ABSTRACT
Epithelial ovarian cancer (EOC) is the most common and lethal cancer-related death among females in the world. Asparaginyl endopeptidase (AEP) is a member of C13 family peptidases and expressed in the extracellular matrix and tumor cells. The aim of this article is to explore the function of asparaginyl endopeptidase in epithelial ovarian cancer. The expression of AEP was examined in 20 EOC samples, 3 EOC metastasis samples, 6 fallopian tube metastasis samples, 4 peritoneum metastasis samples and 20 benign ovarian tumor samples by immunohistochemistry. The expression of AEP was also evaluated in serum and ascites of EOC patients by elisa. And we used a lentiviral vector to overexpress AEP in human epithelial ovarian cancer cell lines SKOV3ip and detected the function of AEP-SKOV3ip cells both in vitro and in vivo. The growth of AEP-SKOV3ip cells was observed by MTT, migration and tube formation assays in vitro. Additionally, the subcutaneous mice model was used to identify the tumor growth and metastasis in vivo. Mice tumors were stained for CD31 to determine the microvessel density (MVD). We demonstrated that AEP was highly expressed in the EOC patient tissues and ascites. The AEP transfected SKOV3ip cells could both promote tumor growth in vitro and in vivo. The MVD in AEP-SKOV3ip group was higher than that in NC-SKOV3ip group. Therefore, our results demonstrated that AEP could induce EOC growth and progressionboth in vitro and in vivo.
Introduction
Epithelial ovarian cancer (EOC) is the most common and lethal cancer-related death among females in the world.Citation1 Both environmental and genetic factors can lead to the progression of EOC. EOC patients always have poor prognosis.Citation2–4 The 5 y survival rate of the EOC patients in the early stage (FIGO I and II) is about 70%, and the 5 y survival rate of the patients in the advanced stage (FIGO III and IV) is about 30%.Citation1 However, only about 19% EOC patients were diagnosed in the early stage due to the lack of diagnostic technique and high metastasis rate.Citation5 Thus, exploring the underlying mechanism of EOC progression is important for early diagnosis, aggressive treatment and novel target therapy.
Asparaginyl endopeptidase (AEP) is a member of C13 family peptidases and specificity for asparagines bond cleavage.Citation6 AEP is made as an inactive zymogen and its activation requires sequential removal N- and C-terminal propeptidase from pro-AEP. It is a lysosomal protease and is identified in the lysosome. AEP plays an important role in kidney physiology.Citation7 AEP is also expressed in the extracellular matrix and tumor cells. Recently, it is reported that AEP is highly expressed in many solid tumors, such as hepatocellular cancer, gastric cancer, breast cancer, colorectal cancer and lung cancer.Citation8-13 However, the mechanism of AEP in epithelial ovarian cancer remains unknown.
In the current study, we found that AEP were highly expressed in the EOC tissues than benign ovarian tumor tissues. Considering the high expression of AEP in EOC, we hypothesized that AEP might lead to EOC progression. Thus, we performed the present study that overexpression of AEP in epithelial ovarian cancer cell lines SKOV3ip to explore its function in EOC development both in vitro and in vivo.
Results
The expression of AEP in EOC patient samples was significantly higher than those in benign ovarian tumor patient samples
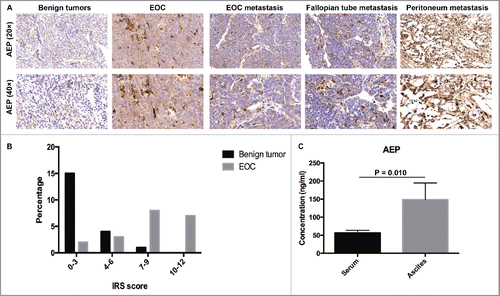
Twenty tissue sections from EOC patients, 3 tissue sections from EOC metastasis, 6 tissue sections from fallopian tube metastasis, 4 tissue sections from peritoneum metastasis and 20 tissue sections from benign ovarian tumor patients were evaluated through immunohistochemistry to analyze the expression of AEP (). To evaluate the expression of AEP, we use IRS scoring system(IRS = SI(stainingintensity)×PP(percentage of positive cells)).Citation14 The percentage in the benign ovarian tumor patient samples and EOC patient samples was shown in . The expression of AEP was confirmed significantly higher in EOC tissues and metastasis tissues compared with those in benign ovarian tumor tissues ( and ). Moreover, the expression of AEP was higher in ascites (148.71 ± 46.02) than in serum of EOC patients (56.43 ± 7.22, p = 0.010, Mann-Whitney U Test) (). Thus, the result might suggest that the expression of AEP was associated with EOC progression.
Figure 1. The expression of AEP in EOC patient samples was higher than those in benign ovarian tumor patient samples. (A) AEP was stained in the benign ovarian tumor, EOC and EOC metastasis tissues by immunohistochemistry. (B) Representative IHC staining of AEP protein levels in EOC tissues (n = 20), EOC metastasis (n = 3), fallopian tube metastasis (n = 6), peritoneum metastasis (n = 4) and benign ovarian tumor tissues (n = 20). IRS scoring system was used to evaluate the expression of AEP. (C) The expression of AEP was detected in serum and ascites of EOC patients by Elisa.
The establishment of AEP-SKOV3ip cells
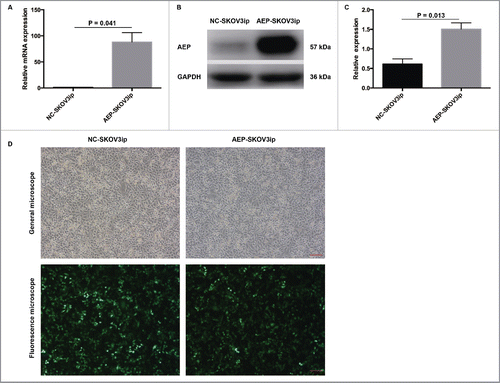
To investigate the AEP function in EOC, an AEP-overexpressing SKOV3ip cells was generated in vitro. The transfection efficiency of AEP-SKOV3ip cells was detected by Real-time PCR and western blot (). The expression of AEP was significantly higher in AEP-SKOV3ip cells (88.08 ± 18.22) than that in NC-SKOV3ip cells (1.00 ± 0.00, p = 0.041, Levene's Test) () by Real-time PCR. The same result was observed in Western blot ( and ). The relative expression of AEP was significantly higher in AEP-SKOV3ip cells (1.50 ± 0.16) than that in NC-SKOV3ip cells (0.61 ± 0.13, p = 0.013, Levene's Test) (). The transfection efficiency in SKOV3ip cells was detected under a fluorescence microscope after 24 hours (). The establishment of AEP-HO8910 was shown in the Fig. S1.
Figure 2. The establishment of AEP-SKOV3ip cells by stable transfection. (A) The transfection efficiency of AEP-SKOV3ip cells was measured by Real-time PCR. (B) The transfection efficiency of AEP-SKOV3ip cells was measured by Western Blot. (C) Quantitative data for the western blot was measured by ImageJ software. (D) The transfection efficiency in AEP-SKOV3ip-GFP cells and NC-SKOV3ip-GFP cells were detected under a fluorescence microscope. Scale bar, 100μm.
Overexpressed AEP in human ovarian cancer cell lines SKOV3ip and the proliferative ability changed
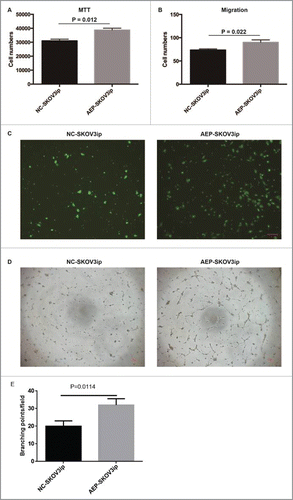
To examine whether AEP-SKOV3ip cells would promote growth potential, we evaluated the cell proliferation and migration of AEP-SKOV3ip cells and NC-SKOV3ip cells by MTT assay and migration assay. The cell numbers of AEP-SKOV3ip cells (38736.20 ± 1313.99) was increased faster as compared with NC-SKOV3ip cells (31000.97 ± 1211.08, p = 0.012, Levene's Test, ) by MTT assay. The cell numbers of AEP-SKOV3ip cells (90.00 ± 5.33) was more than NC-SKOV3ip cells (73.50 ± 2.38, p = 0.022, ANOVA, and ) by migration assay. Next, Huvec tube formation assay were performed and the supernatant of AEP-SKOV3ip cells could increase the tube forming capacity of HUVECs( and ). These results demonstrated that the expression of AEP would effect on the proliferation of SKOV3ip cells in vitro. The results of AEP-HO8910 were similar to the AEP-SKOV3ip cell line. Data were shown in the Fig. S2.
Figure 3. Overexpressed AEP in human SKOV3ip cells and the proliferative ability changed. (A) The cell numbers of AEP-SKOV3ip cells was increased faster as compared with NC-SKOV3ip cells by MTT assay. (B) The cell numbers of AEP-SKOV3ip cells was more than NC-SKOV3ip cells by migration assay. (C) Migration capacity of AEP-SKOV3ip cells was performed using transwell assay. (D) The supernatant of AEP-SKOV3ip cells could increase the tube forming capacity of HUVECs by tube formation assay. (E) Grapical illustration of the AEP-SKOV3ip cells on the branching points of the capillary-like structures in HUVECs. Scale bar, 100µm.
AEP-SKOV3ip cells promotes growth potential in vivo
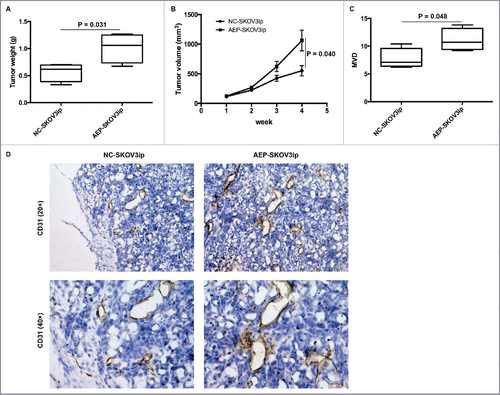
We established a subcutaneous mice model (N = 4) to investigate the function of AEP expression in vivo. The mice from AEP-SKOV3ip group and NC-SKOV3ip group were injected with 0.20 ml of DMEM containing 1.5 × 106 cells of AEP-SKOV3ip cells or NC-SKOV3ip cells. The AEP-SKOV3ip group demonstrated increased tumor weights (g) and volumes (mm3) in mice model. The tumor weights of the AEP-SKOV3ip group (1.02 ± 0.14) g were significantly higher than those of the NC-SKOV3ip group (0.57 ± 0.08) g at the time of sacrifice (p = 0.031, ANOVA, ). Moreover, the tumor volumes of AEP-SKOV3ip group (1062.38 ± 175.04) mm3 were also significantly higher than those of the NC-SKOV3ip group (550.99 ± 86.76) mm3 (p = 0.040, ANOVA, ) in the 4th week. Further, we also analyzed the microvessel density (MVD) in tumors to evaluate whether the tumors of AEP-SKOV3ip would promote angiogenesis. We used CD31 staining in the tumor tissues. The microvessel density of the AEP-SKOV3ip group (11.10 ± 1.01) was higher than that of the NC-SKOV3ip group (7.70 ± 0.93) in mice tumor sections (p = 0.048, ANOVA, and ) at ×200 magnifications. Therefore, the data showed that the expression of AEP would promote the growth and progression of EOC in vivo.
Figure 4. AEP-SKOV3ip cells promote growth potential in mice subcutaneous model. (A) The tumor weights (g) of AEP-SKOV3ip group were significant heavier than that of NC-SKOV3ip group. (B) The tumor volumes of AEP-SKOV3ip group were larger than that of NC-SKOV3ip group. (C) The MVD of AEP-SKOV3ip group was significant higher than that of NC-SKOV3ip group. (D) The expression of MVD in AEP-SKOV3ip and NC-SKOV3ip group was evaluated by immunohistochemistry at ×200 magnifications. Scale bar, 100µm.
Discussion
Epithelial ovarian cancer is the leading cause of cancer deaths in women in the world.Citation15,16 EOC is often treated with chemotherapy and surgery. However, the above therapeutic methods are limited to the most patients since patients are usually diagnosed in the advanced stage and chemoresistance.Citation17-19 Thus, the clinical biomarker of EOC will play an important role in judging the ideal therapeutic method and time.
Asparaginyl endopeptidase (AEP), which is also called legumain (LGMN), is in the cleaving substrates N-terminally of asparagines.Citation20 AEP has many functions, such as modulating fibronectin degradation, processing antigens, participating in tumor associated macrophage function and activating matrix metalloproteinases.Citation11,13,21,22 AEP has recently captured the attention of researchers since some reports have identified that it is expressed higher in many solid tumor tissues compared with normal tissues.Citation9,10 Moreover, some studies have reported that AEP was related to tumor cell proliferation.Citation8,23 And blocking of AEP with antibody was reported to inhibit tumor progression and metastasis. Once AEP combined with its substrate recognition, it could be upregulated in solid tumors. This made AEP a viable candidate for prognostic evaluation and a hot spot for tumor treatment. However, the function of AEP in EOC remains elusive.
In the present study, we confirmed that higher levels of asparaginyl endopeptidase were expressed in the EOC patient tissues compared with benign ovarian tumor tissues. Moreover, the expression of AEP was higher in ascites than in serum of EOC patients. This is similar to some previous reports.Citation10 This result might suggest AEP would be a prognostic biomarker in EOC. And based on this hypothesis, we detected that the high expression of AEP could enhance the ability of SKOV3ip cells progression both in vitro and in vivo. We found that the AEP-SKOV3ip cells increased the proliferation and migration ability in vitro. The supernatant of AEP-SKOV3ip cellscould also increase the tube forming capacity of HUVECs. Moreover, the tumor weights of AEP-SKOV3ip group were heavier compared with the NC-SKOV3ip group. The tumor volumes of AEP-SKOV3ip group were larger compared with the NC-SKOV3ip group. We further demonstrated that the MVD of tumors in the AEP-SKOV3ip group was more than that in the NC-SKOV3ip group. Our research identified that the high expression of AEP might increase EOC progression.
Taken together, our study helped to clarify that the expression of AEP might promote EOC growth and progression, which suggested that AEP might play a vital role in EOC early diagnosis and might be a potential target for EOC novel therapies.
Materials and methods
Patients
Twenty EOC samples, 3 EOC metastasis samples, 6 fallopian tube metastasis samples, 4 peritoneum metastasis samples and 20 benign ovarian tumor samples were obtained from Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine (Shanghai, China) between Jan 2014 and 2015. The ethical approval for the study was approved by the Institutional Review Board of Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine.
Immunohistochemistry
AEP and CD31 were evaluated by immunohistochemistry. To block endogenous peroxide activity and non-specific binding, tumor tissues were incubated in 3% hydrogen peroxide and 5% BSA (SIGMA, USA). Tumor tissues were incubated with primary antibodies (Goat anti-human AEP 1: 150, R&DSystems, USA, rabbit anti-mouse CD31 1:70, ABCAM, USA) overnight at 4°C. After that, secondary antibody (donkey anti-goat IgG, Proteintech, CHINA, goat anti-rabbit IgG, VECTOR, USA) and DAB kits (VECTOR, USA) were added into the tissues. All the reagents were used except the AEP and CD31 for controls. The AEP and CD31 were evaluated in the magnification of 200× and 400× microscopically.
ELISA assays
Blood serum samples of 5 healthy persons and 5 EOC patients' ascites samples were collected to detect AEP in blood serum and ascites. The quantification of AEP expression levels was evaluated by ELISA according to the manufacturer's instructions (Uscn Life Science Inc., CHINA).
Cell lines
The human ovarian cancer cell line SKOV3ip was obtained from ATCC and cultured in Dulbecco's Modified Eagle Medium with 10% FBS (GIBCO, USA). The SKOV3ip cells were transfected withtherecombinant AEP-expression lentivirus (Lentiviral vectors carrying a green fluorescent protein GFP sequence, Hanyin Co. Shanghai, China) andthe negative control lentivirus (NC-lentivirus; Hanyin Co. Shanghai,China) in the presence of 5µg/ml polybrene for 8 hours. Then the medium was replaced by DMEM with 10% FBS. The AEP-transfected SKOV3ip cells and mock-transfected SKOV3ip cells were incubated in 37°C with 5% CO2.
Real-time PCR
The RNA was extracted from AEP-SKOV3ip cells and NC-SKOV3ip cells by Trizol. The TAKARA Retroviral Reverse Transcriptase Kit (Takara, Dalian, China) was then used to synthesize cDNA according to the manufacturer's instruction. Primers were designed as: Forward primer:, 5′-AACCCCAGAGAGTCGTCCTAC-3′; Reverse primer, 5′-ATCCAGTTGACGCTGTACCAG-3′ for AEP gene.
Western blot
AEP-SKOV3ip cells and NC-SKOV3ip cells were treated with total protein lysis buffer (Beyotime, China). Detection of AEP was performed with primary antibody against AEP (1:1000, R&DSystems, USA) by Western blot.
MTT Assay
The AEP-SKOV3ip cells and NC-SKOV3ip cells at a density of 3000 cells/well were seeded in the 96-well plates for 48 hours. A3-(4,5-dimethylthazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) solution (30µl, 5 mg / ml, Sigma–Aldrich) was added to the wells and incubated for 3 hours at 37°C. After that, dissolving the remaining crystals in DMSO. The absorbance was measured at 490 nm using a Thermo Scientific Multiskan. Draw the standard curve by cell numbers and absorbance. Then measured the cells number by the standard curve. (Thermo Fisher Scientific, USA). The MTT assays had been performed for 3 times.
Cell migration assay
Cell migration was detected using transwell filters (BD Biosciences, USA). AEP-SKOV3ip cells (10 × 104) and NC-SKOV3ip cells (10 × 104) in 200μL DMEM medium without serum were added into the upper chamber,800μL medium with 10% FBS (Fetal bovine serum) was in the lower chamber. The cells migrated into the lower compartment and stained with Giemsa for 1 hour after incubation in a humidified 5% CO2 incubator at 37°C for 16 hours. The migrated cells were counted in random 5 fields. Cell counting was detected under a Leica microscope (Germany).
Tube formation assay
AEP-SKOV3ip cells and NC-SKOV3ip cells were cultured as above. The culture mediums were changed into serum-free DMEM when AEP-SKOV3ip cells and NC-SKOV3ip cells were reached 80% confluence. The supernatants were collected and stored at −20°C. HUVECs were suspended in the density of 3000cells/100μl in the supernatants and added into matrigel (BD Biosciences, USA)-coated membrane. Tube images were taken with a microscope. The quantitative assessment of tube formation assay was evaluated via measuring the branching points using the Scion Image analysis program.
The subcutaneous animal model of EOC
Female nude mice (5 weeks old, n = 4) weighing about 18 g were purchased from Tongji University School of Medicine. The nude mice were housed with a constant humidity of 70% and a room temperature of 20°C. They were bred under SPF conditions. The animal protocol was approved by the Institutional Review Board of Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine. The mice were divided into 2 groups as: AEP-SKOV3ip group and NC-SKOV3ip group. The mice from each group were injected with 0.20 ml of DMEM containing 1.5 × 106 cells of AEP-SKOV3ip cells or NC-SKOV3ip cells to establish a subcutaneous model. The nude mice were evaluated weekly for tumor growth. The mice were killed in 4 weeks after cell injection. The tumor weights and diameters were recorded. The volume of the tumors was analyzed using the formula: V = 1/2×length×width2. Tumors were prepared for CD31 immunohistochemical staining.
Statistical analysis
Statistical significance of data were analyzed with one-way analysis of variance (ANOVA), the Levene's test and Mann-Whitney U test using SPSS 20.0. Data and figures were presented as Mean ± Standard error of the mean (SEM). Two-sided of P values < 0.05 was considered to be statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Figures.zip
Download Zip (3.3 MB)Funding
This work was supported by grants from the National Natural Science Foundation of China (81372787, 81072136), the Shanghai Municipal Bureau of Health (20134033), the Shanghai Health and Family Planning Committee research project(20164Y0024) and the Shanghai Health System joint research project (2013ZYJB0201).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5-29; PMID:25559415; http://dx.doi.org/10.3322/caac.21254
- Kwon JS. Improving survival after endometrial cancer: the big picture. J Gynecol Oncol 2015; 26:227-31; PMID:26197859; http://dx.doi.org/10.3802/jgo.2015.26.3.227
- Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol 2013; 129:258-64; PMID:23266352; http://dx.doi.org/10.1016/j.ygyno.2012.12.016
- Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011; 474:609-15; PMID:21720365; http://dx.doi.org/10.1038/nature10166
- Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet Gynecol 2015; 126:491-7; PMID:26244529; http://dx.doi.org/10.1097/AOG.0000000000000981
- Cui Y, Wang Y, Li H, Li Q, Yu Y, Xu X, Xu B, Liu T. Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget 2016; 7(23):34356-70; PMID:27102302
- Miller G, Matthews SP, Reinheckel T, Fleming S, Watts C. Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J 2011; 25:1606-17; PMID:21292981; http://dx.doi.org/10.1096/fj.10-172312
- Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM, Robert M, Nathanson MH, Leite F. Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol 2011; 55:626-35; PMID:21237226; http://dx.doi.org/10.1016/j.jhep.2010.12.022
- Guo P, Zhu Z, Sun Z, Wang Z, Zheng X, Xu H. Expression of legumain correlates with prognosis and metastasis in gastric carcinoma. PloS One 2013; 8:e73090; PMID:24023813; http://dx.doi.org/10.1371/journal.pone.0073090
- Li N, Liu Q, Su Q, Wei C, Lan B, Wang J, Bao G, Yan F, Yu Y, Peng B, et al. Effects of legumain as a potential prognostic factor on gastric cancers. Med Oncol 2013; 30:621; PMID:23740003; http://dx.doi.org/10.1007/s12032-013-0621-9
- Lin Y, Qiu Y, Xu C, Liu Q, Peng B, Kaufmann GF, Chen X, Lan B, Wei C, Lu D, et al. Functional role of asparaginyl endopeptidase ubiquitination by TRAF6 in tumor invasion and metastasis. J Natl Cancer Inst 2014; 106:dju012; PMID:24610907; http://dx.doi.org/10.1093/jnci/dju012
- Haugen MH, Boye K, Nesland JM, Pettersen SJ, Egeland EV, Tamhane T, Brix K, Maelandsmo GM, Flatmark K. High expression of the cysteine proteinase legumain in colorectal cancer - implications for therapeutic targeting. Euro J Cancer 2015; 51:9-17; PMID:25466510; http://dx.doi.org/10.1016/j.ejca.2014.10.020
- Lin Y, Wei C, Liu Y, Qiu Y, Liu C, Guo F. Selective ablation of tumor-associated macrophages suppresses metastasis and angiogenesis. Cancer Sci 2013; 104:1217-25; PMID:23691974; http://dx.doi.org/10.1111/cas.12202
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015; 527:100-4; PMID:26479035; http://dx.doi.org/10.1038/nature15376
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/10.1002/ijc.25516
- Zhang W, Barger CJ, Eng KH, Klinkebiel D, Link PA, Omilian A, Bshara W, Odunsi K, Karpf AR. PRAME expression and promoter hypomethylation in epithelial ovarian cancer. Oncotarget 2016; 7(29):45352-69; PMID:27322684; http://dx.doi.org/10.18632/oncotarget.9977
- Foley OW, Rauh-Hain JA, del Carmen MG. Recurrent epithelial ovarian cancer: an update on treatment. Oncology 2013; 27:288-94, 298; PMID:23781692
- Ribeiro JR, Schorl C, Yano N, Romano N, Kim KK, Singh RK, Moore RG. HE4 promotes collateral resistance to cisplatin and paclitaxel in ovarian cancer cells. J Ovarian Res 2016; 9:28; PMID:27184254; http://dx.doi.org/10.1186/s13048-016-0240-0
- Hou MM, Wang Z, Janku F, Piha-Paul S, Naing A, Hong D, Westin S, Coleman RL, Sood AK, Tsimberidou AM, et al. Continuous anti-angiogenic therapy after tumor progression in patients with recurrent high-grade epithelial ovarian cancer: phase I trial experience. Oncotarget 2016; 7(23):35132-43; PMID:27147567; http://dx.doi.org/10.18632/oncotarget.9048
- Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, Hewitt E, Watts C, Barrett AJ. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem 1997; 272:8090-8; PMID:9065484; http://dx.doi.org/10.1074/jbc.272.12.8090
- Morita Y, Araki H, Sugimoto T, Takeuchi K, Yamane T, Maeda T, Yamamoto Y, Nishi K, Asano M, Shirahama-Noda K, et al. Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett 2007; 581:1417-24; PMID:17350006; http://dx.doi.org/10.1016/j.febslet.2007.02.064
- Sepulveda FE, Maschalidi S, Colisson R, Heslop L, Ghirelli C, Sakka E, Lennon-Duménil AM, Amigorena S, Cabanie L, Manoury B. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity 2009; 31:737-48; PMID:19879164; http://dx.doi.org/10.1016/j.immuni.2009.09.013
- Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, et al. A census of human soluble protein complexes. Cell 2012; 150:1068-81; PMID:22939629; http://dx.doi.org/10.1016/j.cell.2012.08.011
