gene FGFR1, located in 8p11, and several partner genes, including ZMYM2 (previously known as ZNF198).Citation1 According to the World Health Organization (WHO) 2008 classification of tumors, EMS is included in a group of hematological disorders termed myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1, which are clinically characterized by eosinophilia and genetically associated with a collection of fusion genes involving one of the above-mentioned genes.Citation2 Previous studies have shown that EMS is usually resistant to conventional chemotherapies with a poor median survival rate of less than 12 months.Citation1 Although FGFR1-involved fusion proteins are potential therapeutic targets, no satisfactory results have been achieved using tyrosine kinase inhibitors (TKIs) including imatinibCitation3,4 and dasatinib.Citation5
In EMS, multiple genes, including ZMYM2, FGFR1OP, BCR, NUP98, FGFR1OP2, TRIM24, MYO18A, CPSF6, LRRFIP1, CNTRL, ERVK-6Citation1 and the recently identified SQSTM1,Citation6 were detected as fusion partners with FGFR1 with a breakpoint in the 8p11–12 locus. Among these fusion genes, ZMYM2-FGFR1 is the most common type. The ZMYM2 proline-rich region, which mediates protein oligomerization, and the FGFR1 tyrosine kinase domain are conserved in the fusion protein. The abnormal oligomerization of ZMYM2-FGFR1 leads to constitutive activation of its tyrosine kinase.Citation7
While most of the previous studies have been focused on cytogenetic analysis and RT-PCR with or without Sanger sequencing for sequence verification, data on genomic sequencing showing the precise break points in both genes are sparse. In this study, we report a novel EMS case with the ZMYM2-FGFR1 fusion gene identified by use of whole-genome sequencing (WGS) on a next-generation sequencing platform (NGS). We further confirmed the mRNA transcripts by real-time RT-PCR and Sanger sequencing. Interestingly, this case is not only characterized by leukocytosis and eosinophilia as in most EMS cases, but it also displayed erythrocytosis. Moreover, we also found 2 novel nonsynonymous mutations in IKZF1 and ASXL1, which have never been reported previously for their roles or association with tumorigenesis. These findings warrant future studies to determine whether the mutations are tumorigenic.
Case report
A 62-year-old Chinese woman was admitted to the hospital in January 2015. Her face, lips, and hands were a dark plum color; the physical appearance was accompanied by dizziness and headache that had persisted for more than a month. She also had a 10-year history of hypertension. A Computed Tomography (CT) scan detected mediastinal and bilateral axillary lymphadenopathy. The complete blood count indicated a moderate elevation in the number of white blood cells (WBC, 39.33 × 109 cells/L), and a more dramatic increase in the level of red blood cells (RBC count at 8.13 × 1012 cells/L and HCT at 65.3%) and hemoglobin (208 g/L). Neutrophilia, eosinophilia, and basophilia were also observed in the peripheral blood with elevated absolute cell counts (). Hypercellularity of nucleated bone marrow cells was observed in a bone marrow aspiration, demonstrating leukocytosis with prominent neutrophilia (79%) and eosinophilia (6.5%) (), the characteristics of myeloproliferative neoplasm (MPN).
Figure 1. Peripheral blood and bone marrow reveal pathology. (A-B) Peripheral blood smear showing leukocytosis and erythrocytosis. (C-D) Hypercellular bone marrow demonstrating neutrophilia and eosinophilia. A and C: 40X; B and D: 100X. Wright-Giemsa staining in all panels.
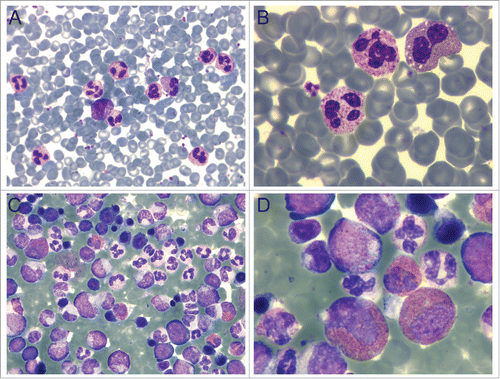
Table 1. Blood counts at the initial diagnosis and after designed treatment.
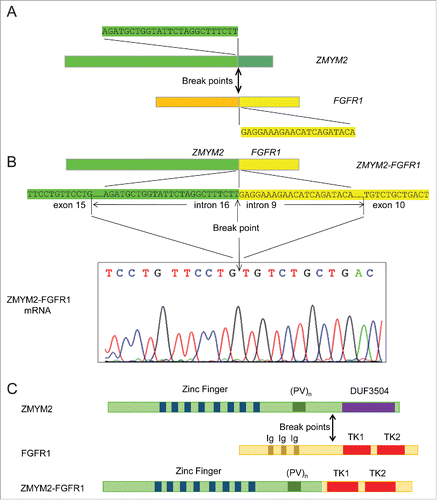
Bone marrow cytogenetic analysis indicated a 46,XX,t(8;13)(p11;q12)[20] karyotype (), a characteristic of 8p11 MPN or myeloid and lymphoid neoplasms with FGFR1 abnormalities, as classified by the WHO.Citation2 Fluorescence real-time RT-PCR and Sanger sequencing analysis did not detect JAK2 mutations (V617F and exon 12), FIP1L1-PDGFRA fusion, or BCR-ABL1 fusion. Using a NGS platform (HiseqX platform, Illumina, San Diego, CA), we performed whole-genome sequencing (WGS) on bone marrow genomic DNA. An in-frame ZMYM2-FGFR1 gene rearrangement was detected (). Moreover, 2 nonsynonymous mutations/SNPs (IKZF1 exon4: c.A490G:p.R164G rs10899750 on Chr7 and ASXL1 exon12:c.T2444C:p.L815P on Chr20) and several synonymous mutations (2 in JAK2, 1 in IDH2, and 1 in ASXL1) were also detected. The ZMYM2-FGFR1 transcript was identified using customized primers targeting sequences flanking the fusion point (). Based on its mRNA sequence, the new fusion protein is predicted to retain the FGFR1 TK domain and the proline-rich ZMYM2 oligomerization domain, while the Ig2_FGFR and Ig3_FGFR domains from FGFR1 and the DUF3504 domain from ZMYM2 are excluded.
Figure 2. Karyotype shows a chromosomal translocation. Bone marrow cytogenetic analysis indicated a characteristic EMS 46,XX,t(8;13)(p11;q12) translocation between chromosomes 8 and 13. Arrows indicate translocation sites.
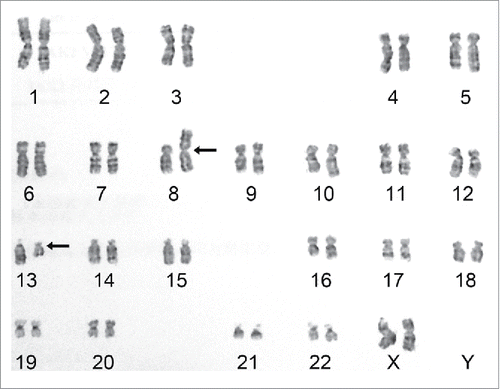
Figure 3. ZMYM2-FGFR1 gene rearrangement, its mRNA transcript, and predicted protein. (A) Diagram showing break points and partial sequences of the 2 participating fusion partner genes. (B) Top diagram shows the fusion of ZMYM2 and FGFR1 genes and the sequence around the break point that were detected by NGS. The DNA sequence in the exon adjacent to the involved intron in each gene is also shown. The fusion transcript was verified by RT-PCR and Sanger sequencing as shown in the bottom chromatogram. (C) Diagrams showing the protein structure of ZMYM2, FGFR1, and their fusion product. (PV)n, proline-valine repeats; DUF3504, domain of unknown function 3504; Ig, Ig-like domains; TK1, TK2, tyrosine kinase domains.
Due to the high levels of RBCs and hemoglobin, the patient was first treated with 3 rounds of RBC apheresis. Through this procedure the hemoglobin level was reduced to close to normal, 155 g/L. The patient was then chronically treated with interferon and hydroxyurea. After about 10 months of treatment, a partial response was achieved. As a result, the patient showed an improved full blood count in the categories of WBC (13.33 × 109 cells/L), hemoglobin (164 g/L, HCT 52.3%) and basophils (1.8%), but not eosinophils (23%) (). Moreover, the patient did not display any signs of mediastinal or bilateral axillary lymphadenopathy. The patient continues to be closely monitored for further improvement or disease progression but has shown no further changes at the time of the completion of this manuscript.
Discussion
In the current study, we report a novel EMS case involving a ZMYM2-FGFR1 fusion and displaying unique clinical characteristics. EMS was first reported in 1977Citation8 and in 1995 Macdonald et al. first suggested using the term “8p11 myeloproliferative syndrome” to describe the entity.Citation9 EMS is an extremely rare hematologic malignancy found in fewer than 100 patients world-wide.Citation1 Among these cases, about half of them are associated with ZMYM2-FGFR1 fusion genes.Citation1 It is a very aggressive disease with a high rate of progression to acute myeloid leukemia (AML).Citation1,10,11 Using an NGS platform, we performed WGS on the genomic DNA of the patient of this report and identified a ZMYM2-FGFR1 fusion, which is consistent with the cytogenetic results. This methodology represents a very powerful tool to reveal tumorigenic mutations as our initial screens using qPCR and Sanger sequencing for BCR-ABL fusions, FIP1L1-PDGFRA fusions, and JAK2 mutations were all negative. Therefore, the use of NGS as a primary screening tool in such rare and difficult cases can save both money and precious time, which can be a crucial factor in the design and outcome of treatments.
Through NGS, we also detected additional genomic abnormalities, including 2 nonsynonymous mutations in IKZF1 and ASXL1 that have never been reported before. Recurrent somatic ASXL1 mutations are associated with poor outcome in patients with myelodysplastic syndrome (MDS), MPN, and AML.Citation12 For example, a study has shown that ASXL1 exon 12 mutations, which are frequently detected in AML with an intermediate risk karyotype, are independently associated with an unfavorable outcome.Citation13 It was also reported that genetic alterations of ASXL1 were good indicators of poor outcomes in AML.Citation14 Thus, ASXL1 mutations may play a critical role in the characteristic clinical manifestations of EMS associated with ZMYM2-FGFR1 fusions, including drug resistance/poor outcome and high rate of transformation to AML. In contrast, IKZF1 mutations are associated with poor outcomes in B-cell–progenitor ALL.Citation15 The clinical significance of mutations in both genes, including these 2 novel mutations identified in our case, remains to be determined.
EMS is characterized by eosinophilia, which is observed in 85% of cases, while basophilia is usually absent. The patient described here displayed both eosinophilia and basophilia. Furthermore, a striking yet distinctive clinical feature in this case is that this patient showed a dramatic elevation in both red blood cell count and hemoglobin level in her peripheral blood. These results support the heterogenetic nature of this entity.Citation1,16 Consistent with most of the reports showing hypercellular bone marrow, this patient's bone marrow aspirate analysis demonstrated marked hypercellularity with nucleated bone marrow cells. Lymphadenopathy is common in EMS patients: approximately 2 thirds present with generalized or localized lymphadenopathy, while mediastinal lymphadenopathy is uncommon.Citation1 In the case presented in this report, both mediastinal and bilateral axillary lymphadenopathy were detected by CT scan. While splenomegaly has been reported in 58% of patients, it was absent in this patient. In summary, this patient exhibited both common and distinctive clinical characteristics.
In theory, the tyrosine kinase receptor FGFR1 is an attractive target for therapy with TKIs. However, there are currently no drugs available for clinical usage that effectively inhibit FGFR1 tyrosine kinase activity, despite some promising in vitro and animal results involving ponatinib (AP24534) and dovitinib (TKI-258).Citation17-20 Other TKIs, including imatinib and dasatinib, that have been used in individual cases involving ZMYM2-FGFR1 fusions were also ineffective.Citation3-5 EMS cases are also highly resistant to conventional chemotherapy. Favorable outcomes have only been reported in a few cases using allogeneic stem cell transplantation.Citation1 In our case, the patient seems to have responded, at least partially, to our treatments, including initial RBC apheresis and long-term application of interferon and hydroxyurea. Stem cell transplantation will still be considered in the future if necessary.
In conclusion, we detected a ZMYM2-FGFR1 fusion using NGS technology in a novel case of EMS, which exhibited not only eosinophilia, but also erythrocytosis. Cases of EMS have been reported with different translocations involving FGFR1 where polycythemia vera was diagnosed.Citation21,22 In the present case, RBC apheresis followed by chemotherapy using interferon and hydroxyurea have been effective, at least partially, to improve symptoms associated with erythrocytosis and leukocytosis. We determined the precise break points on the chromosomes as well as the fusion sequence on the hybrid transcript. Moreover, we discovered 2 novel nonsynonymous mutations on 2 genes known to be tumorigenic in leukemias. Our study shed light on the genetics and etiology of EMS and should inspire new studies addressing the underlying molecular mechanisms.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank pathologists Drs. Wanxin Chen and Mei Xue for their contribution in the preparation of pathology images used in this report.
Funding
This work was partly supported by grant 2011CDB099 from the Natural Science Foundation of Hubei Province and grant 81201866 from the Natural Science Foundation of China.
References
- Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol 2010; 41:461-76; PMID:20226962; http://dx.doi.org/10.1016/j.humpath.2009.11.003
- Bain B, Gilliland D, Horny H-P, Vardiman J. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB and FGFR1. In: Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al., eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC Press, 2008:68-73.
- Buijs A, van Wijnen M, van den Blink D, van Gijn M, Klein SK. A ZMYM2-FGFR1 8p11 myeloproliferative neoplasm with a novel nonsense RUNX1 mutation and tumor lysis upon imatinib treatment. Cancer Genet 2013; 206:140-4; PMID:23751892; http://dx.doi.org/10.1016/j.cancergen.2013.04.001
- Chao H, Zhou M, Zhang R. A case with ZNF198-FGFR1 gene rearrangement presenting as acute eosinophil myeloid leukemia. Chin Med J (Engl) 2015; 128:131-2; PMID:25563327; http://dx.doi.org/10.4103/0366-6999.147863
- Savage NM, Johnson RC, Gotlib J, George TI. Myeloid and lymphoid neoplasms with FGFR1 abnormalities: diagnostic and therapeutic challenges. Am J Hematol 2013; 88:427-30; PMID:22886804; http://dx.doi.org/10.1002/ajh.23296
- Nakamura Y, Ito Y, Wakimoto N, Kakegawa E, Uchida Y, Bessho M. A novel fusion of SQSTM1 and FGFR1 in a patient with acute myelomonocytic leukemia with t(5;8)(q35;p11) translocation. Blood Cancer Journal 2014; 4:e265; PMID:25501022; http://dx.doi.org/10.1038/bcj.2014.86
- Xiao S, McCarthy JG, Aster JC, Fletcher JA. ZNF198-FGFR1 transforming activity depends on a novel proline-rich ZNF198 oligomerization domain. Blood 2000; 96:699-704; PMID:10887137
- Manthorpe R, Egeberg J, Hesselvik M, Videbaek A. Unique eosinophil granules in a case of T-cell lymphoma. Scand J Haematol 1977; 19:129-44; PMID:897557; http://dx.doi.org/10.1111/j.1600-0609.1977.tb02338.x
- Macdonald D, Aguiar RC, Mason PJ, Goldman JM, Cross NC. A new myeloproliferative disorder associated with chromosomal translocations involving 8p11: a review. Leukemia 1995; 9:1628-30; PMID:7564500
- Cross NC, Reiter A. Fibroblast growth factor receptor and platelet-derived growth factor receptor abnormalities in eosinophilic myeloproliferative disorders. Acta Haematol 2008; 119:199-206; PMID:18566537; http://dx.doi.org/10.1159/000140631
- Macdonald D, Reiter A, Cross NC. The 8p11 myeloproliferative syndrome: a distinct clinical entity caused by constitutive activation of FGFR1. Acta Haematol 2002; 107:101-7; PMID:11919391; http://dx.doi.org/10.1159/000046639
- Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, Pandey S, Patel JP, Chung YR, Koche R, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell 2012; 22:180-93; PMID:22897849; http://dx.doi.org/10.1016/j.ccr.2012.06.032
- Schnittger S, Eder C, Jeromin S, Alpermann T, Fasan A, Grossmann V, Kohlmann A, Illig T, Klopp N, Wichmann HE, et al. ASXL1 exon 12 mutations are frequent in AML with intermediate risk karyotype and are independently associated with an adverse outcome. Leukemia 2013; 27:82-91; PMID:23018865; http://dx.doi.org/10.1038/leu.2012.262
- Pratcorona M, Abbas S, Sanders MA, Koenders JE, Kavelaars FG, Erpelinck-Verschueren CA, Zeilemakers A, Lowenberg B, Valk PJ. Acquired mutations in ASXL1 in acute myeloid leukemia: prevalence and prognostic value. Haematologica 2012; 97:388-92; PMID:22058207; http://dx.doi.org/10.3324/haematol.2011.051532
- Mullighan CG, Su X, Zhang J, Radtke I, Phillips LAA, Miller CB, Ma J, Liu W, Cheng C, Schulman BA, et al. Deletion of IKZF1 and Prognosis in Acute Lymphoblastic Leukemia. N Engl J Med 2009; 360:470-80; PMID:19129520; http://dx.doi.org/10.1056/NEJMoa0808253
- Bain BJ. Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or FGFR1. Haematologica 2010; 95:696-8; PMID:20442440; http://dx.doi.org/10.3324/haematol.2009.021675
- Lierman E, Smits S, Cools J, Dewaele B, Debiec-Rychter M, Vandenberghe P. Ponatinib is active against imatinib-resistant mutants of FIP1L1-PDGFRA and KIT, and against FGFR1-derived fusion kinases. Leukemia 2012; 26:1693-5; PMID:22301675; http://dx.doi.org/10.1038/leu.2012.8
- Chase A, Grand FH, Cross NC. Activity of TKI258 against primary cells and cell lines with FGFR1 fusion genes associated with the 8p11 myeloproliferative syndrome. Blood 2007; 110:3729-34; PMID:17698633; http://dx.doi.org/10.1182/blood-2007-02-074286
- Wasag B, Lierman E, Meeus P, Cools J, Vandenberghe P. The kinase inhibitor TKI258 is active against the novel CUX1-FGFR1 fusion detected in a patient with T-lymphoblastic leukemia/lymphoma and t(7;8)(q22;p11). Haematologica 2011; 96:922-6; PMID:21330321; http://dx.doi.org/10.3324/haematol.2010.036558
- Chase A, Bryant C, Score J, Cross NC. Ponatinib as targeted therapy for FGFR1 fusions associated with the 8p11 myeloproliferative syndrome. Haematologica 2013; 98:103-6; PMID:22875613; http://dx.doi.org/10.3324/haematol.2012.066407
- Vizmanos JL, Hernández R, Vidal MJ, Larráyoz MJ, Odero MD, Marín J, Ardanaz MT, Calasanz MJ, Cross NC. Clinical variability of patients with the t(6;8)(q27;p12) and FGFR1OP-FGFR1 fusion: two further cases. Hematol J 2004; 5:534-7; PMID:15570299; http://dx.doi.org/10.1038/sj.thj.6200561
- Popovici C, Zhang B, Grégoire MJ, Jonveaux P, Lafage-Pochitaloff M, Birnbaum D, Pébusque MJ. The t(6;8)(q27;p11) translocation in a stem cell myeloproliferative disorder fuses a novel gene, FOP, to fibroblast growth factor receptor 1. Blood 1999; 93:1381-9; PMID:9949182
