ABSTRACT
The Hippo kinases MST1/2 and LATS1/2 inhibit the oncoproteins TAZ/YAP and regulate T cell function. Hippo kinases also cooperate with the ATR-Chk1 and ATM-Chk2 pathways, central orchestrators of the DNA damage response (DDR). We hypothesized that MST1/2 and LATS1/2 localization differently impacts the efficacy of neoadjuvant therapy (NAT) in breast cancer, being protective when expressed in the cytoplasm of tumor cells and in tumor-infiltrating lymphocytes, whereas representing molecular determinants of chemoresistance when present in the nucleus as a consequence of their cooperation with the DDR. Diagnostic biopsies from 57 HER2-positive and triple-negative breast cancer patients treated with NAT were immunostained for evaluating the expression of phosphorylated MST1/2 (pMST1/2) and LATS1/2 (pLATS1/2) in tumor-infiltrating lymphocytes (TILs) and in cancer cells. TAZ and Chk1 immunostaining was exploited for investigating subcellular compartment-dependent activity of Hippo kinases. Nuclear pMST1/2 (pMST1/2nuc) expression was significantly associated with nuclear expression of Chk1 (p = 0.046), whereas cytoplasmic pMST1/2 (pMST1/2cyt) expression was marginally associated with cytoplasmic TAZ staining (p = 0.053). Patients whose tumors expressed pMST1/2nuc were at increased risk of residual disease after NAT (pCR ypT0/is ypN0: OR 4.91, 95%CI: 1.57–15.30; pCR ypT0 ypN0: OR 3.59, 95%CI 1.14–11.34). Conversely, exclusive cytoplasmic localization of pMST1/2 (pMST1/2cyt)seemed to be a protective factor (pCR ypT0/is ypN0: OR 0.34, 95%CI: 0.11–1.00; pCR ypT0 ypN0: OR 0.31, 95%CI 0.10–0.93). The subcellular localization-dependent significance of pMST1/2 expression suggests their involvement in different molecular networks with opposite impact on NAT efficacy. Larger studies are warranted to confirm these novel findings.
Introduction
The evolutionary conserved Hippo pathway is a central regulator of tissue growth and cell fate.Citation1 In recent years, a wave of studies in animal models has demonstrated that its perturbation triggers tumorigenesis.Citation2 The core of the pathway (regulatory module) comprises the serine/threonine kinases mammalian STE20-like protein kinase 1 and 2 (MST1/2) and large tumor suppressor homolog 1 and 2 (LATS1). Hippo kinases, together with the adaptor proteins Salvador homolog 1 (SAV1) and MOB kinase activator 1A and 1B (MOB1A and MOB1B), mediate an inhibitory phosphorylation of 2 homologous oncoproteins: the transcriptional co-activator with PDZ-binding motif (TAZ) and Yes-associated protein (YAP).Citation1,2 When TAZ/YAP are phosphorylated by Hippo kinases, they are retained in the cytoplasm, excluded from the nucleus, and undergo β-TRCP (β-transducin repeat-containing E3 ubiquitin protein ligase)-dependent degradation by the proteasome machinery.Citation1,2 Thus, it is generally accepted that the regulatory module exerts tumor-suppressive activities by negatively regulating the oncogenic Hippo transducers TAZ/YAP.
Overwhelming preclinical evidence linked Hippo pathway deregulation to breast cancer (BC).Citation3,4 Among the plethora of tumor-promoting functions elicited by alterations in the Hippo signaling, particularly remarkable are those at the breast cancer stem cell (BCSC) level.Citation5-9 Indeed, studies investigating the biologic consequences of aberrant Hippo activity in BCSCs configured a scenario where activation of TAZ/YAP-driven gene transcription promotes self-renewal, epithelial-mesenchymal transition (EMT), therapeutic resistance and distant dissemination.Citation5-9 Consistently, proof-of-concept, retrospective studies performed by our group suggested that the expression of TAZ/YAP is associated with adverse therapeutic and survival outcomes in BC patients.Citation10-12 Nevertheless, the connection between the Hippo cascade and cancer extends beyond the canonical functions of the pathway. For instance, Hippo is involved in the biology of non-malignant cells residing in the tumor microenvironment. In the context of the immune system, a non-canonical, Hippo/MST pathway is emerging as a central orchestrator of T cells activities, being implicated in an array of functions spanning from T cells development and activation to survival and trafficking.Citation13 Another level of regulation of core Hippo kinases refers to their cooperation with central nodes of the DNA damage response (DDR) machinery, chiefly the ataxia telangiectasia and Rad3-related protein (ATR)-Checkpoint kinase 1 (Chk1) and ataxia telangiectasia mutated (ATM)-Checkpoint Kinase 2 (Chk2) signaling avenues.Citation14 The ATR-Chk1 and ATM-Chk2 pathways are deputed to initiate DNA repair upon genotoxic injuries, and their over-activation confers chemoresistant features.Citation15 Mechanistic studies unveiled that the Hippo-DDR cooperation promotes replication fork stability, cell-cycle checkpoint activation and DNA repair.Citation14
On this premise, we hypothesized that the expression of phosphorylated MST1/2 (pMST1/2) and LATS1/2 (pLATS1/2) may be associated with the efficacy of neoadjuvant therapy (NAT) in BC patients in a context-dependent manner. In greater detail, we envisioned the following “Janus-faced” role for Hippo kinases: i) protective when expressed in tumor-infiltrating lymphocytes (TILs), as involved in T cells activation, ii) protective when expressed in the cytoplasm of cancer cells, where they supposedly act in the canonical Hippo signaling inhibiting oncogenic TAZ/YAP, and iii) detrimental when localized in the nucleus of cancer cells, as a consequence of their interactions with central components of the DDR cascade that, in turn, fuel chemoresistance. To test this hypothesis, the expression of pMST1/2 and pLATS1/2 was evaluated by immunohistochemistry in diagnostic biopsies from 57 HER2-positive and triple-negative breast cancer (TNBC) patients treated with NAT. A subset of samples was immunostained for TAZ and Chk1,Citation10,16 enabling us to investigate whether subcellular localization of Hippo kinases is consistent with their participation in different molecular circuits, namely the Hippo signaling cascade for cytoplasmic localization and the DDR for nuclear localization. The choice of the aforementioned BC molecular subtypes is rooted into i) the emerging relationship between deregulation of the Hippo machinery and the most aggressive BC subtypes,Citation3,6,17 ii) a wider use of NAT in HER2-positive and TNBC patients compared with patients diagnosed with HER2-negative/hormone receptor positive diseases, and iii) our previous studies providing initial hints on the predictive significance of Hippo biomarkers in the HER2-positive and TNBC backgrounds.Citation10,11
Materials and methods
Patients
For this retrospective study, 57 patients with histologically confirmed HER2-positive or triple-negative BC were included. Patients were considered eligible if they completed the planned treatment, data on clinical-pathological features including stage, estrogen receptor (ER) status, progesterone receptor (PgR) status, Ki-67, HER2, tumor grade, and pCR were available, and if both pMST1/2 and pLATS1/2 were evaluable in the cellular compartments of interest (tumor cells and TILs). NAT consisted in anthracycline-taxane-based chemotherapy, together with trastuzumab for patients with HER2-postive tumors, as detailed elsewhere.Citation10,11,16 The impact of pMST1/2 and pLATS1/2 on pCR was evaluated considering the 2 most common definitions of pCR: i) no invasive or noninvasive residual cancer in breast or nodes (ypT0 ypN0), according to the definition prevalently adopted by the German Breast Group, and ii) no residual invasive tumor in both breast and axilla irrespective of the presence of ductal carcinoma in situ (ypT0/is ypN0). This study has been conducted in accordance to the Declaration of Helsinki and approved by the Ethics Committee of the “Regina Elena” National Cancer Institute of Rome, the coordinating center (CEC/532/15, 09–06–2015). Written informed consents were obtained before chemotherapy.
Immunohistochemistry
The immunohistochemical assessment of pMST1/2 and pLATS1/2 was performed in formalin-fixed paraffin-embedded (FFPE) tissues, related to diagnostic biopsies, using the following antibodies: anti-MST1/2 (phospho T183) rabbit polyclonal antibody (Abcam) at the dilution of 1:50 (pH 8); and anti-LATS1/2 (phospho T1079 and T1041) rabbit polyclonal antibody (Abcam) at the dilution of 1:100 (pH 6). In cancer cells, pMST1/2 and pLATS1/2 staining intensity was considered as negative (0), weak (1+), moderate (2+) or strong (3+). The variables pMST1/2cyt and pLATS1/2cyt were generated consideringa distinct cytoplasmic immunoreactivity of any intensity in at least 10% of tumor cells, in the absence of nuclear staining. The variables pMST1/2nuc and pLATS1/2nuc were obtained considering a distinct nuclear immunoreactivity of any intensity in at least 10% of tumor cells, independently from the presence of concomitant cytoplasmic staining. Stromal TILs were considered as positive when representing ≥ 50% of the stromal surface area.Citation18 pMST1/2 and pLATS1/2 expression in TILs was considered positive when they were expressed in at least 10% of stromal TILs. Immunoreactions were scored independently by 2 investigators (CE and ADB) blinded to treatment outcomes and discordant cases were reviewed for the final assessment.
Statistical analysis
Characteristics of the study participants were summarized by descriptive statistics. The relationships between pMST1/2 and pLATS1/2, clinical-molecular features and pCR were assessed with the Pearson's Chi-squared (Chi2) test of independence (2-tailed), the Fisher Exact test (F) when required on the basis of the size of the groups compared, or the Mann-Whitney test depending on the nature of the variables considered. The relationship between continuous variables was assessed with the Pearson correlation coefficient (r2). Clinical and molecular factors potentially impacting the outcome of interest (pCR) were tested in univariate logistic regression models whose estimates were reported as Odds Ratio (OR) and 95% Confident Interval (CI). p values inferior to 0.05 were considered as statistically significant. Statistical analyses were performed with SPSS software (SPSS version 21, SPSS Inc., Chicago, IL, USA).
Results
Baseline clinical-pathological characteristics of participants are summarized in . Twenty-eight (49%) and 23 (40%) patients achieved a pCR according to the definition ypT0/is ypN0 and ypT0 ypN0, respectively. Immunohistochemical staining of 2 representative cases is illustrated in . Expression of pMST1/2 and pLATS1/2 in TILs was recorded in 14 (25%) and 24 (42%) tumor samples, respectively (). pMST1/2 was expressed in the nucleus (pMST1/2nuc) in 25 (44%) tumors, and 25 (44%) cases displayed an exclusive cytoplasmic localization (pMST1/2cyt) (). A lower number of tumors had exclusive pLATS1/2 cytoplasmic expression (pLATS1/2cyt), whereas nuclear expression of pLATS1/2 (pLATS1/2nuc) was recorded in 45 (78%) cases ().
Table 1. Baseline characteristics of breast cancer patients included in this study (n = 57).
Figure 1. Representative examples of immunohistochemical expression of pMST1/2 and pLATS1/2 in 4 breast cancer cases (a,b,c,d). Panels a and b show 2 cases with cytoplasmic pMST1/2 (a) and cytoplasmic pLATS1/2 (b) expression with concomitant expression in stromal TILs. Panels c and d show 2 cases with nuclear pMST1/2 (c) and nuclear pLATS1/2 (d) expression with concomitant expression in stromal TILs. Slide magnification x 40, inset magnification x 20. Scale bar 30 μm.
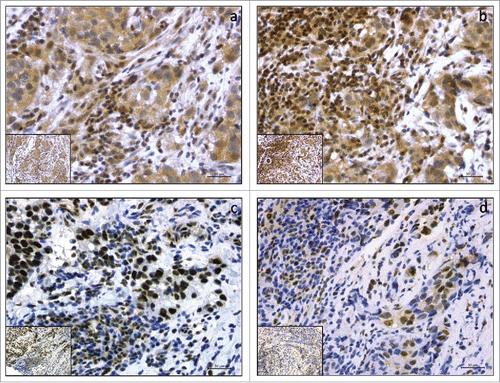
Table 2. expression pattern of pMST1/2 and pLATS1/2 in HER2-positive and triple-negative breast cancer (n = 57).
Significant or borderline significant associations between the investigated molecular markers and standard clinical-pathological features are presented in . pMST1/2nuc expression was significantly more frequent in TNBC (Chi2 p = 0.001); conversely, pMST1/2cyt expression was significantly more frequent in the HER2-positive background (Chi2 p = 0.010). Moreover, a significant positive correlation was seen between the percentage of pMST1/2-expressing stromal TILs and the percentage of stromal TILs (r2 = 0.395; p = 0.002; data available upon request).
Table 3. Significant or borderline significant associations between the molecular markers of interest (pMST1/2 and pLATS1/2) and clinical-pathological factors.
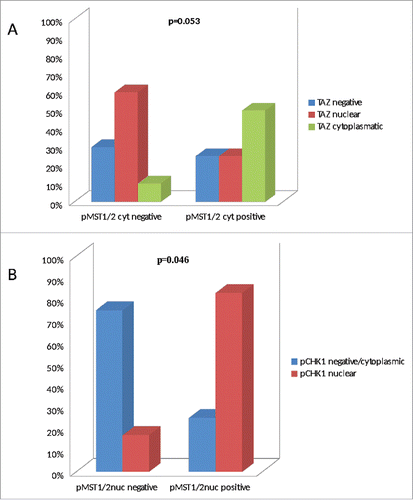
As illustrated in , a borderline significant association was observed between pMST1/2cyt and cytoplasmic TAZ expression (Chi2 p = 0.053; N = 42). Moreover, a significant association was observed between pMST1/2nuc and nuclear expression of phosphorylated Chk1 (pChk1) (F p = 0.046; N = 22) (). These data suggest that subcellular localization of pMST1/2is consistent with their involvement in the canonical Hippo cascade and the DDR network when present in the cytoplasm and in the nucleus, respectively.
Figure 2. Bar charts illustrating the association between pMST1/2cyt and TAZ (panel A), and between pMST1/2nuc and pChk1 (panel B).
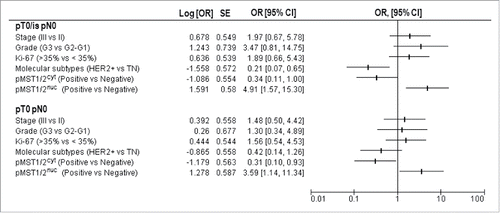
We next investigated the predictive significance of pMST1/2 and pLATS1/2 expression. We did not record any significant relationship between pLATS1/2 and pCR, irrespective of whether we considered their expression in the cytoplasm or in the nucleus (data available upon request). Likewise, neither pLATS1/2 nor pMST1/2 expression in stromal TILs predicted pCR (data available upon request). Conversely, a significant lower rate of pCR (ypT0/is ypN0) was observed for tumors expressing pMST1/2nuc (Chi2 p = 0.005), and this association was maintained even when considering pCR as ypT0 ypN0 (Chi2 p = 0.026) (). Interestingly, an opposite pattern emerged when we addressed the predictive ability of pMST1/2cyt, as the pCR rate was significantly higher in patients whose tumors carried pMST1/2cyt (Chi2 p = 0.047 and p = 0.033 for ypT0/is ypN0 and ypT0 ypN0, respectively) (). Univariate analyses, presented in , indicated that patients with pMST1/2nuc-expressing tumors were at increased risk of residual cancer after NAT (OR 4.91, 95%CI: 1.57–15.30 for pCR evaluated as ypT0/is ypN0; OR 3.59, 95%CI 1.14–11.34 for pCR evaluated as ypT0 ypN0), whereas those expressing pMST1/2cyt had an opposite outcome (OR 0.34, 95%CI:0.11–1.00 for pCR evaluated as ypT0/is ypN0; OR 0.31, 95%CI 0.10–0.93 for pCR evaluated as ypT0 ypN0).
Table 4. Relationship between pMST1/2 expression and pCR (n=57).
Figure 3. Univariate regression models for pCR (pT0/is pN0 and pT0 pN0) illustrating OR and 95%CI: clinical-molecular variables are reported including stage (III vs II), grade (G3 vs G1–2), Ki-67 levels (high vs low), molecular subtypes (HER2-positive vs triple-negative), pMST1/2cyt (positive vs negative) and pMST1/2nuc (positive vs negative).
Discussion
In the present study, we investigated the expression of activated core Hippo pathway kinases (pMST1/2 and pLATS1/2) in a series of 57 HER2-positve and triple-negative BC patients who received NAT. The message conveyed by the present study is that: i) the co-expression pattern observed between pMST1/2nuc and nuclear pChk1 expression, and between pMST1/2cyt and cytoplasmic TAZ expression, support the hypothesis that MST1/2 participate in different molecular networks, ii) the reduced pCR rate observed in patients with pMST1/2nuc-expressing tumors plausibly reflects their connection with the DDR, and the related increased ability of cancer cells to protect their genome when challenged with chemotherapy, and iii) the increased pCR rate recorded in patients with exclusive pMST1/2 cytoplasmic expression is consistent with their function in the canonical Hippo cascade, resulting in the inhibition of nuclear accumulation of tumor-promoting Hippo transducers. The logic behind the assessment of Hippo kinases in TILs also deserves to be mentioned. First, a link is emerging between stromal TILs and pCR in the HER2-positive and triple-negative backgrounds.Citation19,20 Second, our earlier data pointed to the activation of the immune-related Hippo/MST pathway as a potential protective factor in cervical cancer patients treated with NAT.Citation21 Thus, we also strove to investigate whether molecular characterization of TILs improve the predictive significance of current methods of stromal TILs assessment. Even though we did not appreciate any significant impact of Hippo kinases on pCR when expressed in stromal TILs, the positive correlation recorded between stromal TILs and pMST1/2 expression might suggest activation of the non-canonical, immune-related Hippo/MST pathway. On this basis, molecular characterization of TILs, relying on a more extensive Hippo pathway analysis, is advised to add granularity to current criteria of TILs evaluation, as well as to provide further insight into the molecular signals that either boost or depress the anticancer immune response. It is worth mentioning that various types of immune cells cohabit the tumor microenvironment (e.g. T cells, macrophages and myeloid-derived suppressor cells), and that the presence of different immune cells may have different impact on clinical outcomes.Citation22 Even though the nature of the immune infiltrate was not specifically assessed in the present study, a more thorough characterization of immune cells was planned in our ongoing studies, focusing on pathways potentially regulating the antitumor immunity.
We acknowledge that this study, when considering its retrospective design and the size of the cohort examined, needs to be considered as proof-of-concept. Nevertheless, the subcellular localization-dependent association between pMST1/2 and pCR, together with the different distribution of Hippo kinases, more frequently expressed in the nucleus in TNBC and in the cytoplasm in the HER2-positive setting, suggest that Hippo kinases may be endowed of subtype-specific functions. Recollecting this evidence, we envisioned that, while Hippo kinases may be intertwined with cell cycle checkpoints and DNA damage repair effectors in TNBC, they prevalently operate in the context of the canonical Hippo machinery in HER2-driven BC. This is consistent with preclinical studies describing that key Hippo pathway components are targeted by the ATM/Chk2 and ATR/Chk1 pathways,Citation23-26 as well as with our previous findings describing a lower pCR rate in TNBC patients with elevated expression levels of DDR-linked biomarkers in their tumors.Citation16 Next, evidence that both estrogens, via G protein-coupled estrogen receptor (GPER), and HER2 via mechanotransduction, intersect the Hippo pathway corroborates the hypothesis of molecular subtype-dependent levels of Hippo pathway regulation in BC.Citation27,28
The data herein presented provide hints which may help shape a molecular scenario where Hippo kinases exert distinct functions in relation to the underlying molecular background (subtype) of BC. However, the limitations potentially arising from the use of one single investigational approach, i.e., immunostaining, along with the hypothesis generating nature of the evidence provided, prompted the design of ad hoc studies in adequately sized cohorts to investigate the topic of interest to a deeper extent, with special emphasis being placed on TNBC given the well-established defects in the DDR machinery carried by this disease. To this end, an extensive pathway-level analysis will be performed, envisioning the evaluation of several molecular processes related to the DDR and genomic stability that include, beyond central Hippo and DDR kinases, the assessment of biomarkers related to oncogene-induced replication stress and mitotic catastrophe.Citation29,30 In doing so, immunohistochemical data will be integrated by the evidence from dedicated platforms for genomic and transcriptomic analyses. We will rely on samples collected in the context of a prospective, observational trial where neoadjuvant chemotherapy consists in a modified carboplatin-containing regimen, thus enabling us to gather information from patients treated with a more effective regimen than anthracycline- and taxane-containing chemotherapy.Citation31,32
Overall, our data point to the Hippo kinases MST1/2 as potential predictive biomarkers in HER2-positive and triple-negative BC patients candidate to receive NAT. The complexity of molecular stimuli that tune Hippo pathway effectors and the different circuits where they operate deserves further consideration in future studies, and requires the assessment of a wider number of biomarkers for mapping specific molecular networks and functions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Tania Merlino and Ana Maria Edlisca for editorial assistance.
Funding
This study was supported by an intramural research grant to PV and MM-S.
References
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 2014; 13:63-79; PMID:24336504; https://doi.org/10.1038/nrd4161
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014; 94:1287-312; PMID:25287865; https://doi.org/10.1152/physrev.00005.2014
- Maugeri-Saccà M, Barba M, Pizzuti L, Vici P, Di Lauro L, Dattilo R, Vitale I, Bartucci M, Mottolese M, De Maria R. The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications. Expert Rev Mol Med 2015; 17:e14; PMID:26136233; https://doi.org/10.1017/erm.2015.12
- Maugeri-Saccà M, De Maria R. Hippo pathway and breast cancer stem cells. Crit Rev Oncol Hematol 2016; 99:115-22; PMID:26725175; https://doi.org/10.1016/j.critrevonc.2015.12.004
- Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 2015; 34:681-90; PMID:24531710; https://doi.org/10.1038/onc.2014.5
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011; 147:759-72; PMID:22078877; https://doi.org/10.1016/j.cell.2011.09.048
- Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, Bullen JW, Samanta D, Liang H, Semenza GL. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget 2014; 5:12509-27; PMID:25587023; https://doi.org/10.18632/oncotarget.2997
- Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, Ingerpuu S, Patarroyo M, Cao S, Lim E, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes Dev 2015; 29:1-6; PMID:25561492; https://doi.org/10.1101/gad.253682.114
- Nandy SB, Arumugam A, Subramani R, Pedroza D, Hernandez K, Saltzstein E, Lakshmanaswamy R. MicroRNA-125a influences breast cancer stem cells by targeting leukemia inhibitory factor receptor which regulates the Hippo signaling pathway. Oncotarget 2015; 6:17366-78; PMID:25962054; https://doi.org/10.18632/oncotarget.3953
- Vici P, Mottolese M, Pizzuti L, Barba M, Sperati F, Terrenato I, Di Benedetto A, Natoli C, Gamucci T, Angelucci D, et al. The Hippo transducer TAZ as a biomarker of pathological complete response in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Oncotarget 2014; 5:9619-25; PMID:25294813; https://doi.org/10.18632/oncotarget.2449
- Vici P, Ercolani C, Di Benedetto A, Pizzuti L, Di Lauro L, Sperati F, Terrenato I, Gamucci T, Natoli C, Di Filippo F, et al. Topographic expression of the Hippo transducers TAZ and YAP in triple-negative breast cancer treated with neoadjuvant chemotherapy. J Exp Clin Cancer Res 2016; 35:62; PMID:27039292; https://doi.org/10.1186/s13046-016-0338-7
- Di Benedetto A, Mottolese M, Sperati F, Ercolani C, Di Lauro L, Pizzuti L, Vici P, Terrenato I, Sperduti I, Shaaban AM, et al. The Hippo transducers TAZ/YAP and their target CTGF in male breast cancer. Oncotarget 2016; 7(28):43188-98. [Epub ahead of print].
- Du X, Yu A, Tao W. The non-canonical Hippo/Mst pathway in lymphocyte development and functions. Acta Biochim Biophys Sin (Shanghai) 2015; 47:60-4; PMID:25487919; https://doi.org/10.1093/abbs/gmu112
- Pefani DE, O'Neill E. Hippo pathway and protection of genome stability in response to DNA damage. FEBS J 2016; 283:1392-403; PMID:26607675; https://doi.org/10.1111/febs.13604
- Jeggo PA, Pearl LH, Carr AM. DNA repair, genome stability and cancer: a historical perspective. Nat Rev Cancer 2016; 16:35-42; PMID:26667849; https://doi.org/10.1038/nrc.2015.4
- Vici P, Di Benedetto A, Ercolani C, Pizzuti L, Di Lauro L, Sergi D, Sperati F, Terrenato I, Dattilo R, Botti C, et al. Predictive significance of DNA damage and repair biomarkers in triple-negative breast cancer patients treated with neoadjuvant chemotherapy: An exploratory analysis. Oncotarget 2015; 6(40):42773-80; PMID:26544894; https://doi.org/10.18632/oncotarget.6001
- Díaz-Martín J, López-García MÁ, Romero-Pérez L, Atienza-Amores MR, Pecero ML, Castilla MÁ, Biscuola M, Santón A, Palacios J. Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer. Endocr Relat Cancer 2015; 22(3):443-54; PMID:25870251; https://doi.org/10.1530/ERC-14-0456
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; https://doi.org/10.1093/annonc/mdu450
- Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 2015; 1:448-54; PMID:26181252; https://doi.org/10.1001/jamaoncol.2015.0830
- Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-113; PMID:19917869; https://doi.org/10.1200/JCO.2009.23.7370
- Buglioni S, Vici P, Sergi D, Pizzuti L, Di Lauro L, Antoniani B, Sperati F, Terrenato I, Carosi M, Gamucci T, et al. Analysis of the hippo transducers TAZ and YAP in cervical cancer and its microenvironment. Oncoimmunology 2016; 5:e1160187; PMID:27471633; https://doi.org/10.1080/2162402X.2016.1160187
- Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75(11):2139-45; PMID:25977340; https://doi.org/10.1158/0008-5472.CAN-15-0255
- Hamilton G, Yee KS, Scrace S, O'Neill E. ATM regulates a RASSF1A-dependent DNA damage response. Curr Biol 2009; 19:2020-5; PMID:19962312; https://doi.org/10.1016/j.cub.2009.10.040
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007; 316:1160-6; PMID:17525332; https://doi.org/10.1126/science.1140321
- Pefani DE, Latusek R, Pires I, Grawenda AM, Yee KS, Hamilton G, van der Weyden L, Esashi F, Hammond EM, O'Neill E.. RASSF1A-LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat Cell Biol 2014; 16:962-71; PMID:25218637; https://doi.org/10.1038/ncb3035
- Aylon Y, Yabuta N, Besserglick H, Buganim Y, Rotter V, Nojima H, Oren M. Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation. Oncogene 2009; 28:4469-79; PMID:19855428; https://doi.org/10.1038/onc.2009.270
- Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest 2015; 125:2123-35; PMID:25893606; https://doi.org/10.1172/JCI79573
- Lin CH, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, LaBarge MA. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell 2015; 26:3946-53; PMID:26337386; https://doi.org/10.1091/mbc.E15-07-0456
- Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer 2015; 15:276-289; PMID:25907220; https://doi.org/10.1038/nrc3916
- Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 2011; 12:385-92; PMID:21527953; https://doi.org/10.1038/nrm3115
- von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto;GBG 66): A randomised phase 2 trial. Lancet Oncol 2014; 15:747-56; PMID:24794243; https://doi.org/10.1016/S1470-2045(14)70160-3
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dosedense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33:13-21; PMID:25092775; https://doi.org/10.1200/JCO.2014.57.0572
