ABSTRACT
Increasing evidence demonstrated that long non-coding RNA ANRIL serves as a fatal oncogene in many cancers, including nasopharyngeal carcinoma (NPC). However, little is known whether ANRIL regulated NPC cell radioresistance. Quantitative real-time PCR (qRT-PCR) was performed to examine the expression of lncRNA ANRIL and miR-125a in NPC tissues and cell lines. MTT assay was conducted to measure the cell viability of CNE2 and HONE1 cells. The apoptotic rate of CNE2 and HONE1 cells was determined by flow cytometry analysis. Colony survival was determined by clonogenic assay. Luciferase reporter assay was performed to verity the direct target of miR-125a. LncRNA ANRIL was evidently elevated in NPC tissues and cell lines. ANRIL inhibition suppressed proliferation, induced apoptosis, and enhanced radiosensitivity in NPC. Moreover, ANRIL could negatively modulate miR-125a expression. Furethermore, ANRIL upregulation reserved the inhibited proliferation, induced apoptosis, and enhanced radiosensitivity triggered by miR-125a overexpression. The expression of lncRNA ANRIL was upregulated in NPC tissues and cells. Moreover, knockdown of ANRIL repressed proliferation, promoted apoptosis, and improved radiosensitivity in NPC via functioning as a miR-125a sponge.
Introduction
Nasopharyngeal carcinoma (NPC) is a highly metastatic and invasive malignancy, prevalent in South Asia, North Africa and Southern China.Citation1 So far, radiotherapy is still the preferred treatment of NPC patients. Although great advances have been made in radiation techniques and radiotherapeutic strategies, the outcomes in NPC patients remain unsatisfactory, which results from the radioresistance. Increasing literatures have shown that some RNA and protein molecules could influence radioresistance by regulating radioresistance-associated processes, including cell cycle arrest, apoptosis, DNA repair capacity, epithelial to mesenchymal transition and protective autophagy.Citation2 However, the precise molecular mechanisms underlying radioresistance in NPC remain largely unknown. Therefore, it is still urgent to identify molecules involved in NPC radioresistance, providing novel therapeutic targets for NPC patients.
Long non-coding RNAs (lncRNAs) are transcripts greater than 200 nucleotides in length without obvious protein-coding functions.Citation3 LncRNAs play vital regulatory roles in several biologic processes, including genomic imprinting, gene expression, cell metastasis, and so on.Citation4 Accumulating evidence indicates that lncRNAs are commonly deregulated in the malignant transformation and progression of various cancers, including NPC.Citation5-7 These lncRNAs function as tumor suppressors or oncogenes in cancers.Citation8,9 Recently, Ren et al. identified a novel lncRNA n375709 by next generation deep sequencing, which was overexpressed and led to paclitaxel resistance in NPC.Citation10 LncRNA ANRIL (CDKN2B antisense RNA 1) was located a 42-kb stretch on the chromosome 9p21, which was originally identified from familial melanoma patients with germline deletion in the INK4B-ARFINK4A gene cluster.Citation11,12 Subsequently, accumulating documents have indicated that ANRIL is deregulated in various malignancies, such as lung cancer, breast cancer, gastric cancer.Citation13-15 Moreover, a recent study demonstrated that ANRIL was overexpressed in NPC cell lines and NPC tissues and promoted NPC progression through improving cell proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells.Citation16 Growing evidence suggests that ANRIL functions as a fatal oncogene in many cancers, however, limited knowledge is available concerning whether lncRNA ANRIL affects the radiosensitivity of NPC.
MicroRNAs (miRNAs) are a class of small non-coding RNAs, which could regulate the post-transcriptional level of gene expression through binding to target mRNAs, leading to target mRNA degradation or translation suppression.Citation17 Many miRNAs have been showed to be abnormally expressed in NPC, such as miR-21, miR-26a, miR-1 and miR-125a, which play vital roles in many processes of NPC carcinogenesis, including cell proliferation, invasion and angiogenesis.Citation18-21 Consequently, miRNAs could serve as critical therapeutic targets for NPC treatment.Citation22 miR-125a is located on chromosomes 19, 11 and 21. Previous studies have demonstrated that miR-125a is involved in the proliferation, apoptosis, migration and invasion in numerous cancers. Moreover, miR-125a was crucial for paclitaxel sensitivity in colon cancerCitation23 and cisplatin sensitivity in NPC.Citation24 However, whether miR-125a could affect the radiosensitivity in cancers has not been studied.
In the present study, we aimed to explore the function and underlying molecular mechanism of lncRNA ANRIL in NPC.
Materials and methods
Tissue specimens and cell culture
Thirty-five NPC patient tissue samples and 35 normal nasopharyngeal tissue samples were obtained from Huaihe Hospital of Henan University. This study was approved by the Huaihe Hospital Ethic Review Committees.
The human nasal epithelial cell (HNEpC) and nasopharyngeal carcinoma cell lines 5–8F, CNE1, CNE2 and HONE1 was derived from the American Type Culture Collection (ATCC). All cells were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) (Invitrogen) and 1% penicillin/streptomycin (Invitrogen) in a humidified 5% CO2 incubator at 37°C.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the tissue samples and NPC cell lines using the TRIzol reagent (Invitrogen). The cDNA of ANRIL was generated using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). MiR-125a cNDA was generated by TaqMan miRNA Reverse Transcription Kit (Applied Biosystems). A SYBR Premix Ex Taq™ kit (Takara Bio, Otsu, Japan) was used to examine ANRIL and β-actin expression, while TaqMan miRNA assays (Applied Biosystems) was performed to detect miR-125a and U6 expression. qRT-PCR was conducted on the 7500 Real Time PCR System (Applied Biosystems).
Cell transfection
ANRIL was amplified from the cDNA of CNE2 and HONE1 cells and cloned into the pcDNA3.1 plasmid. Two ANRIL siRNAs, Si-control, miR-125a mimic and miR-control were purchased from GenePharma (Shanghai, China). Cells transfection was conducted using Lipofectamine 2000 (Invitrogen).
Cell proliferation assay
CNE2 and HONE1 cells were seeded in 96-well plates and cultured for 24 h. After transfection for 24 h, 48 h and 72 h, MTT assay (Sigma, St. Louis, Missouri, USA) was performed to determine the cell viability. The optical density was determined at 450 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA).
Apoptosis assay
CNE2 and HONE1 cells were plated in 6-well plates. At 24 h after transfection, the cells were harvested and stained with Annexin V-FITC and propidium iodide (PI) (BD Biosciences, San Jose, CA, USA). Then, the flow cytometry data was analyzed by BD FACSDiva software V6.1.3 (BD Biosciences).
Clonogenic assay
The cells were seeded into 6-well plates. After 24 h, they were exposed to different radiation doses (0, 2, 4, 6 and 8 Gy). After an incubation period of 14 days, the colonies were fixed with methanol and stained with crystal violet. Colonies of more than 50 cells were counted and analyzed.
Luciferase reporter assays
CNE2 and HONE1 cells were co-transfected with miR-control or miR-125a and the wild type or mutant ANRIL (ANRIL-WT or ANRIL-MUT) reporter plasmid using lipofectamine 2000 reagent (Invitrogen). The luciferase activities were measured using the Dual-Glo luciferase reporter assay kit (Promega, Madison, WI, USA) 48 h post transfection.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL, USA). Results are expressed as means ± SD. Student's t-test and one-way ANOVA were used to evaluate the significance. Differences were considered to be significant when P < 0.05.
Results
The ANRIL level is negatively correlated with miR-125a expression in NPC tumor tissues
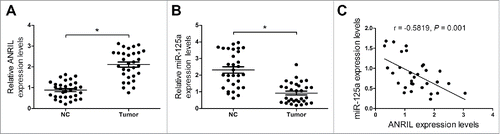
Real-time PCR assay was conducted to detect lncRNA ANRIL and miR-125a expression level in NPC patient samples (n = 35) and normal nasopharyngeal tissue samples (n = 35). The result showed that ANRIL expression was markedly elevated and the expression of miR-125a was significantly decreased in NPC tumor tissues compared with normal tissues (). Moreover, ANRIL expression was negatively correlated with miR-125a expression in NPC tissues ().
Figure 1. Negative Correlation between ANRIL and miR-125a in NPC tumor tissues. (A and B) qRT-PCR analysis of ANRIL and miR-125a expression in NPC patient tumor tissue samples (n = 35) and normal nasopharyngeal tissue samples (n = 35). (C) The ANRIL level and miR-125a expression had a negative correlation in NPC tumor tissues. *P < 0.05 vs. NC.
ANRIL expression is elevated while miR-125a expression is downregulated in NPC cell lines
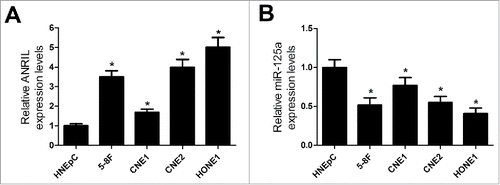
Additionally, the expression level of ANRIL and miR-125a was examined in NPC cell lines (5–8F, CNE1, CNE2 and HONE1) and HNEpC. qRT-PCR analysis revealed that the ANRIL level was evidently increased and miR-125a expression was drastically lowered in all NPC cell lines compared with HNEpC (). All these data suggested that aberrant ANRIL and miR-125a expression may play important roles in NPC tumorigenesis.
Figure 2. ANRIL expression is upregulated and miR-125a expression is downregulated in NPC cell lines. (A) ANRIL expression was examined by qRT-PCR analysis in 5–8F, CNE1, CNE2 and HONE1 cells or HNEpC. (B) qRT-PCR analysis was conducted to determine the expression of miR-125a in 5–8F, CNE1, CNE2 and HONE1 cells or HNEpC. *P < 0.05 vs. HNEpC.
ANRIL knockdown inhibits proliferation of NPC cells
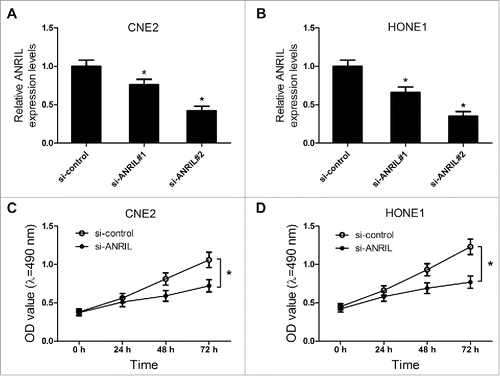
Considering CNE2 and HONE1 cells had higher ANRIL expression among NPC cell lines, we chose CNE2 and HONE1 cells for further study. To explore the function of ANRIL in NPC, the ANRIL knockdown CNE2 and HONE1 cells were established through transfection with si-ANRILs. The interference effect was shown in . It was reported that ANRIL silencing repressed the proliferation of NPC cells.Citation16 In our study, the results of MTT assay also revealed that ANRIL knockdown led to the proliferation inhibition of NPC cells (). All these data revealed that downregulation of ANRIL suppressed proliferation of NPC cells.
Figure 3. ANRIL knockdown inhibits proliferation of NPC cell lines CNE2 and HONE1. CNE2 and HONE1 cells were transfected with si-control or si-ANRIL. (A and B) The level of ANRIL was detected by western blot in CNE2 and HONE1 cells. (C and D) MTT assay was performed to detect the cell viability at 24, 48 and 72 h after transfection in CNE2 and HONE1 cells. *P < 0.05 vs. si-control.
ANRIL knockdown induces apoptosis, and enhances radiosensitivity in NPC cells
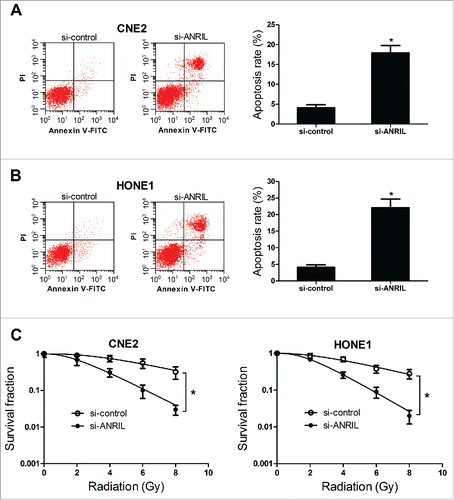
To further explore the effect of ANRIL knockdown on the apoptosis of NPC cells, flow cytometry analysis was performed. The result indicated that the cell apoptosis was induced when knockdown of ANRIL in CNE2 and HONE1 cells (). The colony survival assay is considered as a canonical standard to determine radiosensitivityCitation25 (miR-124 radiosensitizes human glioma cells by targeting CDK4). Therefore, the effect of ANRIL knockdown on the colony survival of NPC cells was explored in the presence of ionizing radiation (IR). Clonogenic assay revealed that si-ANRIL could enhance the radiosensitivity of CNE2 and HONE1 cells (). All these results demonstrated that downregulation of ANRIL induced apoptosis, and enhanced radiosensitivity in NPC cells.
Figure 4. ANRIL downregulation induces apoptosis, and enhances radiosensitivity in NPC cell lines CNE2 and HONE1. CNE2 and HONE1 cells were transfected with si-control or si-ANRIL. (A and B) Cell apoptosis was determined by flow cytometry analysis at 48 h after transfection in CNE2 and HONE1 cells. (C) The colony survival was determined by clonogenic assay in CNE2 and HONE1 cells treated with IR (0, 2, 4, 6 and 8 Gy). *P < 0.05 vs. si-control.
ANRIL inhibits miR-125a expression in NPC cells
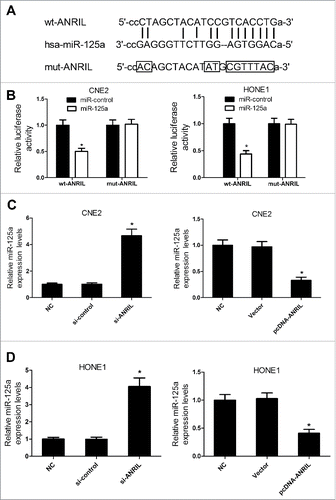
Recent studies indicated that lncRNAs could function as miRNA sponges and modulated miRNAs expression. miRNAs with the binding site of ANRIL were predicted by starBase v2.0. As shown in , miR-125a was a predicted target of ANRIL. Then, luciferase reporter assay was conducted to confirm whether the miR-125a binding site on ANRIL was functional. The results suggested that co-transfected with miR-125a and ANRIL-WT strongly reduced the luciferase activity, while other groups did not change the luciferase activity (). Further, qRT-PCR analysis was performed to explore the effect of ANRIL on miR-125a expression. ANRIL overexpression led to the reduction in miR-125a expression while downregulation of ANRIL caused the increased miR-125a expression in CNE2 and HONE1 cells (). Taken together, these results revealed that ANRIL suppressed miR-125a expression in NPC cells.
Figure 5. ANRIL represses the miR-125a expression in CNE2 and HONE1 cells. (A) Putative miR-125a binding sequence of ANRIL was shown. (B) The relative luciferase activity was detected in CNE2 and HONE1 cells co-transfected with miR-125a mimic or miR-control and wt or mut ANRIL. (C and D) qRT-PCR analysis of miR-125a expression in si-ANRIL or pcDNA-ANRIL transfecting CNE2 and HONE1 cells. *P < 0.05 vs. controls.
ANRIL reverses the effect of miR-125a on proliferation, apoptosis, and radiosensitivity of NPC cells
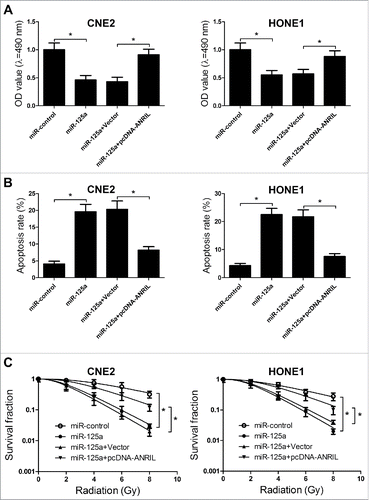
Considering that ANRIL could repress the expression of miR-125a, we further confirmed whether ANRIL exerted its function in NPC by regulating miR-125a expression. MTT assay suggested that miR-125a overexpression resulted in the proliferation inhibition in CNE2 and HONE1 cells, while the inhibitory effect of miR-125a was abolished when ANRIL expression was upregulated (). Moreover, flow cytometry analysis revealed that overexpressed miR-125a could induce the cell apoptosis of CNE2 and HONE1 cells, which was reversed by ANRIL overexpression (). Furthermore, clonogenic assay illuminated that restoration of miR-125a expression enhanced the radiosensitivity of CNE2 and HONE1 cells, however, co-transfected miR-125a and pcDNA-ANRIL could inversed the enhancement effect of miR-125a on radiosensitivity (). All these results demonstrated that downregulation of ANRIL inhibited proliferation, induced apoptosis, and enhanced radiosensitivity of NPC cells through negatively modulating miR-125a expression.
Figure 6. Knockdown of ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in NPC cells through negatively regulating miR-125a expression. CNE2 and HONE1 cells were were transfected with miR-125a or co-transfected with pcDNA-ANRIL and miR-125a. (A) MTT assay was performed to determine the cell viability of CNE2 and HONE1 cells. (B) Flow cytometry analysis was performed to measure the cell apoptosis of CNE2 and HONE1 cells at 48 h after transfection. (C) Clonogenic assay was conducted to determine the colony survival in CNE2 and HONE1 cells treated with IR (0, 2, 4, 6 and 8 Gy). *P < 0.05 vs. controls.
Discussion
Recently, emerging evidence suggested that aberrant expression of lncRNA ANRIL may meaningfully affect the cell proliferation and apoptosis of tumor cells.Citation26 NPC is a highly metastatic and invasive malignancy and is usually resistant to radiotherapy. Therefore, more efforts are urgently needed to elucidate the mechanisms of radioresistance and provide effective molecular therapy targets to sensitize NPC cells to radiotherapy. However, current knowledge about the function of lncRNA ANRIL in NPC is still preliminary. Therefore, defining whether lncRNA ANRIL is essential for NPC tumorigenesis and radioresistance may provide a promising therapeutic target. Our study found that lncRNA ANRIL expression was significantly elevated in NPC tissues and cell lines when compared with those in nasopharyngeal tissues and HNEpC. Functional and mechanistic investigation demonstrated that silencing of lncRNA ANRIL suppressed proliferation, induced apoptosis, and enhanced radiosensitivity in NPC cells via functioning as a miRNA sponge to negatively modulate miR-125a expression.
Increasing data have indicated that LncRNA ANRIL was deregulated and functioned as an oncogene in various cancers.Citation26 Silencing ANRIL expression usually suppressed cell proliferation, migration and invasion and induced apoptosis in many cancers. For instance, Zhang et al.Citation15 confirmed that ANRIL was overexpressed in gastric cancer, which promoted cancer cell proliferation by epigenetic suppression of miR-449a/miR-99a through binding to PRC2. In non-small cell lung cancer (NSCLC), ANRIL was upregulated and knockdown of ANRIL suppressed NSCLC cell migration, proliferation and invasion and promoted apoptosis.Citation27,28 Downregulation of ANRIL repressed cell proliferation by inhibiting Bcl−2 expression and induced cell apoptosis via promoting expression of PARP, cleaved caspase-9, Bax and Smac and caspase-3, and the cytoplasmic cytochrome c in bladder cancer.Citation29 Moreover, another study revealed that depletion of ANRIL blocked ovarian cancer cell invasion and migration, which was mediated by MMP3 and MET.Citation30 Parallelly, our study found that ANRIL was extremely elevated in NPC tissues and cell lines, and knockdown of ANRIL suppressed proliferation and induced apoptosis of NPC cells. In agreement with our results, Zou et al. revealed that ANRIL was upregulated in NPC cell lines and tissues, and ANRIL inhibition suppressed proliferation of NPC cells.Citation16 Moreover, our study also demonstrated that downregulation of ANRIL enhanced radiosensitivity of NPC cells. To the best of our knowledge, this was the first study revealed that ANRIL was involved in the radioresistance of cancers. Coincidentally, another lncRNA, LincRNA-RoR, was confirmed to promote the radioresistance in human colorectal cancer through the negative regulation of p53/miR-145.Citation31 Moreover, a recent study revealed that upregulation of lncRNA HOTAIR enhanced breast cancer radioresistance by targeting HOXD10.Citation32 All the studies suggested that lncRNAs exerted important role in the regulation of cancer radioresistance, which opened up a new avenue for investigating the molecular mechanisms underlying radioresistance in cancers.
Recent studies have demonstrated that lncRNAs could negatively regulate miRNA expression by acting as miRNA sponges.Citation33 Although ANRIL was predicted to harbor the binding sites of many miRNAs. we focused on miR-125a because of its inverse expression and function compared with ANRIL in NPC as reported in previous studies.Citation16,34 In the present study, the luciferase activity assay confirmed the direct binding of miR-125a to ANRIL. Moreover, functional analysis revealed that ANRIL suppressed miR-125a expression. Furthermore, ANRIL overexpression reversed the inhibited proliferation, promoted apoptosis, and enhanced radiosensitivity caused by miR-125a overexpression. All these data demonstrated that knockdown of lncRNA ANRIL suppressed proliferation, promoted apoptosis, and enhanced radiosensitivity in NPC through acting as a sponge to negatively modulate miR-125a expression at the post-transcriptional level. However, previous studies indicated that lncRNA ANRIL could negatively regulate miR-99a/miR-449a expression in epigenetic level by binding to PRC2.Citation15,35 All these data revealed that lncRNA ANRIL could modulate miRNA expression through various mechanisms. In a previous study, lncRNA MALAT1 knockdown was confirmed to enhance the radiosensitivity of NPC cells by sponing miR-1.Citation36 Moreover, downregulation of lncRNA NEAT1 sensitized NPC cells to radiation through negatively modulating miR-204 expression.Citation37 These studies together with ours illuminated that lncRNAs could function as miRNA sponges to modulate the radioresistance in NPC, which may be underlying therapeutic targets for NPC radioresistance.
In summary, our findings suggested that ANRIL was overexpressed in NPC tissues and cell lines. Moreover, knockdown of ANRIL inhibited proliferation, promoted apoptosis, and enhanced radiosensitivity in NPC via negatively modulating miR-125a. Furthermore, our data suggested that targeting the ANRIL/miR-125a axis may be a novel therapeutic application to NPC radioresistance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Huijuan Jiang designed and performed the experiment. Xiaojun Jiang analyzed the data. Xigang Hu supervised the study and wrote the manuscript.
References
- Yoshizaki T, Ito M, Murono S, Wakisaka N, Kondo S, Endo K.. Current understanding and management of nasopharyngeal carcinoma. Auris Nasus Larynx 2012; 39:137-44; https://doi.org/10.1016/j.anl.2011.02.012
- Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thoraic Dis 2017; 9:849-59; https://doi.org/10.21037/jtd.2017.03.23
- Yu X, Li Z. Long non-coding RNA growth arrest-specific transcript 5 in tumor biology (Review). Oncol Lett 2015; 10:1953-8; PMID:26622780; https://doi.org/10.3892/ol.2015.3553
- Autuoro JM, Pirnie SP, Carmichael GG. Long noncoding RNAs in imprinting and X chromosome inactivation. Biomolecules 2014; 4:76-100; PMID:24970206; https://doi.org/10.3390/biom4010076
- Schmitt AM, Chang HY. Gene regulation: Long RNAs wire up cancer growth. Nature 2013; 500:536-7; PMID:23945584; https://doi.org/10.1038/nature12548
- Yang QQ, Deng YF. Genome‐wide analysis of long non‐coding RNA in primary nasopharyngeal carcinoma by microarray. Histopathology 2015; 66:1022-30; PMID:25406670; https://doi.org/10.1111/his.12616
- Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non‐coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer sci 2013; 104:458-64; PMID:23281836; https://doi.org/10.1111/cas.12092
- Di Gesualdo F, Capaccioli S, Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget 2014; 5:10976-96. PMID:25428918; https://doi.org/10.18632/oncotarget.2770
- Yang X, Xie X, Xiao YF, Xie R, Hu CJ, Tang B, Li BS, Yang SM. The emergence of long non-coding RNAs in the tumorigenesis of hepatocellular carcinoma. Cancer Lett 2015; 360:119-24. PMID:25721084; https://doi.org/10.1016/j.canlet.2015.02.035
- Ren S, Li G, Liu C, Cai T, Su Z, Wei M, She L, Tian Y, Qiu Y, Zhang X. Next generation deep sequencing identified a novel lncRNA n375709 associated with paclitaxel resistance in nasopharyngeal carcinoma. Oncol Rep 2016; 36:1861-7; PMID:27498905; https://doi.org/10.3892/or.2016.4981
- Pasmant E, Laurendeau I, Héron D, Vidaud M, Vidaud D, Bièche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res 2007; 67:3963-9; PMID:17440112; https://doi.org/10.1158/0008-5472.CAN-06-2004
- Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol 2008; 28:3457-64; PMID:18332116; https://doi.org/10.1128/MCB.02019-07
- Lu Y, Zhou X, Xu L, Rong C, Shen C, Bian W. Long noncoding RNA ANRIL could be transactivated by c-Myc and promote tumor progression of non-small-cell lung cancer. Onco Targets Ther 2016; 9:3077; PMID:27307748; https://doi.org/10.2147/OTT.S102658
- Meseure D, Vacher S, Alsibai KD, Nicolas A, Chemlali W, Caly M, Lidereau R, Pasmant E, Callens C, Bieche I. Expression of ANRIL–Polycomb Complexes–CDKN2A/B/ARF Genes in Breast Tumors: Identification of a Two-Gene (EZH2/CBX7) Signature with Independent Prognostic Value. Mol Cancer Res 2016; 14:623-33; PMID:27102007; https://doi.org/10.1158/1541-7786.MCR-15-0418
- Zhang EB, Kong R, Yin DD, You LH, Sun M, Han L, Xu TP, Xia R, Yang JS, De W. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 2014; 5:2276-92; PMID:24810364; https://doi.org/10.18632/oncotarget.1902
- Zou ZW, Ma C, Medoro L, Chen L, Wang B, Gupta R, Liu T, Yang XZ, Chen TT, Wang RZ. LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget 2016; 7:61741-54; PMID:27557514; https://doi.org/10.18632/oncotarget.11437
- Ambros V. The functions of animal microRNAs. Nature 2004; 431:350-5; PMID:15372042; https://doi.org/10.1038/nature02871
- Ou H, Li Y, Kang M. Activation of miR-21 by STAT3 induces proliferation and suppresses apoptosis in nasopharyngeal carcinoma by targeting PTEN gene. PloS one 2014; 9:e109929; PMID:25365510; https://doi.org/10.1371/journal.pone.0109929
- Yu L, Lu J, Zhang B, Liu X, Wang L, Li SY, Peng XH, Xu X, Tian WD, Li XP. miR-26a inhibits invasion and metastasis of nasopharyngeal cancer by targeting EZH2. Oncology Lett 2013; 5:1223-8; PMID:23599767; https://doi.org/10.3892/ol.2013.1173
- Lu J, Zhao FP, Peng Z, Zhang MW, Lin SX, Liang BJ, Zhang B, Liu X, Wang L, Li G. EZH2 promotes angiogenesis through inhibition of miR-1/Endothelin-1 axis in nasopharyngeal carcinoma. Oncotarget 2014; 5:11319-32; PMID:25237831; https://doi.org/10.18632/oncotarget.2435
- Gao W, Chan JYW, Wong TS. Curcumin exerts inhibitory effects on undifferentiated nasopharyngeal carcinoma by inhibiting the expression of miR-125a-5p. Clin Sci 2014; 127:571-9; PMID:24896104; https://doi.org/10.1042/CS20140010
- He ML, Luo MX, Lin MC, Kung HF. MicroRNAs: potential diagnostic markers and therapeutic targets for EBV-associated nasopharyngeal carcinoma. Biochim Biophys Acta 2012; 1825:1-10; PMID:21958739; https://doi.org/10.1016/j.bbcan.2011.09.001
- Chen J, Chen Y, Chen Z. MiR-125a/b regulates the activation of cancer stem cells in paclitaxel-resistant colon cancer. Cancer Invest 2013; 31:17-23; PMID:23327190; https://doi.org/10.3109/07357907.2012.743557
- Chen JJ, Liu SX, Chen MZ, Zhao ZY. Has-miR-125a and 125b are induced by treatment with cisplatin in nasopharyngeal carcinoma and inhibit apoptosis in a p53-dependent manner by targeting p53 mRNA. Mol Med Rep 2015; 12:3569-74; PMID:26017674; https://doi.org/10.3892/mmr.2015.3863
- Yaromina A, Krause M, Thames H, Rosner A, Krause M, Hessel F, Grenman R, Zips D, Baumann M. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother Oncol 2007; 83:304-10; PMID:17517444; https://doi.org/10.1016/j.radonc.2007.04.020
- Li Z, Yu X, Shen J. ANRIL: A pivotal tumor suppressor long non-coding RNA in human cancers. Tumor Biol 2016; 37:5657-67; PMID:26753962; https://doi.org/10.1007/s13277-016-4808-5
- Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia R, Liu YW, Liu XH, Zhang EB, Lu KH. Long noncoding RNA ANRIL promotes non–small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. MolCancerTher 2015; 14:268-77; PMID:25504755; https://doi.org/10.1158/1535-7163.MCT-14-0492
- Lin L, Gu ZT, Chen WH, Cao KJ. Increased expression of the long non-coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol 2015; 10:14; PMID:25889788; https://doi.org/10.1186/s13000-015-0247-7
- Zhu H, Li X, Song Y, Zhang P, Xiao Y, Xing Y. Long non-coding RNA ANRIL is up-regulated in bladder cancer and regulates bladder cancer cell proliferation and apoptosis through the intrinsic pathway. Biochem Biophys Res Commun 2015; 467:223-8; PMID:26449463; https://doi.org/10.1016/j.bbrc.2015.10.002
- Qiu JJ, Lin YY, Ding JX, Feng WW, Jin HY, Hua KQ. Long non-coding RNA ANRIL predicts poor prognosis and promotes invasion/metastasis in serous ovarian cancer. Int J Oncol 2015; 46:2497-505; PMID:25845387; https://doi.org/10.3892/ijo.2015.2943
- Yang P, Yang Y, An W, Xu J, Zhang G, Jie J, Zhang Q. The long non‐coding RNA‐ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the P53/miR‐145 pathway. J Gastroenterol Hepatol 2016; 32(4):837-45; PMID:27696511; https://doi.org/10.1111/jgh.13606
- Zhou Y, Zhang C, Qin Q, Zhu H, Liu J, Cheng H, Sun X. Overexpression of long non-coding RNA HOTAIR enhances breast cancer radioresistance via RhoC-Akt pathway by targeting HOXD10. Int J Clin Exp Pathol 2016; 9:1-9.
- Militello G, Weirick T, John D, Döring C, Dimmeler S, Uchida S. Screening and validation of lncRNAs and circRNAs as miRNA 455 sponges. Brief Bioinform 2016; bbw053. PMID:27373735; https://doi.org/10.1093/bib/bbw053
- Gao W, Chan JY, Wong TS. Curcumin exerts inhibitory effects on undifferentiated nasopharyngeal carcinoma by inhibiting the expression of miR-125a-5p. Clin Sci 2014; 127:571-9; 24896104; https://doi.org/10.1042/CS20140010
- Xu Z, Chen J, Luk JM, De W. LncRNA ANRIL indicates a potential prognostic biomarker in gastric cancer and promotes tumor growth by silencing of miR-99a/miR-449a. Cancer Res 2015; 75:157; https://doi.org/10.1158/1538-7445.AM2015-157
- Jin C, Yan B, Lu Q, Lin Y, Ma L. The role of MALAT1/miR-1/slug axis on radioresistance in nasopharyngeal carcinoma. TumorBiol 2016; 37:4025-33; PMID:26482776; https://doi.org/10.1007/s13277-015-4227-z
- Lu Y, Li T, Wei G, Liu L, Chen Q, Xu L, Zhang K, Zeng D, Liao R. The long non-coding RNA NEAT1 regulates epithelial to mesenchymal transition and radioresistance in through miR-204/ZEB1 axis in nasopharyngeal carcinoma. TumorBiol 2016; 37:11733-41; PMID:27020592; https://doi.org/10.1007/s13277-015-4773-4
