ABSTRACT
The purpose of this study was to test the effect of MK2206, an allosteric inhibitor of AKT, on the growth and invasion of patient-derived xenografts (PDX) of endometrial cancer. Three PDX lines, USC1 (uterine serous), EEC2 (endometrioid grade 2) and EEC4 (endometrioid grade 3) of endometrial cancer were grafted under the renal capsule of NSG mice. After 2 weeks of tumor growth the mice were treated with vehicle or 120mg/kg MK2206 twice a week for 3 weeks. Growth of all 3 PDX lines of different type and grade was significantly inhibited in response to MK2206 compared with vehicle control. Histological analysis revealed invasion and spread of EEC2 and EEC4 tumors were significantly decreased with MK2206 treatment. Immunohistochemical analysis showed a decrease in Ki67 in EEC2 upon MK2206 treatment, while USC1 and EEC4 tumors did not show differences in Ki67 levels. PR levels were evident in EEC2 which dramatically increased upon MK2206 treatment. In vitro analysis of EEC4 and AN3CA cells showed a dose-dependent decrease in p(Ser473)-AKT and p(Thr308)-AKT with MK2206. Invasion of EEC4 and AN3CA cells also significantly decreased after 36h and 72h of MK2206 treatment. PDX tumors provide an appropriate model for the testing of compounds that incorporates the heterogeneous nature of endometrial cancer. Further studies to determine efficacy of MK2206 alone or in combination with other compounds can also identify predictors of response to these pathway inhibitors.
Introduction
Endometrial cancer is the most common gynecologic malignancy in the United States. In 2016 the American Cancer Society estimates there will be more than 60,000 new cases of endometrial cancer and will result in over 10,000 deaths.Citation1 These statistics increase each year.
Approximately 80% of all endometrial carcinomas are endometrioid type, which arise from endometrial glands. These tumors, also known as Type I endometrial carcinomas, are associated with unopposed estrogen action. Although more than half of these cancers are diagnosed in postmenopausal women from ages of 55–74, about 5% of women with this disease are diagnosed before the age of 40 y and 20–25% are diagnosed before menopause.Citation2 This paradigm shift to younger ages will continue due to an increase in risk factors such as marked obesity, diabetes mellitus, and hypertension.
Type II endometrial cancer is less common but often presents at an advanced stage and is responsible for a disproportionate percentage of uterine cancer related deaths. Data is accumulating that Type II endometrial adenocarcinomas, including serous, clear cell, and high grade endometrioid histologies, share cellular pathway aberrations responsible for a unique molecular landscape.Citation3
PTEN is a common early genetic alteration in endometrioid endometrial carcinoma.Citation4-6 Genomic characterization of endometrial carcinoma by the Cancer Genome Atlas Network identified frequent mutations in PTEN, PIK3CA, CTNNB1, ARID1A, KRAS and ARID5B.Citation5 Data show that greater than 90% of endometrioid endometrial tumors and 60% of copy number high (serous) tumors have some genetic aberration in the PTEN/PI3K pathway, suggesting a potential for targeted therapy with PI3K/AKT pathway inhibitors.
We previously developed patient derived endometrial tumor xenografts in immunocompromised mice.Citation7 In addition, we reported the efficacy of AKT inhibitors in endometrial cancer cell lines.Citation8-13 In this study, we demonstrate using PDX endometrial tumors of Type I and Type II origin, a significant reduction in tumor growth and invasiveness in response to the allosteric AKT inhibitor, MK2206.
Results
PDX endometrial tumors
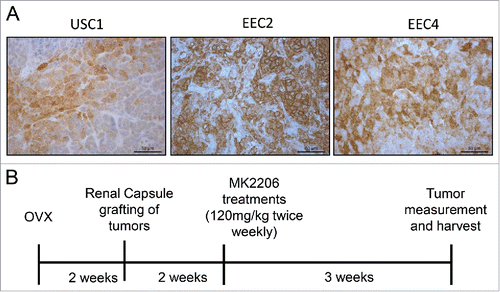
Primary human endometrial carcinoma tissues were established as PDX lines in NSG mice and previously characterized.Citation7 Among these, 3 PDX lines were chosen for this study: USC1 passage 12, derived from a uterine serous cancer, ECC2 passage 6 from a grade 2 endometrioid adenocarcinoma, and ECC4 passage 7 derived from a grade 3 endometrioid adenocarcinoma. Tumor information is summarized in . The tumors stained positively for p(Ser473)-AKT as detected by immunohistochemistry ().
Table 1. The histological characteristics of patient derived endometrial cancer xenografts.
Treatment with MK2206 in vivo
Two weeks after grafting tumor tissues under the renal capsule, mice were treated with either vehicle (30% captisol) or 120mg/kg MK2206 2 times per week for 3 weeks (). Tumors were visualized () and measurements were taken at the end of the experiment. In all PDXs, tumors were smaller in the MK2206 treated mice compared with vehicle treated mice (, ; ). While a wider range of tumor sizes were observed in the vehicle group, all tumors in the MK2206 group remained noticeably small. For USC1 tumors, the median tumor volume was 46.7 mm3 in the vehicle group compared with 8 mm3 in the MK2206 (p < 0.02; ). Similarly, in EEC2, the median tumor volumes were 19.1 mm3 compared with 136.8 mm3 in the MK2206 and vehicle treated mice respectively (p < 0.06). The median tumor volume for ECC4 was 15.8 mm3 in the MK2206 group compared with 125.1 mm3 in the vehicle group (p < 0.0001). In addition to the smaller tumor volumes, the MK2206 treated mice had significantly less invasion of the tumor within the kidney. The aggressive spread and large tumor burden outside of the kidney with the EEC4 tumors previously reportedCitation7 was significantly reduced in the MK2206 treated mice. Overall, the mice appeared to tolerate the MK2206 treatment with no visible signs of distress or weight loss.
Figure 2. Effect of MK2206 treatment on tumor volume. PDX tumors were grafted under the renal capsule of NSG mice. Mice were treated with either vehicle control or 120mg/kg twice a week with MK2206 for 3 weeks. Tumors were (A) visualized and (B) measured. Tumors from USC1, EEC2 and EEC4 are shown (* denotes statistical significance, p < 0.05).
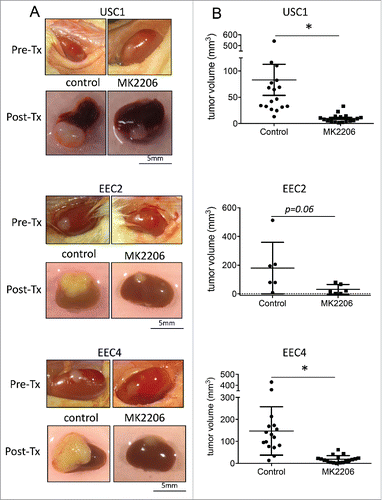
Table 2. Tumor volumes in the PDX tumors after treatment with MK-2206.
Immunohistochemistry for Ki67 and progesterone receptor
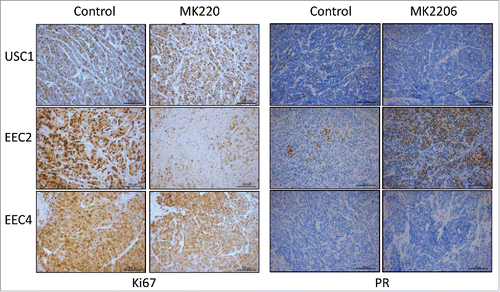
PDX tumors after treatment with MK2206 or vehicle were processed for immunohistochemistry. Strong staining for Ki67, a marker of proliferation, was observed in vehicle treated tumors of USC1, EEC2 and EEC4, indicating active proliferation (). Tumors from MK2206 treated mice exhibited similar staining for Ki67 in USC1 and EEC4, whereas Ki67 staining was significantly lower in MK2206 treated EEC2 tumors. Immunohistochemical staining for PR was also performed given our previous observations that demonstrated an increase in PR levels with AKT inhibitors in endometrial cancer cellsCitation10,12,13 and endometriosis.Citation14,15 As described previously, PR levels were minimal or absent in USC1 and EEC4 while EEC2 expressed PR in patches throughout the tumor ().Citation7 With MK2206 treatment, levels of PR did not change in USC1 and EEC4 while dramatically increased levels of PR were observed in EEC2 tumors.
In vitro response to MK2206
In general, primary cells derived from endometrial cancer tissues do not easily attach and grow in culture, with a few exceptions. Attempts to establish cells in culture from the 3 PDX tumors resulted in successful cell culture from one PDX line, the EEC4 tumor cells. EEC4 cells attached on plastic but did not grow as monolayers as conventional cells lines, rather, these cells grew in piles. With these cells, along with the AN3CA endometrial cancer cell line, in vitro treatment with increasing doses of MK2206 was performed. As a result, levels of p(Ser473)-AKT and p(Thr308)-AKT decreased with increasing concentrations of MK2206 ().
Figure 4. Effect of MK2206 on EEC4 and AN3CA cells in vitro. (A) Cells from EEC4 and AN3CA were cultured in vitro and treated with increasing concentrations of MK2206 (0, 0.01uM, 0.1uM, 1uM, 10uM). Levels of p(Ser473)-AKT, p(Thr308)-AKT and pan AKT were measured by western blot. (B) EEC4 and AN3CA were plated on matrigel coated membranes and treated with 1uM MK2206 for 36h and 72h. Cells on top were scraped off and cells that invaded through to the underside of the membrane was visualized with Diff-Quik stain. Invading cells were visualized under light microscopy and representative pictures are shown of cells treated with 1uM MK2206 for 72h (magnification 200X). The number of invading cells was counted in 4 different fields using ImageJ and the average was taken. Data was log transformed for statistical analysis. Data are presented as percent changes of MK2206 treated compared with vehicle treated cells. (* denotes statistical significance, p < 0.05).
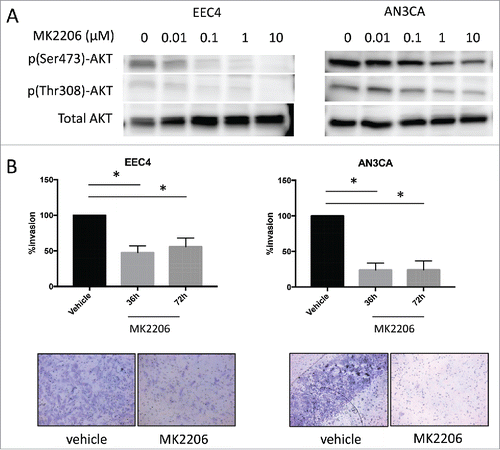
Next, the effect of MK2206 on tumor cell invasion was assessed given the aggressive nature of EEC4 as previously reported.Citation7 EEC4 or AN3CA cells were seeded on matrigel coated inserts and subsequently treated with 1 μM MK2206 or vehicle. Cell invasion was significantly decreased after treatment with MK2206 at 36 and 72 hours for both EEC4 and AN3CA (). The percent tumor invasion was decreased by at least 50% for all cell lines.
Discussion
The constitutively active PI3K/AKT pathway in endometrial cancer due to mutations in one or more members of this pathway in over 90% of endometrial carcinomasCitation5 makes this pathway an attractive target for therapy. To date, there are currently 11 active NCI-supported clinical trial studies with PI3K/AKT/mTOR inhibitors for endometrial cancer (https://www.cancer.gov/about-cancer/treatment/clinical-trials) either alone or in combination with other compounds. There is one phase 2 clinical trial to determine efficacy of MK2206 in patients with recurrent or advanced endometrial cancer (ClinicalTrials.gov Identifier: NCT01307631). Despite the fact that this pathway is overactive in the majority of endometrial cancer cases, as well as in other solid tumors, there are no reliable predictors of response.
PDX models of endometrial cancer that take into consideration the heterogeneity of endometrial tumors have been recently developed but are few in number.Citation7,16-19 Depreeuw et alCitation16 showed that engrafted tumors tend to be enriched with MSI and POLE mutations tumors, compared with non-engrafted samples. In addition, one tumor line from a high grade recurrent endometrioid carcinoma carrying PTEN, PIK3CA and KRAS mutations was treated with the dual pan-PI3K/mTOR inhibitor, NVP-BEZ235 and the MEK1/2 inhibitor, AZD6244. The growth of the high grade tumor was significantly inhibited with both of the agents individually as well as in combination. Bradford et al,Citation19 showed an initial tumorostatic response with the pan PI3K inhibitor, NVP BKM-120, followed by resistance to this agent which can be overcome by combination of NVP BKM-120 with chemotherotherapy. In our previous study, 4 out of 11 cases were established as xenografts and propagated.Citation7 Of these, 3 lines were used to test the efficacy of the allosteric AKT inhibitor, MK2206. Despite the 3 lines originating from tumors of different types or grades (uterine serous, endometrioid grade 2, and endometrioid grade 3) all 3 tumors lines responded to MK2206 treatment of 3 weeks to inhibit tumor growth. Our study is distinct from the others in that the site of grafting occurred under the renal capsule rather than subcutaneously under the skin. The microenvironment influences the behavior of tumors and the renal capsule provides close association of the tumor with blood and lymphatic fluids. For this reason, the renal capsule of a NSG mouse has been a successful site used for tissue grafting of benignCitation20-23 and malignant tissues.Citation7,24-27 We observed aggressive invasion of the EEC2 and EEC4 tumors throughout the kidney while the USC1 line did not invade the kidney.Citation7 MK2206 treated mice exhibited smaller tumors as well as decreased invasion into the kidney and spread throughout the peritoneum. Our in vitro data support the decreased invasive capacity of the EEC4 cells treated with MK2206. Thus, the kidney provided us a model to study not only tumor growth but invasion and tumor spread that was significantly reduced with MK2206. One limitation of grafting on the kidney is the inability to monitor growth during treatment without the addition of a bioluminescent agent. The data was obtained at the end of 3 weeks of treatment and thus the discrepancy of smaller tumors with no difference in Ki67 levels in USC1 and EEC4 was difficult to interpret. It is possible that MK2206 affected proliferation rate early in tumor growth, which was not evident at the end of 3 weeks with Ki67 staining. It is also possible that resistance to MK2206 developed as was shown in the Bradford study.Citation19
The increase in PR protein in EEC2 tumors treated with MK2206 supports our previous lines of investigation with endometrial cancer cell lines as well as endometriosis cells and tissues.Citation10,12-15 Interestingly, in the more advanced tumor lines, USC1 and EEC4, PR was absent or expressed in low levels and treatment with MK2206 did not influence PR levels suggestive of an epigenetic silencing of PR in these cells, such as methylation.Citation28 EEC2 tumors expressed PR and MK2206 increased levels. These data suggest that PR-expressing tumors of endometrioid origin could benefit from MK2206 treatment by increasing progestin sensitivity. Our recent mechanistic studies support the sensitization of endometrial cancer cells to progestins with MK2206 through transcriptional enhancement of a subset of genes affecting angiogenesis, proliferation and others.Citation9 Combination treatment of MK2206 and progestins could be an attractive option for lower grade endometrioid cancers. Currently, the majority of clinical trials in endometrial cancer that are available are targeted for advanced and recurrent cancers. For several reasons, trials for lower grade endometrioid cancers would be difficult to do. PDX endometrial tumors would provide an alternative to test such combinations and would generate important information on mutational and hormone receptor status to better stratify patients for an optimal response.
While the prevalence of mutations in the PI3K/AKT pathway has rendered endometrial cancer attractive to inhibitors to this pathway, response rates differ and more information is needed to identify the patients that will benefit from these inhibitors as single agents or in combination with other treatment strategies. Analysis of PDX tumors would be useful in understanding tumor heterogeneity and to identify predictive markers for response. In turn, sufficient evidence could provide grounds for the development of combination therapies of AKT inhibitor and progestins for Type I endometrial cancer cases that do not respond to progestins alone.
Methods
Patient derived primary tumors
All endometrial tumors were obtained from patients who provided written consent before surgery and has been previously reported.Citation7 This study was approved by the Human Subject Committee of Northwestern University in accordance with US. Department of Health Regulations.
Subrenal grafting of PDX lines
All procedures involving animals were approved by Northwestern University's Animal Care and Use Committee. All surgical procedures were performed under anesthesia by intraperitoneal injection of ketamine/xylazine (90/8 mg/kg) and all efforts were made to minimize suffering. Adult female NOD scid gamma (NSG) mice (Jackson Laboratory, Bar Harbor, ME), were ovariectomized (OVX). Two weeks after OVX, tumors were cut into small fragments (1.5 mm × 1.5 mm) and grafted under the renal capsule of adult female NSG mice (Jackson Laboratory, Bar Harbor, ME) as described previously.Citation7,20 Mice harboring the PDX line EEC2 were given 0.36 mg E2 in the form of 90-day release pellets (Innovative Research of America Inc., Sarasota, Fl) implanted subcutaneously. Tumors were allowed to grow for 2 weeks, after which time the mice were treated for 3 weeks with twice weekly weight based MK2206 (120mg/kg) or 30% captisol as the vehicle control, by oral gavage. Four days after the last treatment, the kidneys were harvested and tumor volumes measured and calculated using the elliptical formula, mm3 = (4/3)πabc.Citation29 Tumor tissues were fixed in 10% neutral buffered formalin containing 3.8% formaldehyde (VWR International, West Chester, PA) and subsequently paraffin embedded for histological analysis.
Cell lysis and immunoblotting
Cell lysis and immunoblotting were performed as described previously.Citation10,22 Membranes were probed with primary antibodies to phospho-Akt Ser473, phospho-Akt Thr308, and pan-Akt from Cell Signaling. Membranes were developed using ECL Reagents (Life Technologies), and protein bands were visualized with a Fuji Film LAS-3000 Imaging System (Stamford, CT, USA).
In vitro matrigel invasion
Matrigel invasion chambers (BD Biosciences, San Jose, CA, USA) were seeded with 3.5 × 105 AN3CA cells or EEC4 cells in serum free DMEM and incubated for 24 hours. Cells were treated with 1uM of MK2206 or DMSO vehicle for 36 or 72 hours in the presence of 10% fetal bovine serum in the bottom chamber as the chemoattractant. Matrigel invasion chambers were then scraped on the top to remove non-invading cells and invaded cells were stained with the Diff-Quik staining kit (Thermo Scientific). The number of invading cells was counted under light microscopy in 4 different 20X fields using ImageJ and was averaged.
Statistical analysis
All statistical analysis was performed using Graphpad Prism version 6.0 (Graphpad Software, La Jolla, CA, USA). Data were considered statistically significant if the p-value was < 0.05. For tumor volumes, the non-parametric Mann Whitney U test was used to compare control and MK2206 treated groups as data did not display normal distribution. For invasion assays, data were first log-transformed and statistical significance between samples was analyzed using the paired student t-test. Data are graphically presented as percent fold changes from vehicle treated cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would also like to thank members of the Kim laboratory for technical assistance and insightful discussions. In addition we thank the research coordinators who consented patients and coordinated tissue collection. We would like to acknowledge the Human Pathology Core at the Robert Lurie Cancer Center, Northwestern University, for assistance in the immunohistochemical staining.
Funding
Funding for this project was provided by a grant from NIH/NCI R01CA155513.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7-30; PMID:26742998; https://doi.org/10.3322/caac.21332
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol 1984; 64:417-20; PMID:6462572
- Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Sundar S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol 2012; 124:15-20; PMID:21864888; https://doi.org/10.1016/j.ygyno.2011.07.030
- Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Weng LP, Eng C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 2000; 92:924-30; PMID:10841828; https://doi.org/10.1093/jnci/92.11.924
- Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497:67-73; PMID:23636398; https://doi.org/10.1038/nature12113
- Levine RL, Cargile CB, Blazes MS, van Rees B, Kurman RJ, Ellenson LH. PTEN mutations and microsatellite instability in complex atypical hyperplasia, a precursor lesion to uterine endometrioid carcinoma. Cancer Res 1998; 58:3254-8; PMID:9699651
- Unno K, Ono M, Winder AD, Maniar KP, Paintal AS, Yu Y, Wei JJ, Lurain JR, Kim JJ. Establishment of human patient-derived endometrial cancer xenografts in NOD scid gamma mice for the study of invasion and metastasis. PLoS One 2014; 9:e116064; PMID:25542024; https://doi.org/10.1371/journal.pone.0116064
- Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013; 34:130-62; PMID:23303565; https://doi.org/10.1210/er.2012-1043
- Lee, II, Maniar K, Lydon JP, Kim JJ. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene 2016; 35:5191-201; PMID:26996671; https://doi.org/10.1038/onc.2016.56
- Pant A, Lee, II, Lu Z, Rueda BR, Schink J, Kim JJ. Inhibition of AKT with the orally active allosteric AKT inhibitor, MK2206, sensitizes endometrial cancer cells to progestin. PLoS One 2012; 7:e41593; PMID:22911820; https://doi.org/10.1371/journal.pone.0041593
- Hoekstra AV, Ward EC, Hardt JL, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1. Gynecol Oncol 2008; 108:609-18; PMID:18234299; https://doi.org/10.1016/j.ygyno.2007.11.007
- Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, Hanifi-Moghaddam P, Schink J, Kim JJ. Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1-59-mediated apoptosis. Horm Cancer 2011; 2:170-81; PMID:21760855; https://doi.org/10.1007/s12672-011-0065-7
- Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology 2008; 149:1942-50; PMID:18096667; https://doi.org/10.1210/en.2007-0756
- Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab 2013; 98:E1871-9; PMID: 24064688; https://doi.org/10.1210/jc.2013-1661
- Kim TH, Yu Y, Luo L, Lydon JP, Jeong JW, Kim JJ. Activated AKT pathway promotes establishment of endometriosis. Endocrinology 2014; 155:1921-30; PMID:24605828; https://doi.org/10.1210/en.2013-1951
- Depreeuw J, Hermans E, Schrauwen S, Annibali D, Coenegrachts L, Thomas D, Luyckx M, Gutierrez-Roelens I, Debruyne D, Konings K, et al. Characterization of patient-derived tumor xenograft models of endometrial cancer for preclinical evaluation of targeted therapies. Gynecol Oncol 2015; 139:118-26; PMID:26232337; https://doi.org/10.1016/j.ygyno.2015.07.104
- Groeneweg JW, Hernandez SF, Byron VF, DiGloria CM, Lopez H, Scialabba V, Kim M, Zhang L, Borger DR, Tambouret R, et al. Dual HER2 targeting impedes growth of HER2 gene-amplified uterine serous carcinoma xenografts. Clin Cancer Res 2014; 20:6517-28; PMID:25294905; https://doi.org/10.1158/1078-0432.CCR-14-1647
- Haldorsen IS, Popa M, Fonnes T, Brekke N, Kopperud R, Visser NC, Rygh CB, Pavlin T, Salvesen HB, McCormack E, et al. Multimodal Imaging of Orthotopic Mouse Model of Endometrial Carcinoma. PLoS One 2015; 10:e0135220; PMID:26252891; https://doi.org/10.1371/journal.pone.0135220
- Bradford LS, Rauh-Hain A, Clark RM, Groeneweg JW, Zhang L, Borger D, Zukerberg LR, Growdon WB, Foster R, Rueda BR. Assessing the efficacy of targeting the phosphatidylinositol 3-kinase/AKT/mTOR signaling pathway in endometrial cancer. Gynecol Oncol 2014; 133:346-52; PMID:24561032; https://doi.org/10.1016/j.ygyno.2014.02.022
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010; 151:2433-42; PMID:20375184; https://doi.org/10.1210/en.2009-1225
- Ono M, Yin P, Navarro A, Moravek MB, Coon JSt, Druschitz SA, Serna VA, Qiang W, Brooks DC, Malpani SS, et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci U S A 2013; 110:17053-8; PMID:24082114; https://doi.org/10.1073/pnas.1313650110
- Sefton EC, Qiang W, Serna V, Kurita T, Wei JJ, Chakravarti D, Kim JJ. MK2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth. Endocrinology 2013; 154:4046-57; PMID:24002033; https://doi.org/10.1210/en.2013-1389
- van Gurp L, Loomans CJ, van Krieken PP, Dharmadhikari G, Jansen E, Ringnalda FC, Beerling E, van Rheenen J, de Koning EJ. Sequential intravital imaging reveals in vivo dynamics of pancreatic tissue transplanted under the kidney capsule in mice. Diabetologia 2016; 59:2387-92; PMID: 27443307; https://doi.org/10.1007/s00125-016-4049-6
- Ishii K, Shappell SB, Matusik RJ, Hayward SW. Use of tissue recombination to predict phenotypes of transgenic mouse models of prostate carcinoma. Lab Invest 2005; 85:1086-103; PMID:15980886; https://doi.org/10.1038/labinvest.3700310
- Lee CH, Xue H, Sutcliffe M, Gout PW, Huntsman DG, Miller DM, Gilks CB, Wang YZ. Establishment of subrenal capsule xenografts of primary human ovarian tumors in SCID mice: potential models. Gynecol Oncol 2005; 96:48-55; PMID:15589579; https://doi.org/10.1016/j.ygyno.2004.09.025
- Parmar H, Young P, Emerman JT, Neve RM, Dairkee S, Cunha GR. A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology 2002; 143:4886-96; PMID:12446616; https://doi.org/10.1210/en.2002-220570
- Press JZ, Kenyon JA, Xue H, Miller MA, De Luca A, Miller DM, Huntsman DG, Gilks CB, McAlpine JN, Wang YZ. Xenografts of primary human gynecological tumors grown under the renal capsule of NOD/SCID mice show genetic stability during serial transplantation and respond to cytotoxic chemotherapy. Gynecol Oncol 2008; 110:256-64; PMID:18547621; https://doi.org/10.1016/j.ygyno.2008.03.011
- Yang S, Jia Y, Liu X, Winters C, Wang X, Zhang Y, Devor EJ, Hovey AM, Reyes HD, Xiao X, et al. Systematic dissection of the mechanisms underlying progesterone receptor downregulation in endometrial cancer. Oncotarget 2014; 5:9783-97; PMID:25229191; https://doi.org/10.18632/oncotarget.2392
- Kashkoush S, El Moghazy W, Kawahara T, Gala-Lopez B, Toso C, Kneteman NM. Three-dimensional tumor volume and serum alpha-fetoprotein are predictors of hepatocellular carcinoma recurrence after liver transplantation: refined selection criteria. Clin Transplant 2014; 28:728-36; PMID:24708263; https://doi.org/10.1111/ctr.12373