ABSTRACT
A 40-year-old Chinese female patient, with radiation-induced brain necrosis after radiosurgery, was treated 6 times with a single dose of 200 mg (3.27 mg/kg) bevacizumab each time, and with an interval of 12–16 weeks between each treatment. Neurological symptoms such as dizziness, fatigue, and headache disappeared after each administration of bevacizumab. The results suggest that repeated bevacizumab treatment using a low-dose and long-dosing interval may significantly alleviate radiation necrosis and its symptoms.
Introduction
Upregulated expression of vascular endothelial growth factor (VEGF) is considered a key factor in radiation-induced brain necrosis.Citation1 Results in clinical trialsCitation2-6 have shown that bevacizumab provides an effective treatment for radiation necrosis by blocking vascular endothelial growth factor-A from binding to its receptors.Citation7,8 Nevertheless, the pharmacodynamics of bevacizumab are not fully understood and a dose–effect relationship has not yet been proven in vivo. Indeed, further improvement in therapeutic efficacy while minimizing side effects is needed, possibly by adjusting the dosage or dosing interval.
Here we report a case of radiation brain necrosis in a 40-year-old female who received radiosurgery for a metastatic brain tumor arising from breast cancer. The patient was treated with bevacizumab 6 times at a dosage of 200 mg (3.27 mg/kg), and with an interval of 12–16 weeks between each treatment. Neuropathological symptoms such as dizziness, fatigue, and headache remain controlled at 17 months following surgery (when this report was written).
Case report
A 40-year-old Chinese female patient was diagnosed with breast carcinoma in October 2003. She received 3 cycles of neoadjuvant chemotherapy (paclitaxel, cyclophosphamide, and capecitabine) along with left axillary lymph node dissection of a 1-cm tumor during breast-conserving surgery in October 2003. Two weeks later, she received 3 cycles of adjuvant chemotherapy (paclitaxel) and left-sided whole breast radiation therapy (50 Gy in 25 fractions). She was treated with tamoxifen for 7 months until she self-withdrew tamoxifen in August 2004. In November 2008, the patient presented with lung metastasis. She received 6 cycles of salvage treatment (paclitaxel and cisplatin) from January 2009 to June 2009 and was withdrawn from this treatment 3 months later due to grade III leucopenia (CTC-scale). Next she was treated with vinorelbine and trastuzumab from August 2009. Vinorelbine was withdrawn 9 months later because of severe fatigue. Computed tomography showed the volume of lung metastasis had reduced to less than 1 cm, which represented a partial response. The patient continued with trastuzumab for 20 months, until she showed multiple brain metastases on magnetic resonance imaging (MRI) ().
Figure 1. The woman was diagnosed with brain metastasis of breast cancer by gadolinium-enhanced T1-weighted MRI (A). After whole brain radiation therapy (40 Gy in 20 fractions), the volume of tumor was reduced significantly (B), and disappeared 8 months after radiotherapy (C). The tumor located in the cerebellum enlarged in the 10th and 11th months after radiotherapy (D and E, respectively). Gadolinium-enhanced T1-weighted MRI showing the reduced size of the tumor at 2 months (F), widespread scattered irregular enhancement at 4 months (G), and increased tumor size at 13 months (H) after gamma knife treatment.
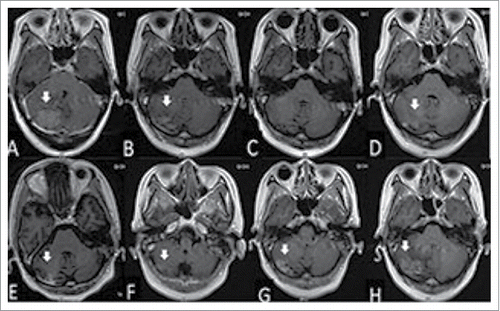
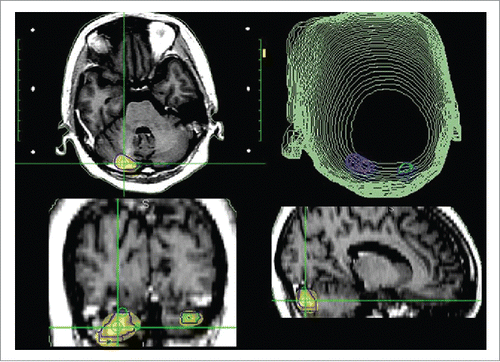
She was treated with whole brain radiation therapy (40 Gy in 20 fractions) in April 2012 and capecitabine for 13 months. Her brain metastases were observed to regress after whole brain radiation therapy (and ). In March 2013, the metastatic tumor in her cerebellum enlarged (and), and she received gamma knife treatment (25 Gy in 5 fractions, 53% isodose curve, ). Three months later, she was treated with 4 cycles of docetaxel from June 2013 to October 2013. Her metastatic lung tumors were then observed to be stable.
Figure 2. The SRS plan of brain tumors; gamma knife treatment (25 Gy in 5 fractions, 53% isodose curve).
Starting in November 2013, the patient was treated orally with anastrozole and goserelin after she showed resistance to docetaxel. In February 2015, she developed symptoms such as dizziness, fatigue, and headache. Her brain MRI showed the metastatic tumor was further enlarged in her cerebellum with widespread scattered irregular enhancement and a large area of edema in the surrounding tissue (and ), suggesting radiation necrosis.
On February 27, 2015, she received single-dose bevacizumab treatment (200 mg, 3.27 mg/kg). Two weeks later, an MRI showed that—compared with pre-treatment (and)—the brain necrosis as well as the volume of edema were significantly reduced (and ). Her symptoms—such as dizziness, fatigue, and headache—had disappeared in the meantime.
Figure 3. The woman was diagnosed with radiation brain necrosis for 1 year. Gadolinium-enhanced T1-weighted MRI in February 2015 showed widespread scattered irregular enhancement (A) and T2-weighted FLAIR MRI showed a large edema in the surrounding tissue (G). After bevacizumab treatment (3.27 mg/kg) in February 2015, the volume of necrosis (B) and edema (H) was reduced. Fifteen weeks after bevacizumab administration, gadolinium-enhanced T1-weighted MRI showed that the volume of necrosis (C) was enlarged and T2-weighted FLAIR MRI showed the edema (I) in the surrounding tissue was enlarged in June 2015, hence bevacizumab treatment (3.27 mg/kg) was given for the second time. At the July 2015 follow-up, the volume of necrosis in gadolinium-enhanced T1-weighted MRI (B) and edema in T2-weighted MRI (J) was reduced significantly again. In the October 2015 follow-up, the volume of necrosis in gadolinium-enhanced T1-weighted MRI (E) and edema in T2-weighted FLAIR MRI (K) was enlarged and the neurological symptoms were aggravated again; thus the patient was treated with bevacizumab (3.27 mg/kg) for the third time. Eight weeks after the third treatment of bevacizumab, the volume of necrosis in gadolinium-enhanced T1-weighted MRI (F) and edema in T2-weighted MRI (L) was reduced again.
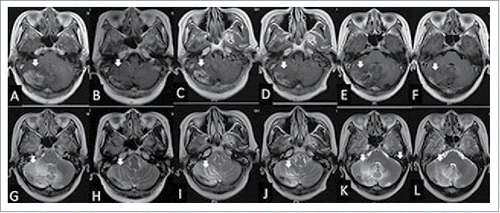
Approximately 4 months later (in June 2015), she gradually exhibited fatigue and headaches again. A brain MRI ( and ) showed a significantly increased volume of necrosis. Therefore, she received 200 mg bevacizumab for the second time, and a subsequent MRI (and) showed that the brain necrosis was significantly improved, and her symptoms disappeared again.
Approximately another 4 months later, the patient presented with similar symptoms as before and was treated with 200 mg bevacizumab for the third time in Oct 2015. The same condition later reoccurred, and she was treated with 200 mg bevacizumab in January 19, 2016, April 22, 2016, and July 22, 2016. Her symptoms significantly improved after each administration of bevacizumab. Eight weeks later, her brain MRI again showed significant alleviation of the brain necrosis (and ).
Discussion
Radiation brain necrosis is a common complication after stereotactic radiotherapy of intracranial tumors,Citation7 and is associated with vascular changes.Citation9 Radiation can induce injuries to astrocytes which can further increase VEGF levels, resulting in increased brain–blood barrier permeability and aggravation of brain edema. As an important anti-angiogenesis drug that can block VEGF release, bevacizumab is a potential candidate for the treatment of radiation-induced brain necrosis.
However, some limitations and unsolved questions are associated with the application of bevacizumab for radiation-induced brain necrosis. First, bevacizumab treatment is known to potentially cause serious side effects—including bleeding, proteinuria, hypertension, gastrointestinal perforation, and thromboembolic events—some of which can be serious or fatal.Citation10,11 Second, two dosing regimens of bevacizumab have been approved by the US. Food and Drug Administration (2.5 mg/kg/week dose equivalent) and European Medicines Agency (5 mg/kg/week dose equivalent) for oncological treatment protocols, and the latter (at 5 mg/kg/week) was more commonly used in previous clinical trials.Citation12-15 Many studies have shown that administration of bevacizumab at a dosage (5 mg/kg every 2–4 weeks) lower than that used for oncological protocols is effective for radiation brain necrosis.Citation2,4,16,17 However, the most appropriate dosage remains to be found. Third, a recent study cautiously showed that repeated bevacizumab treatment may induce drug-resistance following the progression of radiation brain necrosis.Citation18 Therefore, it will be important to explore the ideal dosage and administration interval of bevacizumab to prevent drug-resistance and severe side effects.
In a previous study carried out in our hospital, bevacizumab (5 mg/kg; mean dosing interval: 4 weeks; dosing interval range: 2–9 weeks) was observed to significantly reduce severe brain edema in 10 patients.Citation17 This effect lasted for an extended period after administration (from several weeks to several months in different patients) before the neurological symptoms reappeared. The neurological symptoms associated with radiation-induced brain necrosis could be controlled when the bevacizumab administration was repeated for a second time.
In the present case, radiation-induced brain necrosis was diagnosed based on several reasons as follows. First, the patient accepted whole-brain radiotherapy (40 Gy/20f) in April 2012, followed by 25 Gy/5f re-irradiation SRS in March 2013. Repeated radiotherapy increases the risk for radiation-induced necrosis compared with first-course radiotherapy. Second, her clinical symptoms were significantly relieved after the bevacizumab treatment. In brief, the radiation-induced necrosis was mainly diagnosed basing on our clinical experience, and it was reconfirmed by the way the patient survived with no brain tumor relapse after treatment at a relatively long-term follow-up.
We attempted to treat the patient with bevacizumab at a lower single bolus dosage (3.27 mg/kg) with relatively long dosing intervals (every 12–16 weeks). This treatment regimen was observed to effectively alleviate radiation-induced brain necrosis in this patient and improve her symptoms successively, without inducing any apparent side effect. Moreover, neurological symptoms such as dizziness, fatigue, and headache have been controlled for 17 months to date (when this case report was written).
This case report provides a useful reference for the clinical management of patients with radiation-induced brain necrosis. However, it is worth mentioning that whether the low-dose and long-interval regimen can avoid drug-resistance of bevacizumab is not yet known. Furthermore, the radiation brain necrosis was diagnosed empirically basing on the patients clinical manifestations, responses to treatment and radiographic findings. Without pathology, there is a risk of mis-diagnosing the progression of necrosis. This is an inherent limitation of both this case study, and the indeed real world practice. The small sample size (one patient) also restricts its power to convince that this bevacizumab administration regimen may be less toxic than using a higher dose. Additional research is required to further investigate these issues.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Youth fund of Affiliated Hospital of Academy of Military Medical Sciences, FC-2014-09.
References
- Nonoguchi N, Miyatake S, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, Kuroiwa T, Tsuji M, Fukumoto M, Ono K. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neuro-Oncol 2011; 105:423-31; PMID:21688077; http://dx.doi.org/10.1007/s11060-011-0610-9
- Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Inter J Radiat Oncol Biol Phys 2007; 67:323-6; PMID:17236958; http://dx.doi.org/10.1016/j.ijrobp.2006.10.010
- Pillay Smiley N, Alden T, Hartsell W, Fangusaro J. Severe radiation necrosis successfully treated with bevacizumab in an infant with low-grade glioma and tumor-associated intractable trigeminal neuralgia. Pediat Blood Cancer 2016; 63(9):1671-3; PMID:27187113; http://dx.doi.org/10.1002/pbc.26055
- Furuse M, Nonoguchi N, Kawabata S, Miyata T, Toho T, Kuroiwa T, Miyatake S. Intratumoral and peritumoral post-irradiation changes, but not viable tumor tissue, may respond to bevacizumab in previously irradiated meningiomas. Radiat Oncol (London, England) 2015; 10:156; PMID:26223253; http://dx.doi.org/10.1186/s13014-015-0446-0
- Sadraei NH, Dahiya S, Chao ST, Murphy ES, Osei-Boateng K, Xie H, Suh JH, Peereboom DM, Stevens GH, Ahluwalia MS. Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol 2015; 38:304-10; PMID:23799286; http://dx.doi.org/10.1097/COC.0b013e31829c3139
- Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Internat J Radiat Oncol Biol Phys 2011; 79:1487-95; PMID:20399573; http://dx.doi.org/10.1016/j.ijrobp.2009.12.061
- Du Four S, Hong A, Chan M, Charakidis M. Symptomatic histologically proven necrosis of brain following stereotactic radiation and ipilimumab in six lesions in four melanoma patients. 2014; 2014:417913.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9:669-76; PMID:12778165; http://dx.doi.org/10.1038/nm0603-669
- Schuttrumpf LH, Niyazi M, Nachbichler SB, Manapov F, Jansen N, Siefert A, Belka C. Prognostic factors for survival and radiation necrosis after stereotactic radiosurgery alone or in combination with whole brain radiation therapy for 1-3 cerebral metastases. Radiat Oncol (London, England) 2014; 9:105; PMID:24885624; http://dx.doi.org/10.1186/1748-717X-9-105
- Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM. Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neuro-Oncol 2011; 105:281-9; PMID:21603965; http://dx.doi.org/10.1007/s11060-011-0579-4
- Amit L, Ben-Aharon I, Vidal L, Leibovici L, Stemmer S. The impact of Bevacizumab (Avastin) on survival in metastatic solid tumors–a meta-analysis and systematic review. PloS One 2013; 8:e51780; PMID:23349675; http://dx.doi.org/10.1371/annotation/e3301fb2-ae1d-471a-aaf7-f38b4c989aff
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007; 25:1539-44; PMID:17442997; http://dx.doi.org/10.1200/JCO.2006.09.6305
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Eng J Med 2006; 355:2542-50; PMID:17167137; http://dx.doi.org/10.1056/NEJMoa061884
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005; 23:792-9; PMID:15681523; http://dx.doi.org/10.1200/JCO.2005.05.098
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Eng J Med 2011; 365:2473-83; PMID:22204724; http://dx.doi.org/10.1056/NEJMoa1104390
- Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, Wang X, Yuan Z, Wang P. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Scient Rep 2016; 6:24364; PMID:27067388; http://dx.doi.org/10.1038/srep24364
- Shen G, Wang YJ, Guan YJ, Dong DP, Yang G, Li D, Hao RM, Sun HR, Zhou M, Wang KP, et al. Relief effect of Bevacizumab on severe edema induced by re-irradiation in brain tumor patients. Chin Med J 2015; 128:2126-9; PMID:26228232; http://dx.doi.org/10.4103/0366-6999.161403
- Zhuang H, Yuan X, Sun D, Bian J, Chang JY, Yuan Z, Wang P. Acquired-resistance of bevacizumab treatment for radiation brain necrosis: a case report. Oncotarget 2016; 7:13265-8; PMID:26933810; http://dx.doi.org/10.18632/oncotarget.7724
