ABSTRACT
There is a growing body of evidence supporting the synergistic roles of radiotherapy and immunotherapy in the treatment of malignancy. Published case studies of the abscopal effect have been reported with the use of ipilimumab and radiotherapy in metastatic melanoma, but evidence supporting the routine use of this combination of therapy is limited. We conducted a retrospective analysis to evaluate patients treated with ipilimumab for advanced melanoma at a single institution from May 2011 to June 2015. Patients were grouped into those who had received concurrent radiotherapy while on ipilimumab (Ipi-RT), and those who did not. We then evaluated the treatment response following completion of ipilimumab. A total of 101 patients received ipilimumab in the prespecified time frame. 70 received Ipi-RT and 31 received ipilimumab without concurrent radiotherapy. Median overall survival (OS) was significantly increased in the concurrent Ipi-RT arm at 19 months vs. 10 months for ipilimumab alone (p = 0.01). Median progression free survival (PFS) was marginally increased in the Ipi-RT group compare with the ipilimumab alone group (5 months vs. 3 months, p = 0.20). Rates of complete response (CR) were significantly increased in the Ipi-RT group vs. ipilimumab alone (25.7% vs. 6.5%; p = 0.04), and rates of overall response (OR) in the groups were 37.1% vs. 19.4% (p = 0.11). No increase in toxicities was observed in the Ipi-RT group compare with ipilimumab alone. Prospective trials are needed to further clarify the role of radiotherapy with ipilimumab, but these encouraging preliminary observations suggest that this combination can induce more durable responses to immunotherapy.
Introduction
Malignant melanoma is a devastating disease due to its strong tendency to metastasize early in its disease course leading to death. Although melanoma represents less than 10% of all skin cancers, it accounts for at least 70% of deaths related to skin cancer.Citation1 In 2015, an estimated 74,000 new cases and 10,000 deaths occurred in the United States, and the estimated lifetime risk of the development of melanoma is about 1 in 50.Citation1,2 More than 90% will be diagnosed early when the disease is resectable.Citation3 However, when discovered in the later stages, the prognosis is poor. Also early stage disease can recur as metastatic disease leading to death with increasing risk correlating with increasing pathologic staging. The historic prognosis ofmetastatic malignant melanoma has a median overall survival of less than 1 y and a 5 y overall survival under 10%.Citation4
Until relatively recently, treatment approaches for malignant melanoma had been limited. In 2011, the Food and Drug Administration approved the first new therapy for melanoma in over a decade, the CTLA-4 inhibitor, ipilimumab. Ipilimumab overcomes the natural CTLA-4 checkpoint in T cells which limits the anti-tumor immune response. Multiple studies showed an overall survival benefit for patients with malignant melanoma receiving ipilimumab.Citation5-8 This was based on 2 phase III studies, which showed a median overall survival of 10–11 months.Citation6,9 A subsequent pooled analysis of all patients from 12 studies utilizing ipilimumab showed a median overall survival of 11.4 months.Citation10 The OR and CR rates however remain reproducibly low on ipilimumab alone; 11% and 1.5% respectively in the phase III trial.Citation6 Clearly, new strategies are required to enhance the durable CR rate of ipilimumab. The introduction of PD-1 blockade drugs has been a major addition to the armamentarium for melanoma but even with the combination of ipilimumab and the PD-1 inhibitor, nivolumab, the CR rate remains only about 11.5% albeit with a much higher overall response rate (ORR) of 57.7%.Citation11 Despite this progress, expanding the proportion of patients that achieve a durable CR (leading to long-term cure) should remain the goal of immunotherapies, highlighting the need for additional strategies.
There is evidence that combining radiotherapy with ipilimumab may induce an abscopal effect, that is, a regression of non-irradiated metastatic lesions distant from the primary tumor site directly subject to irradiation.Citation12-15 The mechanisms remain obscure, although presumably, the radiation damage induced in the target results in immunogenic cell death of tumor cells which serves to immunize the host.Citation16,17 This in situ immunization can result in the activation of immune effector cells systemically which can then attack tumor cells remote from the irradiated target.Citation18-21 There is some pre-clinical evidence that very high (ablative) radiation doses may increase this systemic immune effect.Citation22,23 Retrospective reviews have reported infield response rates of 62%, palliation of symptoms in 77%, and an abscopal response rate of 52%, with a minimal increase in toxicity.Citation13,14 These observations have led to numerous attempts to combine radiotherapy of various schedules with the different available immunotherapy agents.Citation12,15,24-26
Recently, a prospective clinical trial demonstrated that combining radiotherapy with ipilimumab resulted in a higher response rate than reported with ipilimumab alone.Citation27 50% of patients experienced clinical benefit from therapy at median follow-up of 55 weeks, and 27.3% achieved an ongoing systemic CR at a median follow-up of 55 weeks. This rate of CR is much greater than the 1.5–11% rate reported for ipilimumab alone, suggesting that combined ipilimumab and radiotherapy may provide improved benefit for this patient population.Citation6
To investigate the broader implications of these findings, we sought to evaluate the clinical response and overall survival in a large cohort of patients at our institution who had been treated with ipilimumab and concurrent radiotherapy on whom outcomes were available to see if this combination improves the response and survival of patients treated for malignant melanoma.
Methods
Patients
We conducted a retrospective analysis of all patients treated with ipilimumab for metastatic or unresectable melanoma at a single institution from May 2011 through June 2015. A pharmacy database was queried to provide records of all doses of ipilimumab administered at our institution during that time. This retrospective study was determined to pose no more than minimal risk and as such was exempt from IRB approval. Procedures werecarried out ethically and in accordance with the Helsinki Declaration of 1975.Patients were grouped into those who received concurrent radiotherapy to any site of melanoma during or within 2 weeks of ipilimumab treatment (Ipi-RT) and those who received ipilimumab alone.
Treatment
Ipilimumab (3 mg/kg) was administered intravenously, with 3 weeks between a total of 4 cycles. Patients received anywhere from 1 to 4 cycles of ipilimumab (). Patients in the Ipi-RT cohort may have received external beam radiotherapy during or within 2 weeks of ipilimumab treatment. Conventional RT was generally given with palliative intent (painful bone, large nodal mass or brain metastasis). Stereotactic RT was given with ablative intent to brain metastases (Gamma knife) or limited body metastases (Stereotactic Body Radiotherapy).
Table 1. Patient demographics.
Outcome evaluation
Patients' responses to treatment were evaluated based on post-treatment PET or CT following completion of therapy. The survival outcomes included the OS and PFS. OS was defined as the time from ipilimumab initiation to the time of death, and PFS was defined as the time from ipilimumab initiation to the time of progressive disease or death. Response outcomes were the rate of CR and OR measured by RECIST criteria (v1.1). Responding patients continued on regular follow-up including surveillance imaging at regular intervals which determined the durability of the initial responses. An OR was identified if patients had a CR, a partial response, or a mixed response to the treatment. A mixed response occurred if there was a response in one area of the disease, but there was progression in another area of disease. The rates of CR and OR were based on both radiated and distant disease sites. Responses were determined at 3 weeks from the final dose of ipilimumab. Patients with CRs continued to be followed with imaging at 3 month intervals which were eventually lengthened to 6 months for 5 yrs. Patients with less than CR were likewise followed clinically and radiographically and most began subsequent therapies. All patients either continued follow-up at the Penn State Cancer Institute or expired, so were available to analysis.
Statistical analysis
Patients and disease characteristics were analyzed using descriptive measures, and comparison of proportions between 2 groups was performed with a Fisher exact test (2-sided). An odds ratio test was used to measure the association between treatment group and their outcomes. OS was estimated by the Kaplan-Meier method, and curve comparisons were calculated using the log-rank test. The median OS and the median PFS (from the initiation of ipilimumab treatment to data lock in September 2016)was calculated by the survival analysis using Prism software. In all analyses, p values < 0.05 were considered statistically significant.
Results
Patient demographics
A total of 101 patients received ipilimumab from May 2011 through June 2015, with a median follow up time of 19 months. Median potential follow-up time (from the start of ipilimumab treatment to data lock in September 2016) was calculated with the reverse survival method. Baseline patient characteristics were gathered and are shown in . The cohort was comprised of 70 patients who received radiotherapy concurrently with ipilimumab and 31 patients who received ipilimumab alone. The treatment groups were very similar in terms of characteristics and risk factors including known adverse factors such as elevated LDH and presence of visceral metastases. The only statistically significant difference between the Ipi-RT group and the ipilimumab alone group was that the ipilimumab alone group was considerably more likely to have received IL-2 therapy before ipilimumab (p = 0.01). This may reflect that these patients were chronologically from the earlier part of the treatment period and had received IL-2 before ipilimumab was available. Interestingly, both groups received similar types of subsequent therapies suggesting that survival differences were not attributable to these additional agents. Of the patients who received Ipi-RT, 62 received conventional external beam radiation given with palliative intent, and 8 received stereotactic radiosurgery or external beam radiation given with ablative intent, with fraction sizes ≥10 Gy. In the Ipi-RT group, one patient was re-exposed to ipilimumab after relapse and required 4 additional cycles and another patient expired during the course of treatment.
Outcomes
Clinical outcomes are compared in the 2 treatment groups as shown in . The median OS was significantly increased in patients treated with concurrent radiotherapy and ipilimumab. The Kaplan-Meier survival curve is shown in . The median OS for the Ipi-RT group was 19 months compare with 10 months for the ipilimumab alone group (p = 0.01). During the period of our analysis, 40 (57.1%) patients of the Ipi-RT group expired compare with 27 (87.0%) patients in the ipilimumab alone group. At 6 months, there was a 80% survival probability in the Ipi-RT group, while there was a 70% survival probability in the ipilimumab alone group. At 12 months, there was a 72% survival probability in the Ipi-RT group, while there was a 35% survival probability in the ipilimumab alone group.
Table 2. Patient outcomes.
Figure 1. Kaplan-Meier overall survival curve comparing Ipi-RT and ipilimumab alone. Kaplan-Meier overall survival curve: The survival probabilities are plotted over time between the 2 treatment groups. The Ipi-RT group (group 1) had a significantly greater median OS than the group treated with ipilimumab alone (group 0; 19 months vs. 10 months, p = 0.01).
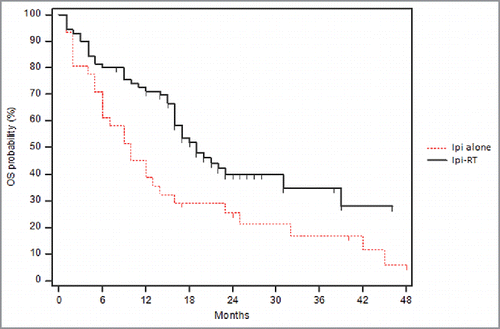
The median PFS was marginally increased in patients treated with concurrent radiotherapy and ipilimumab, though the results were not significant. The Kaplan-Meier survival curve is shown in . The median PFS for the Ipi-RT group was 5 months compare with 3 months for the ipilimumab alone group (p = 0.20). During the period of our analysis, 59 (82.3%) patients of the Ipi-RT group had progressive disease compare with 29 (93.5%) patients in the ipilimumab alone group. At 6 months, there was a 44% PFS probability in the Ipi-RT group, while there was a 29% PFS probability in the ipilimumab alone group. At 12 months, there was a 31% PFS probability in the Ipi-RT group, while there was a 16% PFS probability in the ipilimumab alone group.
Figure 2. Kaplan-Meier progression free survival curve comparing Ipi-RT and ipilimumab alone. Kaplan-Meier progression free survival curve: The survival probabilities are plotted over time between the 2 treatment groups. The Ipi-RT group (group 1) had a marginally greater median PFS than the group treated with ipilimumab alone (group 0;5 months vs. 3 months, p = 0.20).
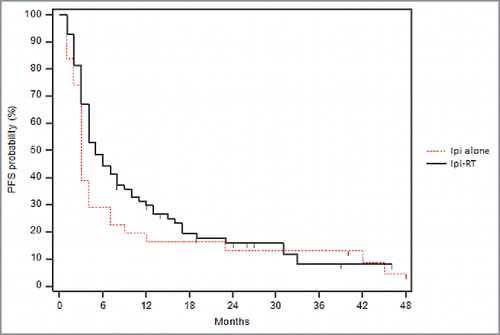
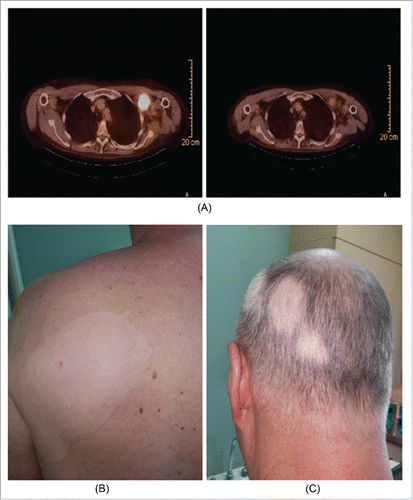
Figure 3. Example of CR due to concurrent radioimmunotherapy associated with acquisition of vitiligo. Radioimmunotherapy: This shows results of radioimmunotherapy in a 62 y old man with locally advanced, BRAF mutation negative melanoma of unknown primary involving bulky left axillary, left supraclavicular and left subpectoral lymph nodes with impingement of neurovascular structures in left axilla and invasion of adjacent chest wall. He was treated with 4 cycles of ipilimumab with concurrent conventional radiotherapy (48 Gy in 20 fractions) to the left axillary mass. He sustained radiographic complete response in all sites of disease and subsequently had a completion left axillary lymph node dissection in region of prior bulk and had no evidence of remaining melanoma consistent with a pathologic complete response. He has remained well since then.
Our secondary outcome was the rate of CR and OR measured by RECIST criteria. Among those who received Ipi-RT, 18 (25.7%) achieved a CR, compare with 2 (6.5%) in the group that received ipilimumab alone [Odds ratio 5.02 (95% CI 1.09–23.2), p = 0.04]. Of the 8patients who received radiotherapy with ablative intent, 3 patients achieved a CR (37.5%), while the other 5 had progressive disease. Patients who received conventional non-ablative radiotherapy had a CR rate of 24.2% (15 out of 62 patients).Furthermore, patients who received Ipi-RT had a marginally increased rate of OR compare with patients who received only ipilimumab (37.1% vs. 19.4%, p = 0.11), though the results were not significant. The rate of a partial response and the rate of a mixed response were not significantly increased after the addition of radiotherapy. The rate of OR was mainly increased in the Ipi-RT group because these patients had a higher rate of CR, while partial and mixed responses were not more likely to occur after the addition of radiotherapy.
The results of therapy in one patient are illustrated in . This patient had locally advanced melanoma considered initially unresectable in the left axilla, left chest wall, and left supraclavicular fossa. He received concurrent ipilimumab and radiotherapy (48 Gy in 20 fractions) and sustained a CR radiographically as noted. This was confirmed as a pathologic CR on exploration of the axilla and adjacent chest wall. Of interest he developed vitiligo in the radiation field as well as abscopal patches of vitiligo on his scalp.
There were no signs of increased toxicities in the combination group compare with the ipilimumab alone group. Typical ipilimumab toxicities were noted in both groups equally and at most were treated with steroids to resolution with sequela ().
Table 3. Adverse effects.
Discussion
In this retrospective analysis, we show that patients treated with concurrent radiotherapy and ipilimumab had superior clinical outcomes compare with those treated with ipilimumab alone. Our OS data and Kaplan-Meier OS curve for the ipilimumab alone group closely matches the recently published pooled long-term survival data for patients treated with ipilimumab alone.Citation10 Furthermore, the rate of CR for the Ipi-RT group in our study was 25.7%, which closely matches the rate of CR in the prospective clinical trial (27.3%).Citation27 The rate of CR in the ipilimumab alone group (6.5%) was also close to the rate of CR reported in a previous phase III trial (1.5–11%).Citation6 This finding suggests that patients in this study who were treated with ipilimumab alone had a similar outcome to patients treated with ipilimumab in previous trials. Taken together, these results indicate that the improved responses seen with addition of radiotherapy in this studyare not explained by reduced efficacy of ipilimumab alone.
Patients treated with Ipi-RT had a significantly greater OS but not a significantly greater PFS. The phenomenon of improved OS without a clear PFS benefit has been observed for immunotherapies, and suggests long-term control not measured by PFS. Examples include sipileucel-T in prostate cancer and GM-CSF with ipilimumab in melanoma.Citation28,29 There was a trend to significance as the p-value was <0.2, and so a larger group may show a PFS benefit. An elevated PFS would indicate an overall enhanced immunotherapeutic effect by radiotherapy, so a larger study may be worthwhile to demonstrate this effect of radiotherapy.
Based on our findings, it appears that that the primary effect of adding radiotherapy onto an ipilimumab regimen is that it increases the rate of CR. There was no significant difference in the rates of partial or mixed responses between the Ipi-RT group and the ipilimumab alone group. However, the rate of CR was increased nearly 4-fold in the Ipi-RT group compare with the ipilimumab alone group (25.7% vs. 6.5%, p = 0.04). This effect may predominantly result in the increased median OS in patients treated concurrently with ipilimumab and radiotherapy. Of interest, the use of ablative intent radiotherapy did increase the rate of CR (37.5%) relative to those treated with conventional radiotherapy (24.2%), which is consistent with the evidence in pre-clinical studies, although given the small number of patients, definitive conclusions cannot be drawn.Citation22,23 The relatively low rate of ablative RT was due to the fact that it was primarily employed for the control of brain metastases (Gamma Knife).It is tantalizing to us that conventional radiotherapy could also induce CR when given with concurrent ipilimumab. We did not observe more toxicity than would be expected from the individual treatments when radiation was combined with ipilimumab ().
While our results show an improvement of clinical outcomes in patients treated with concurrent ipilimumab and radiotherapy, there are significant limitations to our study. The analysis is retrospective, and results will optimally need to be prospectively validated. Patients in the ipilimumab alone group were more likely to be treated with IL-2 before ipilimumab than patients in the Ipi-RT group, and it is possible that this more heavily pre-treated group was less likely to respond to further immunotherapy. However, prior IL-2 receipt did not appear to impair responses to subsequent immunotherapy in other studies, suggesting this should not be a factor.Citation30,31 It is possible that the total dose of radiation, the dose per fraction of radiation, or the timing of radiation in relation to ipilimumab could have an effect on the response to treatment but there seemed to be no pattern of note in the patients receiving conventional RT in this relatively small group of patients. It is worth exploring these variables, however, in order to optimally design further combination trials. These radiation parameters and other variables are currently under study at this institution in a larger group of patients receiving all forms of immunotherapy with radiation.
As in our study, a recent report by Theurich and colleagues also noted that the OS of patients treated with concurrent ipilimumab and local therapy including radiotherapy is significantly greater than that of patients treated with ipilimumab alone.Citation32 In this study, the addition of radiotherapy to ipilimumab significantly prolonged OS after adjusting for various clinical factors such as tumor stage, tumor burden, and central nervous system metastases.Citation32 Of note, our Kaplan-Meier survival curve comparing the survival probabilities between the Ipi-RT group and the ipilimumab alone group very closely resembles the survival curve published in that study suggesting harmony between our studies and their similar conclusions.
Given that ipilimumab is approved to be given concurrently with PD-1 inhibitors, it is feasible to explore the effect of adding radiotherapy to such a combination regimen.Citation33 A recent study showed that in both human and murine models, melanoma expressing high levels of PD-L1 did not respond to concurrent ipilimumab and radiotherapy. It was theorized that PD-L1 on melanoma cells allowed for an immune escape mechanism and that a combination of radiation, anti-CTLA-4, and anti PD-L1 could promote response and immunity through direct mechanisms.Citation34 This is a promising avenue to conduct further studies. Anecdotally, we have noticed a similar benefit in the clinic of adding radiation to combination immunotherapy in terms of CR induction (unpublished observations). This paradigm of radioimmunotherapy could be expanded to treating other tumor types and indeed a trial of the PD-L1 blockade drug, Durvalumab, and radiation for locally advanced bladder cancer has been recently opened to accrual (Monika Joshi. Durvalumab And Radiation Therapy Followed by Adjuvant Durvalumab in Patients With Urothelial Cancer (T2–4 N0–2 M0) of the Bladder (DUART), In: ClinicalTrials.gov [Internet]. NLM Identifier: NCT02891161).
Our study provides strong evidence that combining ipilimumab with radiotherapy improves the clinical outcomes in patients with metastatic melanoma. The median overall survival more than doubled in patients receiving radiation with ipilimumab (19 months vs. 10 months, p = 0.01), and the rate of complete response nearly quadrupled (25.7% vs. 6.5%, p = 0.04). Given the currently suboptimal CR rates achieved by combination immunotherapy alone, such an improvement in treatment response warrants further study. We are hopeful that combining radiotherapy with ipilimumab and other immunotherapeutics will prove to be effective in a prospective setting with negligible additional toxicities.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ferri F. Melanoma. In: Ferri's Clinical Adviser. 2nd ed.; 2017:773-5
- Gangadhar TC, Fecher LA, Miller CJ, et al. Melanoma. Abeloff's Clin Oncol 2014; 1071-91:e3
- Webster RM, Mentzer SE. The malignant melanoma landscape. Nat Rev Drug Discov 2014; 13(7):491-2; PMID:24981356; http://dx.doi.org/10.1038/nrd4326
- Miller AJ, Mihm MC. Melanoma. N Engl J Med 2006; 355(1):51-65; PMID:16822996; http://dx.doi.org/10.1056/NEJMra052166
- Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov 2011; 10:411-2; PMID:21629286; http://dx.doi.org/10.1038/nrd3463
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Lipson EJ, Drake CG. Ipilimumab: An anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 2011; 17(22):6958-62; PMID:21900389; http://dx.doi.org/10.1158/1078-0432.CCR-11-1595
- Callahan MK, Postow MA, Wolchok JD. Immunomodulatory therapy for melanoma: Ipilimumab and beyond. Clin Dermatol 2013; 31(2):191-9; PMID:23438382; http://dx.doi.org/10.1016/j.clindermatol.2012.08.006
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364(26):2517-26; PMID:21639810; http://dx.doi.org/10.1056/NEJMoa1104621
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015; 33(17):1889-94; PMID:25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373(1):23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, Ng AK, Hodi FS, Schoenfeld JD. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015; 4(11):e1046028; PMID:26451318; http://dx.doi.org/10.1080/2162402X.2015.1046028
- Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014; 3:e28780; PMID:25083318; http://dx.doi.org/10.4161/onci.28780
- Barker CA, Postow MA, Khan SA, Beal K, Parhar PK, Yamada Y, Lee NY, Wolchok JD. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res 2013; 1(2):92-8; PMID:24777500; http://dx.doi.org/10.1158/2326-6066.CIR-13-0082
- Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013; 2(6):899-906; PMID:24403263; http://dx.doi.org/10.1002/cam4.140
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31(1):51-72; PMID:23157435; http://dx.doi.org/10.1146/annurev-immunol-032712-100008
- Golden EB, Apetoh L. Radiotherapy and Immunogenic Cell Death. Semin Radiat Oncol 2015; 25(1):11-7; PMID:25481261; http://dx.doi.org/10.1016/j.semradonc.2014.07.005
- Hammerich L, Bhardwaj N, Kohrt HE, Brody JD. In situ vaccination for the treatment of cancer. Immunotherapy 2016; 8(3):315-30; PMID:26860335; http://dx.doi.org/10.2217/imt.15.120
- Pierce RH, Campbell JS, Pai SI, Brody JD, Kohrt HEK. In-situ tumor vaccination: Bringing the fight to the tumor. Hum Vaccines Immunother 2015; 11(8):1901-9; PMID:26055074; http://dx.doi.org/10.1080/21645515.2015.1049779
- Derer A, Deloch L, Rubner Y, Fietkau R, Frey B, Gaipl US. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses - pre-clinical evidence and ongoing clinical applications. Front Immunol 2015; 6:505; PMID:26500646; http://dx.doi.org/10.3389/fimmu.2015.00505
- Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine 2015; 33(51):7415-22; PMID:26148880; http://dx.doi.org/10.1016/j.vaccine.2015.05.105
- Shen RN, Hornback NB, Shidnia H, Lu L, Montebello JF, Brahmi Z. A comparison of lung metastases and natural killer cell activity in daily fractions and weekly fractions of radiation therapy on murine B16a melanoma. Radiat Res 1988; 114(2):354-60. http://www.ncbi.nlm.nih.gov/pubmed/3375430; PMID:3375430; http://dx.doi.org/10.2307/3577230
- Lee Y, Auh SL, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: Changing strategies for cancer treatment. Blood 2009; 114(3):589-95; PMID:19349616; http://dx.doi.org/10.1182/blood-2009-02-206870
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366(10):925-31; PMID:22397654; http://dx.doi.org/10.1056/NEJMoa1112824
- Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: A review of clinical outcomes. Int J Radiat Oncol Biol Phys 2014; 88(5):986-97; PMID:24661650; http://dx.doi.org/10.1016/j.ijrobp.2013.08.035
- Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol 2015; 1:1325-32; PMID:26270858; http://dx.doi.org/10.1001/jamaoncol.2015.2756
- Hiniker SM, Reddy SA, Maecker HT, Swetter SM, Shura L, Knox SJ. A Prospective Clinical Trial Combining Radiation Therapy With Systemic Immunotherapy in Metastatic Melanoma. Radiat Oncol Biol 2015; 93(3):S95; PMID:27681753; http://dx.doi.org/10.1016/j.ijrobp.2015.07.228
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med 2010; 363(5):411-422; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294
- Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 2014; 312(17):1744-53; PMID:25369488; http://dx.doi.org/10.1001/jama.2014.13943
- Kaufman H, Lutzky J, Clark J, et al. Safety and efficacy of ipilimumab in melanoma patients who received prior immunotherapy on phase III study MDX010-020. ASCO Meet Abstr 2013; 31(15_suppl):9050. http://meeting.ascopubs.org/cgi/content/abstract/31/15_suppl/9050.
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 2007; 13(22 Pt 1):6681-88; PMID:17982122; http://dx.doi.org/10.1158/1078-0432.CCR-07-0187
- Theurich S, Rothschild SI, Hofmann M, et al. Immunologic Synergy of Local Tumor Treatment in Combination with Systemic Ipilimumab Immunotherapy Prolongs Overall Survival in Patients with Advanced Malignant Melanoma. Cancer Immunol Res. 2016; 2997:1-12; PMID:27466265; http://dx.doi.org/10.1158/2326-6066.CIR-15-0156
- Koller KM, Wang W, Schell TD, Cozza EM, Kokolus KM, Neves RI, Mackley HB, Pameijer C, Leung A, Anderson B, et al. Malignant melanoma—The cradle of anti-neoplastic immunotherapy. Crit Rev Oncol Hematol 2016; 106:25-54
- Victor CT-S, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520(7547):373-7; PMID:25754329; http://dx.doi.org/10.1038/nature14292
