ABSTRACT
Uveal melanoma (UM) is an intractable disease with a low survival rates, despite adequate local treatment, as a result of its metastatic characteristics. Thus, new therapeutic strategies, including combinations of novel gene therapy and traditional chemotherapy, are under investigation to improve long-term prognosis. Dacarbazine or DTIC, an alkylating agent which results in DNA methylation, is most commonly used to treat melanoma but the response is very limited. The O6-methylguanine DNA methyl transferase (MGMT), a DNA repair protein, is involved in chemoresistance in DTIC treatment. We previously investigated a combination of oncolytic adenovirus H101 and the alkylating agent DTIC in the treatment of UM cells in vitro and observed a synergistic antitumor effect. In this study, we validated this result and report an enhanced therapeutic effect in vivo. Our findings also demonstrated that the oncolytic adenovirus H101 decreased MGMT levels via accumulation of p53 overcoming DTIC chemoresistance. Therefore, the clinical therapeutic efficacy of DTIC in the treatment of UM might be improved using this adenovirus-based combination therapy.
Abbreviations
| DMEM | = | dulbecco's modified Eagle's medium |
| DMEM/F12 | = | dulbecco's modified eagle medium and Ham's F-12 nutrient mixture |
| DTIC | = | dimethyl-triazeno-imidazol carboxamide, Dacarbazine |
| FBS | = | fetal bovine serum |
| H101 | = | a recombinant human type-5 adenovirus |
| HRP | = | horseradish peroxidase |
| MGMT | = | the O6-methylguanine DNA methyl transferase |
| PAGE | = | polyacrylamide gel electrophoresis |
| pfu | = | plague-forming units |
| RT-PCR | = | reverse transcription-polymerase chain reaction |
| SDS | = | sodium dedecyl sulfate |
| SiRNA | = | small interferencing RNA |
| UM | = | uveal melanoma |
Introduction
Uveal melanoma (UM) is the most frequent primary malignant intraocular tumor in adults. It is an intractable disease with a low survival rates, despite adequate local treatment. This is largely a result of its highly metastatic nature.Citation1,2 Thus, new therapeutic strategies, including systemic chemotherapy, immune-therapy, gene therapy and some combination therapies have been investigated to improve the outcome of UM.Citation3 In our previous studies, we have attempted to treat UM cells in vitro using a combination of the oncolytic adenovirus H101 with DTIC, a classic chemotherapeutic agent for melanoma, and were able to report a synergistic antitumor effect without enhanced toxicity to normal cells.Citation4
Dacarbazine or DTIC (dimethyl-triazeno-imidazol carboxamide) is an alkylating agent, which results in DNA methylation, which is commonly used to treat melanoma.Citation5 Previous studies demonstrated that a key mechanism of resistance to alkylating agents is O6-methylguanine DNA methyl transferase (MGMT) overexpression.Citation6 MGMT is a DNA repair protein which can transfer the methyl group from guanine to a cysteine residue, repairing the cytotoxic lesion induced by alkylating agents, such as DTIC, resulting in drug resistance.Citation7 Therefore, downregulation of MGMT expression could enhance the cytotoxicity of alkylating agents in tumor cells.Citation8 There are several mechanisms for the downregulation of MGMT gene expression, all at different levels, which can be used in new strategies to decrease chemoresistance and enhance anti-tumor ability.Citation9 Numerous studies have shown that the methylation of CpG sites in the promoter may result in the silencing of MGMT.Citation10-12 Other research has revealed that transcription factor p53 is also associated with the inhibition of MGMT and consequently changes the sensitivity of tumor cells to alkylating agents.Citation8,13,14 In addition, there is evidence that adenovirus E1A protein could inhibit MGMT expression by binding to and inactivating p300.Citation15,16
H101, an oncolytic adenovirus with no E1B and portions of E3 regions, is designed to replicate in tumor cells that have inactivated p53, but some studies have shown that its replication may be independent of p53.Citation17,18
At present, adenovirus-based combination therapy is a promising avenue for the treatment of malignant tumors. In our previous study, we have shown that DTIC did not affect the entry and replication of H101, enabling their combination in the treatment in UM cells in vitro.Citation4 Therefore, we hypothesized that H101 overcome the chemoresistance of DTIC by promoting antitumor efficiency. In this study, we intended to further validate the synergistic antitumor effect of this adenovirus-based therapy to UM in vivo, and uncover the underlying molecular mechanisms involved.
Results
Combination of H101 and DTIC exhibited enhanced antitumor effect in UM in vivo
Previously, we treated melanoma cells by combining oncolytic virus H101 and DTIC in vitro and observed a synergistic antitumor effect without enhanced toxicity to normal cells.Citation4 In this study, we intended to detect whether the same effect during co-treatment can be observed in vivo using an animal model. SP6.5 cells were implanted in nude mice, when the xenograft was greater than 100 mm3 the animals received intra-tumor injection of H101 and DTIC alone or together. As seen in , monotherapy of H101 or DTIC exerted only a moderate antitumor ability when compared with the PBS group, while co-treatment of H101 and DTIC led to a much stronger antitumor effect when compared with either single treatment. A significant difference in tumor size was observed between the combination group and other groups (**P < 0.01).
Figure 1. Antitumor effect of combinatorial treatment of H101 and DTIC in SP6.5 xenografted mice. (A) Average tumor volume of the mice bearing SP6.5 UM xenograft after treatment with PBS, H101, DTIC, and H101+DTIC. Values represent the means ± SD. **P<0.01 compared with H101+DTIC group. (B) Kaplan–Meier survival curves for overall survival of the tumor-bearing mice.*P<0.05compared to H101+DTIC group.
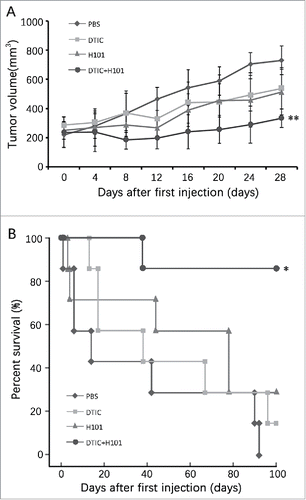
We also measured the survival duration for animals in each group, evaluating the long-term effect of these therapies. During our observation over 100 days, only one animal died (14.3%) on day 38 after the first injection in the combination group. There were 14.3% and 28.6% of animals still alive in H101 or DTIC monotherapy groups respectively, and all the animals died by the day 92 in PBS control group. These findings indicated that the combination of H101 and DTIC could achieve an enhanced antitumor effect in UM in vivo.
H101 overcame chemoresistance to DTIC by downregulation of MGMT expression
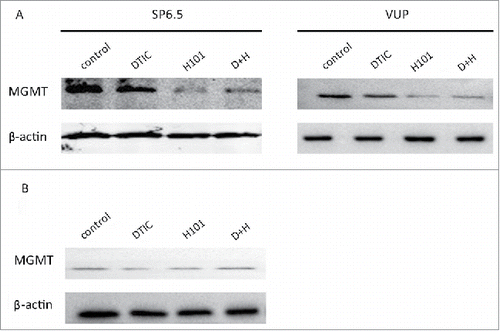
Next, we intended to investigate the underlying mechanism for the improved therapeutic outcome of combination therapy in UM cells. DTIC does not interfere with adenoviral replication,Citation4 therefore we examined whether H101 overcomes chemoresistance to DTIC. It has been shown that MGMT overexpression plays an important role in resistance to alkylating agents in tumor cells. Thus, we analyzed the MGMT expression in SP6.5 and VUP cells treated with DTIC or H101 alone and in combination (). We found both SP6.5 and VUP cells have MGMT protein expression and H101 treatment induced a distinct down-modulation of MGMT expression in both cell lines. Compared with control group, DTIC treatment did not lead to obvious changes in MGMT expression, hence, the downregulation of MGMT in the combination therapy group may be a result of H101. H101 cannot replicate in normal retinal pigment cells (RPE) and did not change the MGMT expression levels in these cells whether applied alone or in combination with DTIC (). These findings indicate that H101 may reduce MGMT expression to overcome chemoresistance to DTIC in UM cells.
Figure 2. Down-regulation of MGMT expression by H101 in UM cells. (A) Western blot analysis of MGMT expression 48 hours after PBS, DTIC, H101, and DTIC+H101 treatment in SP6.5 and VUP cells. (B) Western blot analysis of MGMT expression 48 hours after PBS, DTIC, H101, and DTIC+H101 treatment in human normal pigment epithelial cells RPE.
p53 was involved in downregulation of MGMT induced by H101
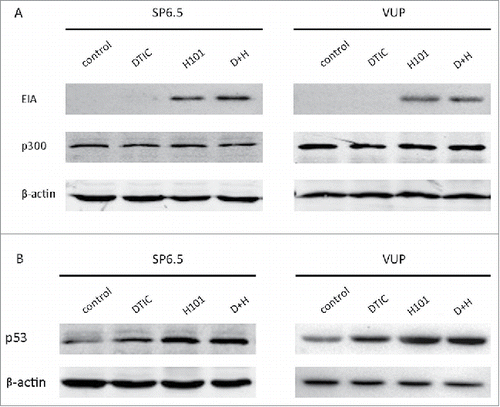
Some studies report that adenovirus E1A inhibits MGMT promoter activity by binding to and inactivating CBP/p300.Citation15,16 To investigate whether the same mechanism was at work in this study, we analyzed the expression level of E1A and p300 in the four different treatment groups in SP6.5 and VUP cells. As seen in , we detected E1A expression in the H101 group and in the combination therapy group. However, there was no p300 inhibition as predicted by E1A expression in the corresponding treatment groups. This indicated that p300 may not be involved in the downregulation of MGMT induced by H101. Thus we had to investigate other potential mechanisms to explain our observations. Transcription factor p53 interacts with the MGMT promoter and downregulates its expression;Citation8,14 we decided to assess the p53 expression in the four treatment groups. There was a significant increase in p53 expression in both the H101 group and the combination therapy group and only a slight increase in DTIC group ().
Silencing of p53 by siRNA increases MGMT expression
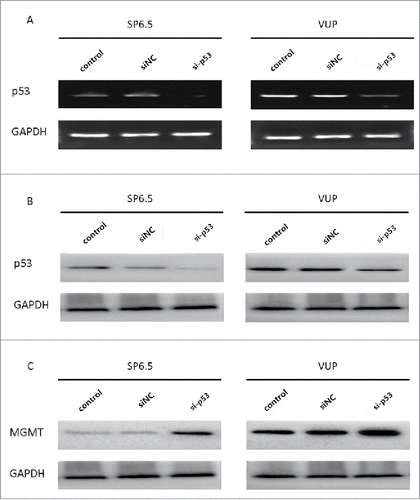
To further validate whether p53 plays a role in MGMT downregulation induced by H101, we tested whether inhibition of p53 could affect the MGMT expression in UM cells. After optimization, p53 expression was silenced by a p53 specific siRNA (si-p53) at both the mRNA and protein level () in SP6.5 and VUP cells. MGMT expression was only detected by western blot 48 hours after si-p53 transfection (). As we had hypothesized, transfection of si-p53 induced a significant increase of MGMT protein expression in both SP6.5 and VUP cells when compared with the siNC and PBS controls, which indicated that p53 may take part in the downregulation of MGMT induced by H101 in UM cells.
Figure 4. siRNA transfection downregulated p53 expression and resulted in an upregulation of MGMT. (A) PCR analysis of p53 expression in VUP and SP6.5 cells 48 h after transfection with p53-specific siRNA (si-p53), negative control siRNA (siNC) or PBS control (control). (B) Western blot analysis of p53 expression in VUP and SP6.5 cells 48 h after transfection. (C) Western blot analysis of MGMT expression in VUP and SP6.5 cells 48 h after transfection.
Discussion
At present, adenovirus-based strategies present promising novel treatment options in many malignancies and are rapidly advancing toward clinical application.Citation19 The popular adenovirus-based combination treatments exploited for cancers involve the use of cytotoxic chemotherapy agents, which may encounter chemoresistance and prominent side effects when used alone.Citation20
In this study, we validated the antitumor efficacy of oncolytic adenovirus H101 in combination with an alkylating agent, DTIC, in the treatment of UM in vivo and received a synergistic effect in the combination therapy group, which was consistent with our previous in vitro study.Citation4 In the animal experiment, the combination treatment showed enhanced tumor inhibition and prolonged survival time compared with the monotherapy and PBS groups ().
However, the underlying mechanism of this combination therapy is not well understood. Since the drug DTIC does not interfere with adenovirus replication in the UM cells in vitro,Citation4 we focused on whether the oncolytic adenovirus H101 played a role in the reduction of chemoresistance to DTIC.
Previous studies revealed a significant association between MGMT expression and DTIC chemoresistance. High MGMT expression levels in tumor cells usually means pronounced resistance to DTIC.Citation6,21 It has been reported that adenovirus sensitized tumor cells to DTIC by downregulation of MGMT.Citation15 In our study, oncolytic adenovirus induced a distinct downregulation of MGMT expression in both SP6.5 and VUP cells, without significant changes in normal cells (). Since H101 was unable to replicate in normal cells and the monotherapy of DTIC did not lead to obvious changes in MGMT levels, the suppression of MGMT in uveal cells may be primarily the result of H101, which allows DTIC to overcome their natural chemoresistance.
There are many mechanisms involved in the downregulation of MGMT expression but so far promoter hyper-methylation has proven to be the major mechanism of MGMT silencing.Citation11,12 In previous studies, adenoviruses express protein E1A inhibiting MGMT promoter activity by removing p300 from the MGMT transcriptional machinery.Citation15,16,22 However, our investigation indicated that the p300 level was not decreased although E1A expression was detected in the H101 monotherapy group and the combination therapy group in both VUP and SP6.5 cells (). Thus it is possible that p300 may not be involved in the MGMT downregulation in the combination treatment of H101 and DTIC in UM.
It has been proven that the adenovirus E1B 55-kDa protein can bind to and block the transcriptional activity of p53. Together with E4–34 kDa, it also promotes ubiquitination and proteasomal degradation of p53 in cytoplasm. Therefore, the infection of E1B-55K mutant adenoviruses led to p53 stabilization and accumulation in p53 positive cells.Citation23,24 H101, which lacks E1B-55K, can specifically lyse tumor cells that have inactivated p53. Previous studies have demonstrated that both UM cells (SP6.5 and VUP) contained mutant p53, thus serving as an ideal target for H101.Citation25,26 With the deletion in E1B region, H101 is incapable of binding and degrading p53 which therefore accumulates in the nucleoplasm after infection ().
In addition, it is reported that tumor suppressor protein p53 may cause the downregulation of MGMT by directly interacting with MGMT promoter.Citation8 To verify the theory, we silenced p53 at the mRNA and protein level using a p53 specific siRNA in SP6.5 and VUP cells () and an increase in MGMT expression was observed in the corresponding si-p53 group (). These findings indicated that the increased p53 in UM cells seems to retain the ability to suppress MGMT expression, which render the UM cells to overcome chemoresistance to DTIC.Citation8 A possible reason for this phenomenon might be that the mutation sites of p53 in UM cells are out of its sequence interacting with MGMT. There is evidence that alkylating agents could also lead to p53 activation and upregulation via AMP-activated protein kinase, and p53 is required for drug-induced cytotoxicity.Citation27 It is interesting to note that p53 expression was slightly increased in the DTIC mono treatment group in our study, while MGMT in the same group did not change significantly (, ).The reason why DTIC-induced p53 accumulation did not down-regulate MGMT was not clear. It is possible that the p53 increase in the DTIC group was too small to induce MGMT downregulation, or p53-induced MGMT downregulation may demand the participation of certain other factors which are produced by the adenovirus, or some mechanism triggered by DTIC prevented the process of p53-mediated MGMT reduction.
Although a positive correlation between MGMT expression and alkylating agents DTIC and temozolomide (TMZ) resistance has been previously demonstrated in glioma and melanoma,Citation6-8 no data are yet available on the relationship between MGMT and DTIC in UM cells in literature. It has been proven that promoter hypermethylation prevents MGMT expression and sensitizes glioma cells to alkylating agent. And p53 is involved in MGMT reduction by directly interacts with MGMT promoter.Citation8 In our study, we demonstrated that the combination treatment downregulated MGMT expression via increasing p53 levels and sensitized UM cells (SP6.5 and VUP) to DTIC, which were in agreement with the previous reports.Citation7,8 Meanwhile, we found that the combined therapy decreased MGMT levels through promoter hypermethylation (Data not shown). Taken together, MGMT may sensitize UM cell lines to DTIC under the same mechanisms as above.
In conclusion, our study validated the adenovirus-based combination strategy for UM and demonstrated a synergistic therapeutic effect in vivo. We also demonstrated that oncolytic adenovirus H101 decreases MGMT levels via accumulation of p53 to overcome chemoresistance to DTIC. Therefore, the clinical therapeutic efficacy of DTIC to UM might be improved by this adenovirus-based combination therapy.
Materials and methods
Cell lines and culture conditions
Human UM cell lines SP6.5 and VUP, as well as the human retinal pigment epithelium cell line, RPE, were generously provided by Prof. Fan (Department of Ophthalmology, Ninth People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China). The UM cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). RPE cells were cultured in DMEM /F12 (1:1) supplemented with 10% fetal bovine serum (FBS). All cell lines were maintained at 37°C with 5% CO2.
Chemicals and virus
DTIC (Nanjing Pharmaceutical Factory, #6) was dissolved in 0.9% saline to produce a 1,000 mg/mL stock solution as described previously.Citation4 Oncolytic adenovirus H101 was purchased from Shanghai Sunway Biotech and maintained under conditions recommended by the manufacturer as described previously.Citation18
Animal studies
SP6.5 cells (1 ×107) were subcutaneously injected into the right flank of thymic nude mice to establish tumor xenografts. Then the tumor-bearing mice were randomly divided into 4 groups when the tumor volume, calculated by the formula: length × width2 × 0.5, was greater than 100 mm3. The H101 plus DTIC group received intra-tumoral injections of DTIC (50 mg/kg) on day 1, 4, 8, 11 and 15 and H101 (1 ×109pfu) on day 2, 5, 9, 1 2 and 16. The DTIC group and the H101 group received 5 injections of DTIC (50 mg/kg) and 5 injections of H101 (1 ×109pfu) respectively. The control group received 5 injections of PBS.Citation17 The tumor growth was monitored by a vernier caliper every 4 d. Animal studies were performed according to institutional guidelines for animal care as described by the Kunming Medical University.
Western blot
SP6.5, VUP and RPE were harvested, separated on 6–12% SDS-PAGE and transferred to polyvinylidene fluoride membranes. Membranes were blocked using PBS containing 5% non-fat milk and 0.1% Tween 20 for 2 h followed by incubation with the primary antibody overnight at 4°C. Primary antibodies used were: MGMT (Cell Signaling Technology, #2739), E1A (Santa Cruz Biotechnology, sc-58658), p300 (Santa Cruz Biotechnology, sc-48343), p53 (Cell Signaling Technology, #2527), β-actin (Santa Cruz Biotechnology, sc-323503). The bands were visualized with HRP -substrate coloring solution and Image J software was used to analyze the density of the bands and predict protein quantity.Citation28
Reverse transcriptase-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, 15596–026) according to the manufacturer's instructions. The cDNA was synthesized from 1 μg of total RNA using RevertAid First Strand cDNA Synthesis Kit (Fermentas, K1621). After denaturation at 95°C for 2 min, cDNA was amplified using a35 cycle regimen consisting of 95°C for 60 s, 54°C for 60 s and 72°C for 60 s, and a subsequent extension at72°C for 10 min. Specific PCR primers were as follows: p53:5′ -TGCTCAGATAGCGATGGTC- 3′ (forward) and 5′ -TTTATGGCGGGAGGTAGA- 3′ (reverse). RT-PCR for GAPDH served as a positive control.
RNA interference
Cells were transfected with 50 to 100 nM p53 siRNA or control siRNA using a riboFECT™ CP Transfection Kit following the manufacturer's instructions (RiboBio, C10511–05) and the knockdown was monitored by RT-PCR for mRNA and western blot analysis for protein. siRNAs against p53 and scrambled siRNA (negative control) were synthetized by RiboBio (siG1111284137, siN05815122147).
Statistical analysis
All experiments were performed in triplicate, and the data are expressed as mean ± SD. The survival curve was assessed according to the Kaplan–Meier method and the data were analyzed by the log-rank test. The other data were analyzed with 2-tailed t-test. All the results were considered statistically significant at p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China grant (81301952, 81372469).
References
- Buder K, Gesierich A, Gelbrich G, Goebeler M. Systemic treatment of metastatic uveal melanoma: Review of literature and future perspectives. Cancer Med 2013; 2:674-86; PMID:24403233; http://dx.doi.org/10.1002/cam4.133
- Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology 2003; 110:962-5; PMID:12750098; http://dx.doi.org/10.1016/S0161-6420(03)00077-0
- Chattopadhyay C, Kim DW, Gombos DS, Oba J, Qin Y, Williams MD, Esmaeli B, Grimm EA, Wargo JA, Woodman SE, et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016; 122:2299-312; PMID:26991400; http://dx.doi.org/10.1002/cncr.29727
- Cun B, Song X, Jia R, Zhao X, Wang H, Ge S, Fan X. Combination of oncolytic adenovirus and dacarbazine attenuates antitumor ability against uveal melanoma cells via cell cycle block. Cancer Biol Ther 2012; 13:77-84; PMID:22336909; http://dx.doi.org/10.4161/cbt.13.2.18436
- Quirin C, Mainka A, Hesse A, Nettelbeck DM. Combining adenoviral oncolysis with temozolomide improves cell killing of melanoma cells. Int J Cancer 2007; 121:2801-7; PMID:17724714; http://dx.doi.org/10.1002/ijc.23052
- Jiang G, Wei ZP, Pei DS, Xin Y, Liu YQ, Zheng JN. A novel approach to overcome temozolomide resistance in glioma and melanoma: Inactivation of MGMT by gene therapy. Biochem Biophys Res Commun 2011; 406:311-4; PMID:21329652; http://dx.doi.org/10.1016/j.bbrc.2011.02.042
- Ma S, Egyhazi S, Ueno T, Lindholm C, Kreklau EL, Stierner U, Ringborg U, Hansson J. O6-methylguanine-DNA-methyltransferase expression and gene polymorphisms in relation to chemotherapeutic response in metastatic melanoma. Br J Cancer 2003; 89:1517-23; PMID:14562026; http://dx.doi.org/10.1038/sj.bjc.6601270
- Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, Yoshida J. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res 2005; 65:7573-9; PMID:16140920; http://dx.doi.org/10.1158/0008-5472.CAN-05-0036
- Cabrini G, Fabbri E, Lo Nigro C, Dechecchi MC, Gambari R. Regulation of expression of O6-methylguanine-DNA methyltransferase and the treatment of glioblastoma (Review). Int J Oncol 2015; 47:417-28; PMID:26035292; http://dx.doi.org/10.3892/ijo.2015.3026
- Chen YP, Hou XY, Yang CS, Jiang XX, Yang M, Xu XF, Feng SX, Liu YQ, Jiang G. DNA methylation and histone acetylation regulate the expression of MGMT and chemosensitivity to temozolomide in malignant melanoma cell lines. Tumour Biol 2016; 37:11209-18; PMID:26943799; http://dx.doi.org/10.1007/s13277-016-4994-1
- Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 1999; 59:793-7; PMID:10029064
- Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 2008; 26:4189-99; PMID:18757334; http://dx.doi.org/10.1200/JCO.2007.11.5964
- Rolhion C, Penault-Llorca F, Kemeny JL, Kwiatkowski F, Lemaire JJ, Chollet P, Finat-Duclos F, Verrelle P. O(6)-methylguanine-DNA methyltransferase gene (MGMT) expression in human glioblastomas in relation to patient characteristics and p53 accumulation. Int J Cancer 1999; 84:416-20; PMID:10404096; http://dx.doi.org/10.1002/(SICI)1097-0215(19990820)84:4<416::AID-IJC15>3.0.CO;2-A
- Blough MD, Zlatescu MC, Cairncross JG. O6-methylguanine-DNA methyltransferase regulation by p53 in astrocytic cells. Cancer Res 2007; 67:580-4; PMID:17234766; http://dx.doi.org/10.1158/0008-5472.CAN-06-2782
- Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res 2007; 67:11499-504; PMID:18089777; http://dx.doi.org/10.1158/0008-5472.CAN-07-5312
- Bhakat KK, Mitra S. Regulation of the human O(6)-methylguanine-DNA methyltransferase gene by transcriptional coactivators cAMP response element-binding protein-binding protein and p300. J Biol Chem 2000; 275:34197-204; PMID:10942771; http://dx.doi.org/10.1074/jbc.M005447200
- Zhang H, Wang H, Zhang J, Qian G, Niu B, Fan X, Lu J, Hoffman AR, Hu JF, Ge S. Enhanced therapeutic efficacy by simultaneously targeting two genetic defects in tumors. Mol Ther 2009; 17:57-64; PMID:19018252; http://dx.doi.org/10.1038/mt.2008.236
- Song X, Zhou Y, Jia R, Xu X, Wang H, Hu J, Ge S, Fan X. Inhibition of retinoblastoma in vitro and in vivo with conditionally replicating oncolytic adenovirus H101. Invest Ophthalmol Vis Sci 2010; 51:2626-35; PMID:20007825; http://dx.doi.org/10.1167/iovs.09-3516
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: Combination therapy with oncolytic viruses. Mol Ther 2010; 18:251-63; PMID:20029399; http://dx.doi.org/10.1038/mt.2009.283
- Wennier ST, Liu J, McFadden G. Bugs and drugs: Oncolytic virotherapy in combination with chemotherapy. Curr Pharmaceutical Biotechnol 2012; 13:1817-33; PMID:21740354; http://dx.doi.org/10.2174/138920112800958850
- Perazzoli G, Prados J, Ortiz R, Caba O, Cabeza L, Berdasco M, Gonzalez B, Melguizo C. Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression. PloS One 2015; 10:e0140131; PMID:26447477; http://dx.doi.org/10.1371/journal.pone.0140131
- Jiang H, Alonso MM, Gomez-Manzano C, Piao Y, Fueyo J. Oncolytic viruses and DNA-repair machinery: Overcoming chemoresistance of gliomas. Expert Rev Anticancer Therapy 2006; 6:1585-92; PMID:17134363; http://dx.doi.org/10.1586/14737140.6.11.1585
- McCormick F. Interactions between adenovirus proteins and the p53 pathway: The development of ONYX-015. Semin Cancer Biol 2000; 10:453-9; PMID:11170867; http://dx.doi.org/10.1006/scbi.2000.0336
- Savelyeva I, Dobbelstein M. Infection with E1B-mutant adenovirus stabilizes p53 but blocks p53 acetylation and activity through E1A. Oncogene 2011; 30:865-75; PMID:20935676; http://dx.doi.org/10.1038/onc.2010.461
- Huang X, Wang L, Zhang H, Wang H, Zhao X, Qian G, Hu J, Ge S, Fan X. Therapeutic efficacy by targeting correction of Notch1-induced aberrants in uveal tumors. PLoS One 2012; 7:e44301; PMID:22937170; http://dx.doi.org/10.1371/journal.pone.0044301
- Wang J, Jia R, Zhang Y, Xu X, Song X, Zhou Y, Zhang H, Ge S, Fan X. The role of Bax and Bcl-2 in gemcitabine-mediated cytotoxicity in uveal melanoma cells. Tumour Biol 2014; 35:1169-75; PMID:24014050; http://dx.doi.org/10.1007/s13277-013-1156-6
- Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, Yang Y. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem 2010; 285:40461-71; PMID:20880848; http://dx.doi.org/10.1074/jbc.M110.164046
- Zhao J, Geng YU, Hua H, Cun B, Chen Q, Xi X, Yang L, Li Y. Fenofibrate inhibits the expression of VEGFC and VEGFR-3 in retinal pigmental epithelial cells exposed to hypoxia. Exp Therapeutic Med 2015; 10:1404-12; PMID:26622498; http://dx.doi.org/10.3892/etm.2015.2697