ABSTRACT
The conjugation of toxins, dyes, peptides, or proteins to monoclonal antibodies is often performed via free thiol groups generated by either partial reduction methods or engineering free cysteine residues into the antibody sequence. Antibodies from the rabbit Oryctolagus cuniculus have an additional intrachain disulfide bond, whereby the light chain variable kappa domain is bridged to the constant kappa region between cysteine residues at positions 80 and 171, respectively. Chimerization of rabbit antibodies with human constant domains allows for the generation of a free thiol group at the light chain position 80 (C80) that can be used for site-specific conjugation. An efficient process for the purification and simultaneous removal of cysteinylation at the C80 site was developed. The unpaired C80 was shown to be efficiently conjugated using several different maleimido-based ligands. REsidue SPEcific Conjugation Technology (RESPECT) antibody-drug conjugates prepared using rabbit-human chimeric anti-human mesothelin rabbit antibodies and maleimido-PEG2-auristatin conjugated to C80 were shown to be highly potent and specific in vitro and effective in vivo in reduction of tumor growth in a highly aggressive mesothelin-expressing xenograft tumor model.
Introduction
The manufacturing of antibody drug conjugates (ADCs) usually involves conjugation of a linker, drug, or other payload to lysine or cysteine residues on the heavy (HC) and light (LC) chains of a monoclonal antibody (mAb).Citation1-4 Lysine conjugation is typically mediated by succinimide (NHS)-based or isothiocyanate-based chemistry. Given the number of exposed lysines on the surface of an antibody, amine-based conjugation approaches result in multiple lysines being modified, each to various degrees in different mAb molecules. Therefore, the final product is a heterogeneous mixture of mAbs with a wide distribution of drug-to-antibody ratios (DAR).
All cysteines within an antibody are involved in either inter- or intra-chain disulfide bonds. Conjugation to cysteines requires partial reduction of the antibody to break a subset of the disulfide bonds, thereby creating free thiol side chains. Thiol-reactive payloads can then react with the free thiol groups on the antibody to generate ADCs. This process has been used successfully in the manufacture of Adcetris®, approved for the treatment of classical resistant Hodgkin's lymphoma and systemic anaplastic large cell lymphoma.Citation5 Partial reduction of the disulfide bonds does not produce an equal number of unpaired cysteine for every mAb in the sample. Therefore, similar to lysine-based conjugation, this process results in a heterogeneous mixture of ADC molecules.
The aforementioned shortcoming has recently incentivized drug developers to devise site-specific conjugation technologies as a way to produce homogeneous ADC product with a defined DAR.Citation6,7 One method includes the incorporation of non-natural amino acids into the mAb during protein synthesis providing amino acid side chains with unique chemistry that can be used as specific sites of conjugation.Citation8 Several groups have also used enzymatic modification of glutamines by transglutaminase to conjugate linkers onto specific glutamine residues engineered into the antibody.Citation9,10 The engineering of an unpaired cysteine in conjunction with partial reduction has been demonstrated as a means to conjugate cysteine-reactive molecules.Citation11 While effective, all of these technologies require introduction of non-native residues either through mutagenesis or incorporation of non-natural amino acids during translation that could lead to potential immunogenicity.
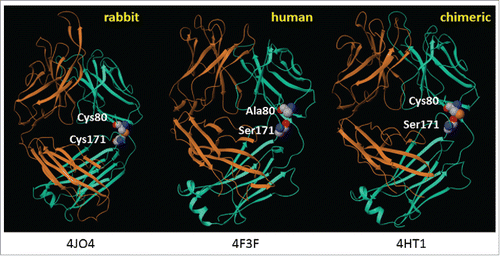
Oryctolagus cuniculus (hereafter referred to as rabbit) antibodies are unique among known species of antibodies in that the variable kappa (Vκ) domain is often covalently linked to the constant kappa (Cκ) region through an intrachain disulfide bond between residues 80 (C80, Kabat numbering) and 171 (C171, EU numbering) ().Citation12 During initial engineering and upon chimerization, we observed that because the rabbit constant domains are genetically replaced with the human gamma and kappa domains, C80 no longer forms a disulfide bond with C171 and is therefore unpaired.
Figure 1. Structures of rabbit, human, and rabbit-human chimeric antibodies. PDB file information is indicated below each structure.Citation13-15
Given that the majority of germline rabbit Vκ families have C80 (Fig. S1), we reasoned that the free thiol group could be exploited to develop a Residue-Specific Conjugation Technology (RESPECT), to generate ADCs with various payloads. In this report, we investigate the feasibility of this approach and describe a process that constitutes a milestone for generating future RESPECT ADC with humanized sequence.
Results
Creation of an unpaired cysteine at light chain position 80 and discovery of its cysteinylation
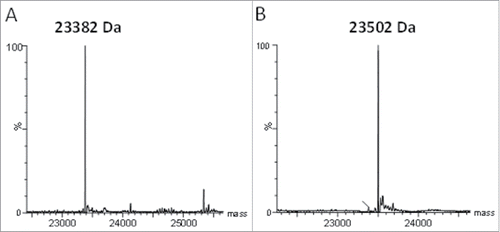
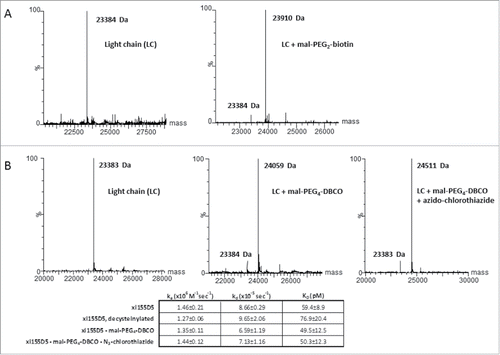
Human carbonic anhydrase 9 (CA9) is a type I transmembrane protein upregulated in the hypoxic environment found in many tumors. Antibodies were raised to CA9 by immunization of rabbits with a monomeric form of the extracellular domain of human CA9, where the cysteine involved in the CA9 homodimerization of the extracellular domain was mutated to serine. The heavy and light variable domains of rabbit anti-human CA9 clone 155D5 were amplified from the cDNA of a clonal rabbit hybridoma and cloned into expression plasmids containing human constant regions. The resultant chimeric antibody (xi155D5) contained an unpaired C80 in the light chain variable domain. Mass spectrometry analysis of the protein A purified mAb showed that when fully reduced (10 mM DTT, 60 °C, 5 minutes), the mass of the light chain was 23,382 Da (). However, when subjected to mild reducing conditions (100 μM DTT, room temperature, 30 minutes) a mass shift of +120 Da was observed () demonstrating that the light chain was cysteinylated. Cysteinylation, a modification where an unpaired cysteine in a protein molecule is linked to a free cysteine via a disulfide bond, has been observed on proteins containing one or more unpaired cysteines, including serum albumin,Citation16 and monoclonal antibodies having engineered cysteines for conjugation.Citation11,17 To determine which residue was cysteinylated, xi155D5 was digested with Asp-N and Glu-C, and the masses of the peptides were analyzed by LC-MS/MS. A cysteinylated peptide fragment corresponding to residues 71 through 80 (FTLTITGVQC) was identified, thereby confirming C80 is the site of cysteinylation (data not shown).
Figure 2. Rabbit-human chimeric 155D5 is cysteinylated at C80. Rabbit-human chimeric anti-human CA9 mAb 155D5 was purified using protein A affinity chromatography, reduced using harsh (20 mM DTT, 60°C, panel (A) or very mild (100 µM DTT, room temperature, panel (B) conditions and analyzed by mass spectrometry. The mass shift of 120 Da indicates the presence of a capping cysteine group on the light chain.
Development of a process to remove cysteinylation at C80
To maximize the potential utility of the C80 residue for conjugation, we integrated a process to remove cysteinylation (decysteinylation) into the mAb purification process. This was achieved by optimizing various conditions, including reagent concentrations as well as reaction pH and temperature. In the final optimized method, we use cysteine as a mild reductant in neutral phosphate buffer containing EDTA to remove the cysteinylation at C80 during purification, followed by an on-column re-oxidation. Antibody is bound to protein A resin and incubated with a buffer containing 5 mM cysteine for 16 h at 4°C to reduce the cysteine-cys80 disulfide bond, followed by re-oxidation with Tris-HCl at neutral pH at 4°C for 60 hr. The decysteinylated and re-oxidized antibody is then eluted in a low pH glycine buffer. Protein recovery averaged 75% using the final developed process, when compared side-by-side with traditional protein A purification. Based on mass analysis of the non-reduced purified antibody, close to 100% of the antibody can be decysteinylated using this process (). Free thiol content analysis indicated 2 thiol groups per mAb, consistent with a free thiol at C80 and efficient reoxidation of any reduced interchain disulfide bonds (data not shown).
Decysteinylated C80 can be conjugated to maleimides
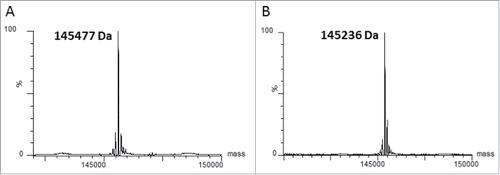
A non-disulfide-bridged cysteine in a protein may not necessarily be accessible to a cysteine-reactive compound.Citation18 In fact, we have attempted the conjugation of unpaired C171 in the xi155D5 (C80P/S171C mutant) mAb after decysteinylation and could not detect appreciable conjugation (Fig. S2). We therefore explored whether C80 in the xi155D5 mAb after decysteinylation could be modified by thiol-reactive molecules. Decysteinylated xi155D5 was incubated with maleimide-PEG2-biotin as described in methods. Mass spectrometry analysis showed that 94% of the antibody was conjugated with maleimide-PEG2-biotin as indicated in , panel A. We further examined the feasibility and efficiency of conjugating a pharmacological agent using strain-promoted alkyne-azide chemistry (SPAAC).Citation19 Dibenzylcyclooctyne (DBCO) was conjugated to the C80 at high efficiency (85%), and an azido-PEG2-chlorothiazide was then conjugated to the DBCO in a facile, efficient manner ().Citation20 Binding analyses of the parent antibody, intermediates and final conjugate demonstrated that antibody affinity was unaffected after conjugation.
Figure 4. A decysteinylated C80 can be efficiently conjugated to maleimides with no impact on antigen binding affinity (A) Mass spectrometry analyses of the light chain of decysteinylated xi155D5 conjugated with maleimido-PEG2-biotin. Efficiency of conjugation was 94%. (B). Mass spectrometry analyses of the light chain of decysteinylated xi155D5, xi155D5 conjugated with maleimido-PEG4- DBCO, and xi155D5-DBCO conjugated with azido-chlorothiazide via SPAAC. Efficiency of maleimido addition to the light chain was 85%. SPR analysis of the binding affinity of cysteinylated, decysteinylated, and decysteinylated/conjugated mAb to soluble CA9 indicated that the decysteinylation procedure as well as subsequent conjugation to C80 had no impact on binding affinity of the xi155D5 antibody to antigen.
RESPECT mAb conjugated to 800CW dye efficiently targets tumors in vivo
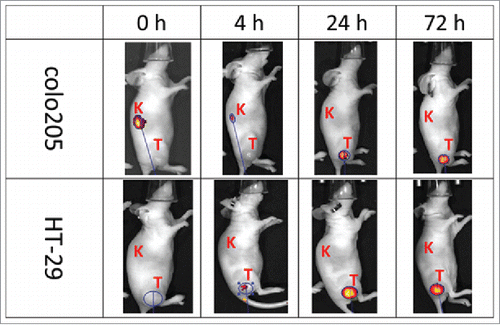
To explore the broad utility of RESPECT mAbs for tumor targeting, xi155D5 was conjugated with an infrared dye using maleimido-IRDye 800CW and tested for its ability to localize in human CA9-positive tumors xenografted in nude mice. SDS-PAGE analysis with fluorescent detection indicated very efficient and specific labeling of the light chain of xi155D5 (Fig. S3). A single dose of xi155D5–800CW was injected into mice implanted with human colon tumor xenografts derived from either colo205 or HT-29 cell lines, both of which have been shown to over-express CA9 in a HIF-1-dependent manner.Citation21,22 xi155D5–800CW strongly localized in both tumors within 24 hours post-injection (). Localization lasted for up to 12 d post-injection.
Figure 5. Tumor-specific localization of anti-human CA9 xi155D5–800CW. Human colo205 (upper panel) and HT-29 (lower panel) cells were grafted into nude mice, which were subsequently injected with xi155D5–800CW. Fluorescent signal (orange-red) was monitored at various time point (shown are 0–72 hours and right flank). Approximate location of kidney (K) and tumor (T) is indicated.
RESPECT mAb conjugated to auristatin F mediates robust anti-tumor effect in vivo
To evaluate the utility of ADCs prepared using conjugation to the C80 site for treatment of tumors, antibodies were generated in rabbits against human mesothelin and were cloned and chimerized in a similar manner to anti-human CA9 xi155D5.
Mesothelin is a naturally occurring ligand for human CA125/MUC16 that is highly overexpressed in pancreatic cancer and mesothelioma.Citation23,24 We and others have reported on the potential utility of biologic agents targeting mesothelin for therapeutic indications in oncology.Citation25-27 Therefore, we sought to generate novel RESPECT mAbs targeting mesothelin. Anti-mesothelin mAbs were generated, screened for specific binding to human mesothelin by SPR (), and several antibodies with sub-nanomolar affinity were selected and further investigated. A non-cleavable maleimido-derivative of auristatin F, created by formation of an amide linkage of PEG2-maleimide to the free acid on auristatin F () was conjugated to C80 within the selected mAbs with very high efficiency. DAR values for all RESPECT mAbs were 2.0, as measured by mass spectrometry () Aggregate levels for conjugated antibodies were typically < 10% and binding to target was fully retained.
Figure 6. In vivo specificity and efficacy of rabbit-human chimeric anti-human mesothelin – auristatin F C80 ADCs. (A) Schematic of conjugation of maleimido-PEG2-auristatin F to C80. (B) Anti-human mesothelin binding, aggregation, and ADC cytotoxicity data. Affinities are shown pre- and post-conjugation. (C) In vivo efficacy of anti-mesothelin auristatin F ADCs. Anti-human endosialin xi1–55–2 – AuF was dosed similarly to anti-mesothelin ADCs.
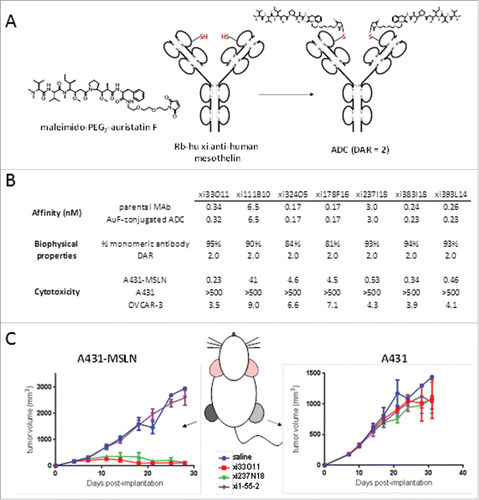
Binding kinetics showed that antigen affinity following conjugation was fully retained. Cytotoxicity analyses were performed with the RESPECT mAbs using a human epidermoid cell line A431-MSLN stably transfected with human mesothelin, along with the parental A431 cell line as control. RESPECT mAbs were found to be highly toxic to the mesothelin-expressing cells, with sub-nanomolar EC50 values for 4 of 7 RESPECT mAbs tested, while no cytotoxicity was observed on the parental A431 cell line (). Moreover, nanomolar-level cytotoxicity was also observed with all 7 auristatin-conjugated ADCs on the ovarian tumor cell line OVCAR-3, which endogenously expresses human MSLN ().Citation23
Two RESPECT mAbs, xi33O11-AuF and xi237I18-AuF, were evaluated in a dual-flank in vivo xenograft model, in which one flank of the mouse was implanted with the A431-MSLN tumor cell line, while the opposite flank was implanted with the parental tumor cell line. When tumors reached 150–250 mm3, treatment was administered q7dx2 at a dose of 10 mg/kg. Both RESPECT mAbs were effective in causing regression of mesothelin-expressing tumors in this highly-aggressive xenograft disease model ().
Development of RESPECT bispecific molecules
As C80 is located in the framework region of the variable domain of the rabbit-human chimeric antibodies, a Fab fragment generated either by partial papain digestion or recombinant expression also retains the single, unpaired C80. In light of this configuration, we reasoned that by using bioorthogonal conjugation chemistry, C80-containing Fabs could be used to generate chemically-conjugated bispecific constructs useful for a variety of tumor targeting strategies, including redirecting effector cells or anti-tumor enhancing proteins to tumor lesions.
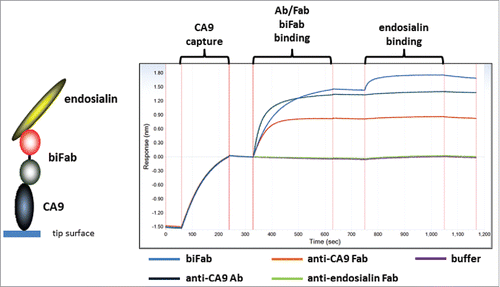
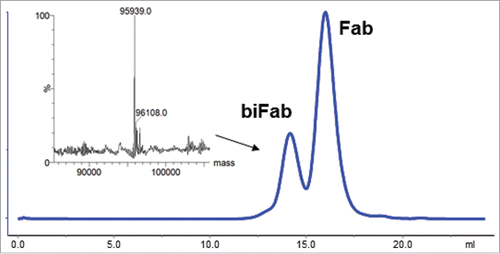
To model this, the anti-CA9 RESPECT mAb, xi155D5, and the anti-endosialin RESPECT mAb, xi1–55–2, were selected. Endosialin is a tumor associated pericyte cell surface protein. Fab was generated using partial papain digestion, followed by protein A chromatography to remove the Fc fragments and undigested antibodies. Mass spectrometry analysis demonstrated that the Fabs were fully decysteinylated using the method described previously above (data not shown). Next, xi155D5 and xi1–55–2 Fabs were conjugated using maleimido-PEG4-DBCO and maleimido-PEG4-azide, respectively. Complete occupancy of the C80 sites was confirmed by mass spectrometry (data not shown). The xi155D5 and xi1–55–2 Fabs were conjugated to each other via SPAAC by incubation in neutral phosphate buffer overnight. Efficiency of conjugation was 20%. This RESPECT bispecific molecule (biFab) was then purified from unconjugated Fabs by gel-filtration chromatography (). Orthogonality of conjugation was confirmed by mass spectrometry ().
Figure 7. Purification of biFab heterodimer. Following overnight incubation of DBCO- and azido-conjugated Fabs, RESPECT bispecific antibody (biFab) was purified by S-200 SEC chromatography. The fractions containing the RESPECT bispecific antibody were pooled and the mass was analyzed by mass spectrometry (inset).
The binding properties of the RESPECT bispecific Fab was analyzed via biolayer inferometry (BLI) analysis using an inverse sandwich assay. RESPECT biFab was shown to first bind to immobilized human CA9 and followed by binding to soluble endosialin (). Immobilized CA9 was bound by both xi155D5 mAb, 155D5 Fab, and the RESPECT biFab. However, only the RESPECT biFab was able to also bind human endosialin, thereby demonstrating the utility of C80-based biFabs as a platform for generating bispecific constructs.
Discussion
Oryctolagus cuniculus antibodies contain an extra disulfide bond between residue 80 in Vκ and residue 171 in Cκ that is not currently known to be found in any other species. When C80 was unpaired through the process of chimerization of the light chain, we discovered that this residue was cysteinylated during the production of the recombinant mAb. We therefore developed an engineering method to remove cysteinylation of C80 during the purification to enable broad conjugation to thiol-reactive compounds.
Cysteinylation is a modification that has been observed on engineered cysteines of monoclonal antibodies,Citation11,17,18 as well as naturally occurring cysteines on albumin,Citation16 peptide toxins,Citation28 and in the CDRs of antibodies.Citation29 In the case of CDR-located cysteines, cysteinylation typically leads to the loss of binding. Removal of cysteinylation is necessary for the subsequent conjugation of thiol-reactive molecules such as maleimides or haloacetamides. Typical processes used for the removal of cysteinylation involve the use of thiol-based or non-thiol-based reductants, removal of the reductant species, followed by re-oxidation. This process has been shown to lead to mixed disulfide formation, dimerization, and misfolding.Citation11 The incorporation of the reduction and re-oxidation steps into the purification process eliminates the necessity of removal of the reductant before re-oxidation. Because the capture is affinity-based, any antibody having a loss of structure due to over-reduction is removed during the process. Careful control over the substitution level of the affinity capture agent, in this case protein A, enables spatial separation of captured antibodies during the re-oxidation step, thereby reducing the formation of intermolecular mixed disulfide bonds between antibodies.
The use of a native cysteine at position 80 as a site of attachment for potent cytotoxic agents, made unpaired via the process of chimerization, offers several advantages over the use of engineered cysteines. These include the ability to avoid high solvent-exposure of engineered cysteines in antibodies that have been shown to lead to the formation of intermolecular disulfides and poor conjugatability.Citation11,18,30,31 Analysis of the crystal structure of chimerized rabbit antibodies and calculation of solvent accessible surface area (SASA) using Bioluminate 2.6 (Schrodinger) suggests that an unpaired C80 will retain solvent exposure without overly-extensive access to solvent (SASA of 34 ± 9 Å2), due to the close proximity of the S171 loop in the human Ck.Citation15,32-37 High solvent exposure (SASA ≥ 100 Å2) is observed in many engineered cysteines present at the apex of antibody surface-exposed loops, resulting in poor conjugatability and/or poor conjugate stability.Citation11,18 Moderately-exposed cysteines as conjugation sites have been demonstrated to lead to ADCs having improved serum stability and pharmacokinetic parameters.Citation11,31,38 The low aggregate levels seen in RESPECT mAbs, as well as their high conjugation efficiency, excellent specificity, and low off-target cytotoxicity suggest that these ADCs exhibit excellent properties for potential therapeutic use.
Conjugation to C80 not only has utility in making site-specific ADCs, but also as a platform for generating bispecific molecules. Traditional amine and thiol conjugation via partial reduction modifies multiple residues, therefore making these methods unsuitable for generating bispecific mAbs because of their propensity of forming heterogeneous and multimeric proteins. In contrast, the process detailed here enables the generation of single-species RESPECT bispecific molecules capable of simultaneously binding 2 antigens. Since the use of a chemical conjugation strategy for generation of bispecific molecules allows for matrix-based screening strategy of several Fab pairs than when using a genetic fusion strategy, this approach may enable rapid multiplex screening and selection of optimal antibody pairings for the desired bispecific application. In the studies detailed here, efficiency of conjugation was limited to 20%. Future studies focusing on improving the efficiency of this process will include linker length optimization and the investigation of orthogonal chemistries with faster kinetics, such as tetrazine – trans-cyclooctene.Citation39
In summary, we have described the novel use of a native unpaired cysteine at light chain position 80 in rabbit-human chimeric antibodies for the purposes of conjugation using thiol-reactive chemistry. Antibody-drug conjugates using this approach have been shown to be highly potent and specific both in vitro and in vivo. By using a mutational and structural approach, we have recently directed our efforts to defining the requirements for humanizing RESPECT antibodies for clinical use, to reduce potential immunogenicity of RESPECT immunoconjugates while retaining both antigen binding and conjugatability.Citation40 These results have revealed the adjacent residue position 83 to be critical to the retention of high conjugatability of humanized RESPECT antibodies and low aggregate levels in immunoconjugates. The results of these studies are detailed in an upcoming publication.Citation41
Experimental procedures
Reagents
All materials used in these studies were obtained as reagent-grade or higher. Maleimido-PEG2-auristatin F was synthesized at Concortis Biosystems, Inc. (San Diego, CA) and was used as provided by the manufacturer.
Expression and purification of recombinant antigen proteins
The extracellular domains of human CA9, human mesothelin, and human endosialin extracellular domains were synthesized with a C-terminal His-tag and subcloned into a pEF-based vector, stably-expressed as 293F pools, similar to antibodies as described below. Conditioned medium from terminal cultures of stable 293F pools was purified using Ni-NTA affinity chromatography (Qiagen) with an FPLC (GE Healthcare) and dialyzed against 1x PBS containing 0.1 mM PMSF. Purified protein was stored at −80C until use.
Antibody generation in rabbits
Antibodies to human endosialin and human carbonic anhydrase 9
New Zealand White rabbits were subcutaneously injected with purified, recombinant human protein antigen every 21 d. Freund's complete adjuvant (FCA) was used in initial injections, followed by Freund's Incomplete Adjuvant (FIA) in subsequent boosts. The pre- and test bleed were collected for the antibody titer testing.
Titer was determined using enzyme-linked immunosorbent assay (ELISA) with coated antigens and HRP-linked anti-rabbit antibody as secondary reagent. When the titer reached 1:15,000 or greater, the rabbits were given a final boost of antigen without adjuvant. Lymphocytes were isolated from spleens and lymph nodes one week following last boost.
Appropriate amount of rabbit splenocytes stimulated with pokeweed mitogen and fusion partner cells CBF7 were mixed at the desired ratio (1:4) in 50 mL tubes.Citation42 CytoPulse cell fusion apparatus CEEF-50 (CytoPulse Sciences) was used for the fusion. The cells were incubated at room temperature for 25 min and then overnight at 37°C, 5% CO2 following the fusion. Cells were plated in IMDM plus10% FBS containing 1X hypoxanthine-aminopterin-thymidine (7,000 cells/well). The plates were incubated at 37°C, 5% CO2 and fed with fresh medium weekly for 3–4 weeks. Hybridoma supernatants were screened for antigen binding by ELISA. Hybridoma cells secreting rabbit mAbs of interest were lysed to extract RNA. RNA was then used for DNA amplification of light chain variable (VL) and heavy chain variable (VH) regions by nested RT-PCR. Fragments were subcloned into an expression plasmid containing a human gamma or kappa constant region using an InFusion HD cloning kit (Clontech). All clones were sequenced to confirm the presence and fidelity of the inserts.
Antibodies to human mesothelin
New Zealand White rabbits were immunized as described above for CA9 and endosialin. The lymphocytes from lymph nodes stimulated with pokeweed mitogen were seeded directly into 384-well plates (5 cells/well) pre-seeded with CHO-K1 cells expressing CD154 as feeder cells. Two weeks after seeding, B-cell culture supernatants were collected. B-cell culture supernatants were first screened for IgG production by rabbit IgG FRET assay. IgG-producing clones were selected and screened by ELISA to determine binding to human mesothelin. RNA was extracted from corresponding B-cell clones and VH/VL regions were amplified and subcloned as described above.
Transfection and stable cell line generation
HEK293 cells were transfected using one of 2 methods. Transient transfections and stable cell lines of 293F cells were performed as previously reported.Citation43 Alternatively, Expi293 cells were transfected using ExpiFectamine according to the manufacturer's protocol (Thermo-Fisher). All cells were maintained in a humidified incubator at 37°C in 8% CO2 with shaking at 125 rpm.
Antibody production
Antibody production from stable pools was performed by one of 2 methods: 1.) Stable-transfected cell line pools were seeded at 0.6 to 1 × 106 cells/mL in 293FreeStyle medium. Two days after the culture reached a density of 1 × 106 cells/mL, cultures were fed as previously reported.Citation43 2.) Stable-transfected cell line pools were centrifuged at 1000 rpm in a Beckman Allegra 6 centrifuge for 5 min. The supernatant was removed, and the cells were resuspended in 1 L expi293 medium (Gibco) at 0.5–0.8 × 106 cells/mL in a 2.8-L shake flask. Cells were incubated at 37°C, 8% CO2, shaking at 125 rpm.
For both methods, the cultures were incubated at 37°C in 8% CO2 with shaking at 125 rpm for 7–10 d. When the cell viability was less than 50%, the cultures were centrifuged for 1 h at 8000 rpm in a Beckman JLA8.1000 rotor. The supernatant was then filtered through a 0.2 μm PES filter and stored at 4°C or −20°C until purification.
Antibody purification by protein A affinity chromatography
Using an ÄKTA Explorer (GE Healthcare), a protein A column (GE Healthcare) was equilibrated with 10 column volumes (CV) of 20 mM sodium phosphate, 10 mM EDTA, pH 7.2. The sample was then loaded, followed by washing unbound material with 10 CV of equilibration buffer. The sample was eluted using 5 CV of 0.1 M glycine pH 2.9. The fractions containing the mAb were pooled and dialyzed in Dulbecco's phosphate buffer (DPBS) using a MWCO 20K Slide-A-Lyzer (Thermo).
Antibody purification with on-column decysteinylation
Using an ÄKTA Explorer (GE Healthcare), a protein A column (GE Healthcare) was equilibrated with 10 column volumes (CV) of 20 mM sodium phosphate, 10 mM EDTA, pH 7.2. The sample was then loaded, followed by washing unbound material with 10 CV of equilibration buffer. The column was washed with 16 CV of 20 mM sodium phosphate, 10 mM EDTA, 5 mM cysteine, pH 7.2 at 0.5 mL/min for 16 hrs. The column was then washed with 60 CV of 20 mM Tris, pH 7.5 at 0.5 mL/min for 60 hrs. The sample was eluted using 5 CV of 0.1 M glycine pH 2.9. The fractions containing the mAb were pooled and dialyzed in DPBS using a MWCO 20K Slide-A-Lyzer (Thermo).
Conjugation to C80
Decysteinylated antibody was brought to 5.0 mg/mL in 1x DPBS, 1 mM EDTA. Typically, maleimido-compound was added to decysteinylated antibody at a molar ratio of 1:4 (mAb:compound) and mixed gently. Conjugation proceeded for 3.5 to 4 hr at room temperature. For more hydrophobic compounds, propylene glycol was included in the conjugation reaction at a final percentage of 25%. Conjugated antibody was purified using HiTrap desalting column(s) (GE Healthcare) with chromatography performed on an FPLC (GE Healthcare) using 1x DPBS as running buffer, to remove maleimido compounds and propylene glycol.
SEC-HPLC analysis
Antibody aggregation was analyzed by size-exclusion high performance liquid chromatography method (SEC-HPLC) using an Agilent 1100. The mAb was diluted to 1 mg/mL in DPBS. Twenty μL of antibody was injected onto a TSKgel SuperSW guard column(4.6 mm × 3.5cm, 4μ) followed by a TSKgel SuperSW3000 column (4.6 mm × 30cm, 4μ). Antibodies were eluted from the column with 0.1 M PBS containing 0.15 M NaCl and 0.05% NaN3, at pH 7.4, at a flow rate of 0.3 mL/min for 20 min. All data were analyzed using Agilent ChemStation software.
UPLC/ESI-MS analysis of conjugation efficiency
Conjugated antibodies were deglycosylated using PNGase F (NEB). G7 buffer (10 μL) and PNGase F (2 μL) were added to the mAb (90 μL). The reaction was incubated in a Discover microwave (CEM) for 2 cycles: 1.) microwave power 10 W, 37°C, 10 min, and then wait for 3–5 min; 2.) microwave power 2 W, 37°C, 10 min. A portion of the sample was reduced by adding DTT to a final concentration of 20 mM, followed by incubation at 60°C for 3 min. Samples were then analyzed using a Waters Acquity UPLC and Q-Tof Premier mass spectrometer. Samples (0.5–2 μg each) were injected onto a MassPrep micro desalting column at 65°C, eluted from the column with a 5 min equilibration in 95% of mobile phase A, a 10 min gradient (5–90% B), and a 10 min re-equilibration in 95% of mobile phase A, at 0.05 mL/min. Mobile phase A was 0.1% formic acid in water. Mobile phase B was 0.1% formic acid in acetonitrile. The Q-Tof mass spectrometer was run in positive ion, V-mode with detection in the range of 500–4000 m/z. The source parameters were as follows: capillary voltage, 2.25 kV (intact antibody)-2.50 kV (reduced antibody); sampling cone voltage, 65.0 V (intact antibody) or 50.0 V (reduced antibody); source temperature, 100°C; desolvation temperature, 250°C; desolvation gas flow, 550 L/hr. The protein peak was deconvoluted using the MassLynx MaxEnt 1 function.
Biacore analysis of mAb: Antigen affinity
Antibody concentrations were adjusted to generate 30–40 RU signal when bound to the antigen. Chimeric mAbs purified by standard protein A affinity chromatography or by the on-column decysteinylation method were injected over an anti-human IgG sensor for 1 min at a flow rate of 10 μL/min. The sensor surface was washed by injecting HBS-P buffer for 1 min at a flow rate of 50 μL/min. To record the antigen association to the captured mAb, a series of increasing concentrations of antigen was injected for 60 sec at a flow rate of 50 μL/min. The dissociation of antigen was monitored for 30 min at the same flow rate. The sensor surface was regenerated by injecting 3 M MgCl2 for 1 min and then 30 sec at a flow rate of 30 μL/min. Sensograms were analyzed with Biacore T100 Evaluation Software using a 1:1 binding model.
Bispecific fab preparation
MAb-derived Fab fragments were prepared separately using immobilized papain, followed by isolation of the pure Fab fragments from Fc and undigested mAb using protein A chromatography. Maleimido-PEG4-azide was synthesized by combining NHS-maleimide and azido-PEG4-amine in DMSO for 1 hr in a 1:1 molar ratio. Unreacted NHS was quenched by the addition of Tris-HCl buffer to prevent homodimerization. Fabs were conjugated to either maleimido-PEG3-azide or maleimido-PEG4- DBCO (Click Chemistry Tools) at a 5:1 molar ratio of maleimide:Fab and reacted for 4 hr at room temperature. The modified Fab fragments were desalted twice each in 1x DPBS to remove all unreacted products, and the Fab fragments were combined at a molar ratio of 1:1 at 2 mg/mL final concentration and allowed to conjugate overnight at room temperature. The reaction was analyzed by SDS-PAGE and conjugation efficiency was estimated at 20%. Conjugate was purified from unreacted monomer by Sephadex-200 gel filtration chromatography with 1x DPBS as running buffer using FPLC (GE Healthcare).
Bispecific binding assay using biolayer inferometry
Biotinylated human CA9 was captured on streptavidin Biosensor tips (Pall) for 4 min. Following incubation in PBS for 2 min, the tips were incubated with the bispecific Fabs, mAb alone, or Fab alone for 5 min. Following incubation in PBS for 2 min, the tips were incubated with human endosialin/TEM-1 for 5 min. Finally, the tips were incubated in PBS for another 2 min. Association and disassociation protein to the tips was measured throughout.
In vivo imaging of human colon carcinoma xenograft tumors using rabbit-human chimeric anti-human CA9 antibody 155D5 labeled at C80 with maleimido-IRDye-800CW
NCR-nude female mice were injected with either colo205 or HT-29 human tumor cells (ATCC) subcutaneously to the right hind limbs. Tumor growth was monitored by caliper measurement. When the tumor volume was 200–300 mm3, xi155D5–800CW was injected through the tail veils at 0.1 mg/200µL/mouse. For monitoring xi155D5–800CW distribution via fluorescent living imaging, animals were placed into an anesthesia chamber for approximately 3–4 minutes using isofluorane/O2 until the animals were unconscious. Animals were imaged using the fluorescence setting of 745 excitation and 840 emission in an IVIS® Lumina II-Kinetic instrument (PerkinElmer, Waltham, MA). Images of the dorsal, right, ventral, and left side were taken at different time points as indicated in the figures. After each successive image the animal were allowed to regain consciousness in a recovery chamber receiving 100% oxygen flush followed by normal air.
Generation of A431-MSLN cell line
A CMV promoter-driven expression plasmids was used to transfect A431 cells in a 6 well plate using lipofectamine 2000 as described by the vendor (Thermo-Fisher). Twenty-four hours after transfection, cells were harvested and reseeded in a T-75 flask with 10 μg/mL zeocin selection. After 2 weeks of selection, the stably transfected pool was subcloned by limiting dilution in 96-well plates. Single colonies were selected, expanded, and analyzed for surface expression of MSLN by flow cytometry using an anti-MSLN antibody. A single clone (A3) expressing surface MSLN was used for subsequent experiments and referred to as A431-MSLN.
In vitro cytotoxicity assay
Cells were sub-cultured and seeded at 5,000 cells/well in complete growth medium in 96-well tissue culture plates, and incubated at 37°C, 5% CO2 overnight. Test reagents were serially diluted and added to the cell plates (initial concentration of 100 nM). Plates were incubated at 37°C, 5% CO2 for an additional 3 d. Medium was discarded, plates were washed once with 200 µL DPBS, stained with 50 µL of 0.2% Crystal Violet solution at room temperature for 15 minutes, and then washed extensively with tap water. Plates were air-dried, and Crystal Violet was dissolved with 200 µL of 1% SDS solution. Plates were read at 570 nm. Data was analyzed using GraphPad Prism 6.
In vivo efficacy of rabbit-human chimeric anti-human mesothelin – auristatin F ADCs conjugated at C80
A431-MSLN cell line is a highly tumorgenic A431 cell line stably expressing human GPI-linked mesothelin. Parental A431 and A431-MSLN cells were propagated in cell culture, suspended in serum-free growth medium, mixed 1:1 with Matrigel™, and 5 million cells/0.2 mL/mouse were implanted subcutaneously into athymic NCr nu/nu mice. A431 cells were implanted on day 1 and A431-MSLN cells on day 4. Tumor volume was determined by caliper measurements (mm) and using the formula for an ellipsoid sphere: Length x Width2/2 = Volume (mm3). When tumor volume reached a range of 100–250 mm3 for each tumor, mice were randomized into treatment groups. The animal body weights and tumor size were recorded twice weekly. ADCs and cytotoxins were administered intravenously Q7D starting on randomization day, 2 doses total.
Disclosure of potential conflicts of interest
All authors were employees of Morphotek, Inc. during the execution of the studies detailed herein and own no equity in the company.
Supplemental_Figures_1-3.docx
Download MS Word (536.3 KB)Funding
All funding for these studies was provided by Morphotek Inc.
References
- Deonarain MP, Yahioglu G, Stamati I, Marklew J. Emerging formats for next-generation antibody drug conjugates. Expert Opin Drug Discov 2015; 10(5):463-81; PMID:25797303; https://doi.org/10.1517/17460441.2015.1025049
- de Goeij BE, Lambert JM. New developments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol 2016 Jun; 40:14-23; PMID:26963132; https://doi.org/10.1016/j.coi.2016.02.008
- Bakhtiar R. Antibody drug conjugates. Biotechnol Lett 2016; 38(10):1655-64; PMID:27334710; https://doi.org/10.1007/s10529-016-2160-x
- Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell 2016; 14; PMID:27743348; https://doi.org/10.1007/s13238-016-0323-0
- Berger GK, McBride A, Lawson S, Royball K, Yun S, Gee K, Bin Riaz I, Saleh AA, Puvvada S, Anwer F. Brentuximab vedotin for treatment of non-Hodgkin lymphomas: A systematic review. Crit Rev Oncol Hematol 2017; 109:42-50; PMID:28010897; https://doi.org/10.1016/j.critrevonc.2016.11.009
- Kline T, Steiner AR, Penta K, Sato AK, Hallam TJ, Yin G. Methods to Make Homogenous Antibody Drug Conjugates. Pharm Res 2015; 32(11):3480-93; PMID:25511917; https://doi.org/10.1007/s11095-014-1596-8
- Sochaj AM, Świderska KW, Otlewski J. Current methods for the synthesis of homogeneous antibody-drug conjugates. Biotechnol Adv 2015; 33(6 Pt 1):775-84; PMID:28178520; https://doi.org/10.1016/j.biotechadv.2015.05.001
- Zimmerman ES, Heibeck TH, Gill A, Li X, Murray CJ, Madlansacay MR, Tran C, Uter NT, Yin G, Rivers PJ, et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug Chem 2014; 25(2):351-61; PMID:24437342; https://doi.org/10.1021/bc400490z
- Jeger S, Zimmermann K, Blanc A, Grünberg J, Honer M, Hunziker P, Struthers H, Schibli R. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew Chem Int Ed Engl 2010; 49(51):9995-7; PMID:21110357; https://doi.org/10.1002/anie.201004243
- Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, Ho WH, Farias S, Casas MG, Abdiche Y, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol 2013; 20(2):161-7; PMID:23438745; https://doi.org/10.1016/j.chembiol.2013.01.010
- Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008; 26(8):925-32; PMID:18641636; https://doi.org/10.1038/nbt.1480
- Strosberg AD, Margolies MN, Haber E. The interdomain disulfide bond of a homogeneous rabbit pneumococcal antibody light chain. J Immunol 1975; 115(5):1422-4; PMID:240892
- Stammers TA, Coulombe R, Rancourt J, Thavonekham B, Fazal G, Goulet S, Jakalian A, Wernic D, Tsantrizos Y, Poupart MA, et al. Discovery of a novel series of non-nucleoside thumb pocket 2 HCV NS5B polymerase inhibitors. Bioorg Med Chem Lett 2013; 23(9):2585-9; PMID:23545108; https://doi.org/10.1016/j.bmcl.2013.02.110
- Ma J, Tang WK, Esser L, Pastan I, Xia D. Recognition of mesothelin by the therapeutic antibody MORAb-009: structural and mechanistic insights. J Biol Chem 2012; 287(40):33123-31; PMID:22787150; https://doi.org/10.1074/jbc.M112.381756
- Lammens A, Baehner M, Kohnert U, Niewoehner J, von Proff L, Schraeml M, Lammens K, Hopfner KP. Crystal Structure of Human TWEAK in Complex with the Fab Fragment of a Neutralizing Antibody Reveals Insights into Receptor Binding. PLoS One 2013; 8(5):e62697; PMID:23667509; https://doi.org/10.1371/journal.pone.0062697
- Lee P, Wu X. Review: modifications of human serum albumin and their binding effect. Curr Pharm Des 2015; 21(14):1862-5; PMID:25732553; https://doi.org/10.2174/1381612821666150302115025
- Buchanan A, Clementel V, Woods R, Harn N, Bowen MA, Mo W, Popovic B, Bishop SM, Dall'Acqua W, Minter R, et al. Engineering a therapeutic IgG molecule to address cysteinylation, aggregation and enhance thermal stability and expression. MAbs 2013; 5(2):255-62; PMID:23412563; https://doi.org/10.4161/mabs.23392
- Junutula JR, Bhakta S, Raab H, Ervin KE, Eigenbrot C, Vandlen R, Scheller RH, Lowman HB. Rapid identification of reactive cysteine residues for site-specific labeling of antibody-Fabs. J Immunol Methods 2008; 332(1–2):41-52; PMID:18230399; https://doi.org/10.1016/j.jim.2007.12.011
- Zheng M, Zheng L, Zhang P, Li J, Zhang Y. Development of bioorthogonal reactions and their applications in bioconjugation. Molecules 2015; 20(2):3190-205; PMID:25690284; https://doi.org/10.3390/molecules20023190
- Zhou Q, Gui J, Pan CM, Albone E, Cheng X, Suh EM, Grasso L, Ishihara Y. Baran PS Bioconjugation by native chemical tagging of C-H bonds. J Am Chem Soc 2013; 135(35):12994-7; PMID:23957305; https://doi.org/10.1021/ja407739y
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 2001; 158(3):905-19; PMID:11238039; https://doi.org/10.1016/S0002-9440(10)64038-2
- Carlin S, Khan N, Ku T, Longo VA, Larson SM, Smith-Jones PM. Molecular targeting of carbonic anhydrase IX in mice with hypoxic HT29 colorectal tumor xenografts. PLoS One 2010; 5(5):e10857; PMID:20523727; https://doi.org/10.1371/journal.pone.0010857
- Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer 2006; 5(1):50; PMID:17067392; https://doi.org/10.1186/1476-4598-5-50
- Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol 2003; 27(11):1418-28; PMID:12883236; https://doi.org/10.1097/00000478-200311000-00003
- Hassan R, Kindler HL, Jahan T, Bazhenova L, Reck M, Thomas A, Pastan I, Parno J, O'Shannessy DJ, Fatato P, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014; 20(23):5927-36; PMID:25231400; https://doi.org/10.1158/1078-0432.CCR-14-0804
- Lindenberg L, Thomas A, Adler S, Mena E, Kurdziel K, Maltzman J, Wallin B, Hoffman K, Pastan I, Paik CH, et al. Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using Single Photon Emission Computed Tomography-Computed Tomography (SPECT-CT) imaging. Oncotarget 2015; 6(6):4496-504; PMID:25756664; https://doi.org/10.18632/oncotarget.2883
- Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J Clin Oncol 2016; 34(34):4171-4179; PMID:27863199; https://doi.org/10.1200/JCO.2016.68.3672
- Gajewiak J, Azam L, Imperial J, Walewska A, Green BR, Bandyopadhyay PK, Raghuraman S, Ueberheide B, Bern M, Zhou HM, et al. A disulfide tether stabilizes the block of sodium channels by the conotoxin μO§-GVIIJ. Proc Natl Acad Sci U S A 2014; 111(7):2758-63; PMID:24497506; https://doi.org/10.1073/pnas.1324189111
- McSherry T, McSherry J, Ozaeta P, Longenecker K, Ramsay C, Fishpaugh J, Allen S. Cysteinylation of a monoclonal antibody leads to its inactivation. MAbs 2016; 8(4):718-25; PMID:27050640; https://doi.org/10.1080/19420862.2016.1160179
- Shopes B. A genetically engineered human IgG mutant with enhanced cytolytic activity. J Immunol 1992; 148(9):2918-22; PMID:1573276
- Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, Parsons-Reponte KL, Tien J, Yu SF, Mai E, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol 2012; 30(2):184-9; PMID:22267010; https://doi.org/10.1038/nbt.2108
- Arai H, Glabe C, Luecke H. Crystal structure of a conformation-dependent rabbit IgG Fab specific for amyloid prefibrillar oligomers. Biochim Biophys Acta 2012; 1820(12):1908-14; PMID:22940003; https://doi.org/10.1016/j.bbagen.2012.08.016
- Pan R, Sampson JM, Chen Y, Vaine M, Wang S, Lu S, Kong XP. Rabbit anti-HIV-1 monoclonal antibodies raised by immunization can mimic the antigen-binding modes of antibodies derived from HIV-1-infected humans. J Virol 2013; 87(18):10221-31; PMID:23864637; https://doi.org/10.1128/JVI.00843-13
- Pan R, Chen Y, Vaine M, Hu G, Wang S, Lu S, Kong XP. Structural analysis of a novel rabbit monoclonal antibody R53 targeting an epitope in HIV-1 gp120 C4 region critical for receptor and co-receptor binding. Emerg Microbes Infect 2015; 4(7):e44; PMID:26251831; https://doi.org/10.1038/emi.2015.44
- Zhai Q, He M, Song A, Deshayes K, Dixit VM, Carter PJ. Structural Analysis and Optimization of Context-Independent Anti-Hypusine Antibodies. J Mol Biol 2016; 428(3):603-17; PMID:26778617; https://doi.org/10.1016/j.jmb.2016.01.006
- Malia TJ, Teplyakov A, Brezski RJ, Luo J, Kinder M, Sweet RW, Almagro JC, Jordan RE, Gilliland GL. Structure and specificity of an antibody targeting a proteolytically cleaved IgG hinge. Proteins 2014; 82(8):1656-67; PMID:24638881; https://doi.org/10.1002/prot.24545
- Bujotzek A, Lipsmeier F, Harris SF, Benz J, Kuglstatter A, Georges G. VH-VL orientation prediction for antibody humanization candidate selection: A case study. MAbs 2016; 8(2):288-305; PMID:26637054; https://doi.org/10.1080/19420862.2015.1117720
- Shinmi D, Taguchi E, Iwano J, Yamaguchi T, Masuda K, Enokizono J, Shiraishi Y. One-Step Conjugation Method for Site-Specific Antibody-Drug Conjugates through Reactive Cysteine-Engineered Antibodies. Bioconjug Chem 2016; 27(5):1324-31; PMID:27074832; https://doi.org/10.1021/acs.bioconjchem.6b00133
- Ramil CP, Lin Q. Bioorthogonal chemistry: strategies and recent developments. Chem Commun (Camb) 2013; 49(94):11007-22; PMID:24145483; https://doi.org/10.1039/c3cc44272a
- Zhang YF, Ho M. Humanization of rabbit monoclonal antibodies via grafting combined Kabat/IMGT/Paratome complementarity-determining regions: Rationale and examples. MAbs 2017; 9:1-11; PMID:28165915; https://doi.org/10.1080/19420862.2017.1289302
- Spidel JL, Albone E, Cheng X, Park YC, Vaessen B, Jacob S, Milinichik A, Verdi A, Kline JB, and Grasso L. Engineering humanized antibody framework sequences for optimal site-specific conjugation of cytotoxins. MAbs 2017; ( in press).
- Niedbala WG, Stott DI. A comparison of three methods for production of human hybridomas secreting autoantibodies. Hybridoma 1998; 17(3):299-304; PMID:9708833; https://doi.org/10.1089/hyb.1998.17.299
- Spidel JL, Vaessen B, Chan YY, Grasso L, Kline JB. Rapid high-throughput cloning and stable expression of antibodies in HEK293 cells. J Immunol Methods 2016; 439:50-8; PMID:27677581; https://doi.org/10.1016/j.jim.2016.09.007