ABSTRACT
Both Pten and Nras are downstream mediators of receptor tyrosine kinase activation that plays important roles in controlling cell survival and proliferation. Here, we investigated whether and how Pten loss cross-talks with Nras activation in driving liver cancer development in mice. Somatic disruption of hepatic Pten and overexpression of Nras were achieved in out-bred immunocompetent CD-1 mice through a hydrodynamic delivery of plasmids carrying Sleeping Beauty transposon-based integration of Nras and the CRISPR/Cas9-mediated Pten knockout system. Concurrent Pten knockout and Nras knock-in induced hepatocellular carcinoma, while individual gene manipulation failed. Tumor development was associated with liver fibrosis, hyperlipidemia, hepatic deposition of lipid droplets and glycogen, and hepatomegaly. At the molecular level, lipid droplet formation was primarily contributed by upregulated expression of genes responsible for lipogenesis and fatty acid sequestration, such as Srebpf1, Acc, Pparg and its downstream targets. Our findings demonstrated that Pten disruption was synergized by Nras overexpression in driving hepatocyte malignant transformation, which correlated with extensive formation of lipid droplets.
Introduction
Pten acts as a phosphoinositide phosphatase in regulating cell survival and lipid metabolism.Citation1,2 Pten overexpression suppresses proliferation of mouse mammary epithelial cells, reduces migration of ovarian cancer cells, and promotes apoptosis of liver cancer cells.Citation3-5 Conversely, Pten deficiency is linked to the pathogenesis of various cancers.Citation1 In humans, hepatic loss of PTEN correlates with poor prognosis of patients with poorly differentiated hepatocellular carcinoma (HCC).Citation6 In animal models, heterozygous deletion of Pten causes neoplasia in multiple organ systems including the thymus and liver.Citation7 Hepatocyte-specific Pten knockout increases insulin sensitivity and induces fatty liver.Citation8 Germline deletion of hepatic Pten causes HCC in mice.Citation9 Somatic disruption of hepatic Pten leads to lipid accumulation in hepatocytes but fails to induce HCC.Citation10
Nras is a proto-oncogene whose activating mutation has been linked to many human cancers including HCC.Citation11,12 Sustained Nras activation resulting from overexpression of constitutively active Nras induces HCC in genetically compromised mice.Citation13,14 In immunocompetent mice, however, the carcinogenic effect of this genetic manipulation is limited due to cell senescence and immune clearance.Citation14,15 Considering that both Pten and Nras are downstream mediators of receptor tyrosine kinase activation, and both factors are implicated in the pathogenesis of HCC, we hypothesize that there is a crosstalk between Pten and Nras in driving initiation and progression of liver tumor.
In the current study, we used the techniques of genome editing and hydrodynamic gene delivery and examined the effects of Sleeping Beauty Transposon-based Nras knock-in, CRISPR-Cas9-mediated knockout of Pten, or combination of both on HCC development in immunocompetent CD-1 mice. We found that hepatic loss of Pten is synergized by Nras activation in driving HCC development and excessive lipid deposition in hepatocytes.
Materials and methods
Materials
The plasmids pT-Nras (#20205), pX330-Pten (#59909), and pCMV-SB-X100 (#34879) were obtained from Addgene (Cambridge, MA). pLIVE-GFP and pLIVE-RFP were prepared by inserting coding sequence of fluorescence protein into the multi-cloning site of pLIVE vectors from Mirus Bio (Madison, WC). Plasmids were prepared using cesium chloride–ethidium bromide density-gradient ultracentrifugation, and kept in saline at −80 °C until use. Purity of each plasmid preparation was examined with absorbency ratio at 260 and 280 nm determined by a GENESYS™ 10S UV-Vis Spectrophotometer from Fisher Scientific (Waltham, MA). The plasmid purity was also measured by 1% agarose gel electrophoresis. Periodic acid–Schiff (PAS) staining kit (#91022) were purchased from Newcomer Supply (Middleton, WI). Masson's trichrome staining kit (#26367) and Oil-red O staining solution (#26503–02) were from Electron Microscopy Sciences (Hatfield, PA). Nile Red staining reagent (#N3013) was purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies for p-Akt (#4060), p-Erk1/2 (#4094), and Pten (#9556), and Alexa Fluor 488-conjugated secondary antibodies were from Cell Signaling Technology (Boston, MA). Antibodies against Nras (sc-519) and Gapdh (sc-25778) were from Santa Cruz (Santa, CA), and anti-α-Sma antibody (#1E12) was from DSHB (Iowa City, IA). IHC detection kit (#ADI-950–122) was from Enzo Life Sciences (Farmingdale, NY). Western blotting substrate (#32209), kits for blood triglyceride (#TR22421), AST (#TR70121), and ALT (#TR71121) determination, and O.C.T. medium (#23–730–571) were from Fisher Scientific (Waltham, MA). Commercial kit for cholesterol determination (#C7510) was from Pointe Scientific (Canton, MI). RNeasy Lipid Tissue Mini Kit (#74804) was from QIAGEN (Valencia, CA). Superscript RT III enzyme kit (#11752–050) was from Life Technologies (Carlsbad, CA). ABI StepOne Plus Real-Time PCR system (#95072–012) using SYBR Green was from Quanta BioSciences (Gaithersburg, MD). PCR primers were synthesized at Sigma-Aldrich (St. Louis, MO).
Animal treatment
Male CD-1 mice (∼22 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed under standard conditions with a 12 h light-dark cycle. All animal procedures performed were approved by the Institutional Animal Care and Use Committee at the University of Georgia, Athens (Protocol number, A2014 07–008-Y1-A2). Hydrodynamic injection of plasmid DNA was performed using a previously established procedureCitation16 with a DNA dose of 20 μg/mouse and injection time of 5–8 s through the tail vein. The control animals were injected with pLIVE empty plasmids and pCMV-SB-X100 (2 μg/mouse).
Histological examination
Animals were killed at selected time points and liver samples collected and fixed using 10% neutral buffered formalin. Fixed tissues were dehydrated through gradient ethanol solutions and embedded in paraffin. Tissue sections were made at 6 μm in thickness. H&E staining was performed following the instruction from the manufacturer. Periodic acid–Schiff (PAS) staining was performed using a commercial kit following the protocol of the manufacture. Masson's trichrome staining was conducted according the instructions provided by Electron Microscopy Sciences. The tissue sections were examined using a Nikon ECLIPSE-Ti optical microscope and pictures were taken using the Nikon NIS-Elements AR system.
Determination of hepatic lipid level
Liver samples were freshly collected and stored at -80 °C until use. These samples were sectioned at 8 μm in thickness using a Cryostat, fixed in 10% neutral buffered formalin, and stained using Oil Red O solution. Nile Red staining was performed according to the manufacturer's instruction. Biochemical determination of hepatic triglyceride content has been previously reported.Citation17 Briefly, liver samples (∼200 mg) were homogenized in 1 ml of mixture of chloroform and methanol (2:1). The homogenates were incubated at 4 °C for 12 h before centrifugation at 12,000 g for 20 min. Supernatants were isolated, dried, and re-dissolved for triglyceride measurement using a commercial kit (#TR22421) from Fisher Scientific.
Immunohistochemistry (IHC) and immunofluorescence assay
Liver samples were collected from animals, embedded in O.C.T. medium, frozen in liquid nitrogen for 10 s, and stored at −80 °C until examination. Tissue sections were made at 8 µm in thickness, fixed in 10% neutral buffered formalin for 30 min, washed 3 time using phosphate buffered saline, and incubated with first antibody for 12 h at 4 °C, and subsequently stained with the second antibodies with appropriate conjugates for calorimetric development. The images were taken using a Nikon ECLIPSE-Ti fluorescence microscope with a Nikon NIS-Elements AR system.
Western blotting assay
Liver samples were homogenized in radioimmunoprecipitation assay buffer (25 mM Tris, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, pH 8.0) for 1 min using a Tissue Homogenizer and centrifuged for 20 min at 12,000 g. The supernatant was collected and loaded on a SDS-PAGE gel (50 μg proteins per lane) for separation. Semi-Dry transfer was performed to transfer the protein bands to PVDF membrane followed by blocking and washing with Tris-buffered saline buffer containing 5% non-fat milk. First antibody (p-Akt, 1:1000; p-Erk1/2, 1:1000; Nras, 1:1000; and Gapdh, 1:1000) staining was performed in blocking buffer for 12 h at 4 °C. The membranes were washed using phosphate buffered saline 3 times and incubated with the secondary antibody at room temperature for 1 h. Following 3 times washing, protein bands were visualized using the Pierce ECL Western Blotting substrate.
Measurements of blood concentrations of glucose, triglyceride, cholesterol, AST, and ALT
Mice were fasted for 4 h for measurement of fasting glucose. Blood samples (∼10 μL) were collected from tail veins of the mice and glucose level was determined using a glucometer. Triglyceride and cholesterol concentrations were determined using commercial kits from Fisher Scientific. Blood concentrations of AST and ALT were determined using commercial kits. All measurements were performed following the protocols provided by the manufactures.
Gene expression analyses
Total RNA was isolated from liver samples using RNeasy Lipid Tissue Mini Kit following the manufacturer's instructions. Two μg of total RNA was used for the first strand cDNA synthesis using a Superscript RT III enzyme kit from Life Technologies. Real-time PCR was performed in an ABI StepOne Plus Real-Time PCR system using SYBR Green as the indicator. The data were analyzed using the ΔΔCt method and normalized to internal control of Gapdh mRNA. Primers were synthesized at Sigma-Aldrich and their sequences are listed in Table S1. Melting curve analyses of all real-time PCR products were conducted and showed a single DNA duplex.
Statistics
All data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using the one-way analyses of variance test (ANOVA). A P value less than 0.05 (P < 0.05) was considered significant.
Results
Delivery and expression of transgenes in the liver by hydrodynamics-based procedure
We first confirmed hydrodynamic gene delivery efficiency and verified whether 2 separate plasmids can be co-delivered into the same set of hepatocytes in mice. Two reporter plasmids were used for this purpose, one carrying GFP and the other RFP (). A mixture of plasmid DNA in a 1:1 molar ratio was hydrodynamically injected into mouse tail vein and mice were killed 3 d later. Florescence photo images shown in suggest a successful delivery of pLIVE-GFP and pLIVE-RFP plasmids into mouse liver. Overlay of the 2 color images provide direct evidence in support that both reporter genes were expressed in the same set of cells. Using the same procedure, 4 groups of animals were injected with different plasmid or in combination as schematically presented in . For Nras knock-in, each mouse received a hydrodynamic injection of pT-Nras (18 µg) and pCMV-SB-X100 (2 µg). For Pten deletion, mice received an injection of pX330-Pten (20 µg per mouse). For animals receiving both Nras and Pten systems, each mouse was injected with pT-Nras (18 µg), pCMV-SB-X100 (2 µg), and pX330-Pten (20 µg). Control mice received pLIVE empty plasmids (18 µg per muse) and pCMV-SB-X100 (2 µg per mouse).
Figure 1. Illustration of the systems used in this study. (A) Schematic illustration of concurrent transfer of GFP and RFP gene into mouse liver via a hydrodynamics-based procedure. (B) Representative images of liver sections showing overexpressed GFP and RFP. Liver samples were collected 3 d after hydrodynamic plasmid injection. Scale bar = 200 μm. (C) Schematic illustration of plasmids for Nras knock-in and Pten knockout.
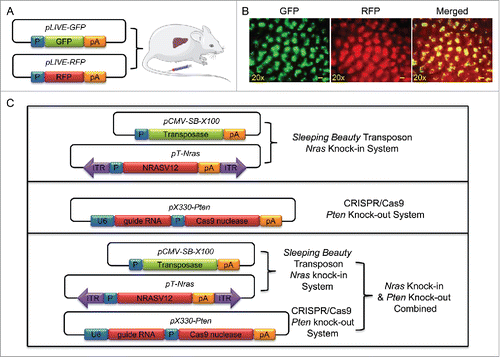
Hydrodynamic delivery of pT-Nras and pX330-Pten plasmids together induces tumor in mouse liver
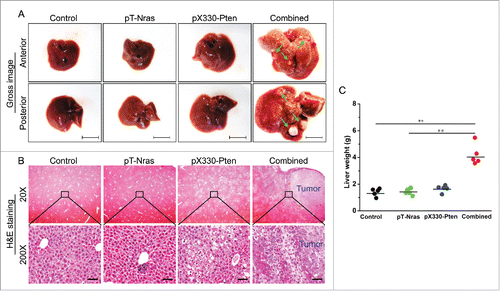
All mice were killed 16 weeks after plasmid injection and livers were collected. Results in show anterior and posterior appearance of the livers. Depending on the plasmids injected, the livers of animals receiving control, pT-Nras/pCMV-SB-X100, or pX330-Pten plasmids appear normal in contrast to those containing numerous tumor nodules of animals receiving combination of pT-Nras/pCMV-SB-X100/pX330-Pten plasmids. H&E staining of the liver sections show tumor nodules in the livers of animals receiving combined plasmids ( and Fig. S1A). Vacuole-like structures were seen in the liver of animals injected with pX330-Pten. Tumor development was associated with severe hepatomegaly which was ∼2.9-fold heavier than the control ().
Figure 2. Pten disruption was synergized by Nras activation in driving liver tumor development. Liver samples from 5 mice in each group were collected 16 weeks after hydrodynamic plasmid injection. (A) Representative images of livers injected with different plasmids. Nodules are indicated by arrows; Bar = 1 cm. (B) Representative images of H&E staining of liver sections. Scale bar = 50 μm. (C) Liver weight. ** P < 0.01 compared with the control. ## P < 0.01 compared with mice injected with pT-Nras.
Tumor development in the liver is associated with overexpression of Nras, lack of Pten and activation of downstream components of receptor tyrosine kinase activated pathways
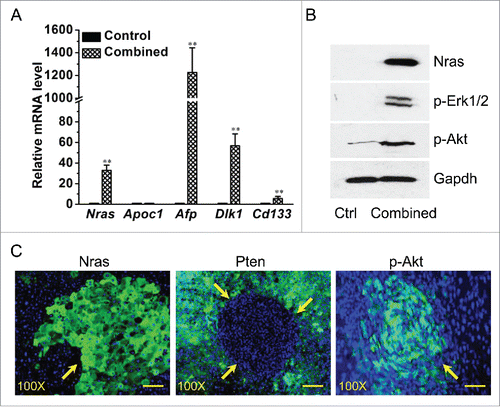
Successful Nras knock-in and Pten knockout was confirmed at both mRNA and protein levels. Results in show a ∼33-fold increase of Nras transcript in the liver with combined treatment comparing to the basal level of the control. As expected, increase in mRNA levels of HCC marker genes including Afp (∼1226.2-fold), Dlk1 (∼56.2-fold), and Cd133 (∼5.7-fold) were also seen in the livers, comparing to no change in mRNA level of Apoc1, a hepatocyte marker gene. The results from a Western blotting assay () show a significant elevation of Nras protein in mice with combined plasmid injection, which is accompanied by an increase of phosphorylated Erk1/2 (p-Erk1/2) and Akt (p-Akt), suggesting Nras overexpression and knockout of Pten has led to activation of other members of the receptor tyrosine kinase pathway. Immunofluorescence microscopy was used to confirm the loss of Pten in the tumor and overexpression of Nras. Liver sections show strong positive of Nras and complete lack of Pten in the tumor nodules (). Activated Akt in phosphorylated form (pAkt) was also detected in the tumor area of liver section ().
Figure 3. Characterization of the liver tumor resulting from combined Nras overexpression and Pten disruption. Liver samples from 5 mice in each group were collected 16 weeks after hydrodynamic plasmid injection. (A) Expression levels of Nras, Apoc1 and liver cancer marker genes. (B). Western blotting determination of Nras, and phosphorylated Akt and Erk1/2. (C) Immunofluorescence microscopy determination of Nras, Pten, and phosphorylated Akt. The green color indicates target proteins of Nras, Pten, and p-Akt in each figure, while the blue color indicates nuclei. Scale bar = 20 μm. Values in (A) represent average ± SD (n = 5). ** P < 0.01 compared with the control.
Pten knockout resulted in hepatic deposition of lipid droplets and glycogen
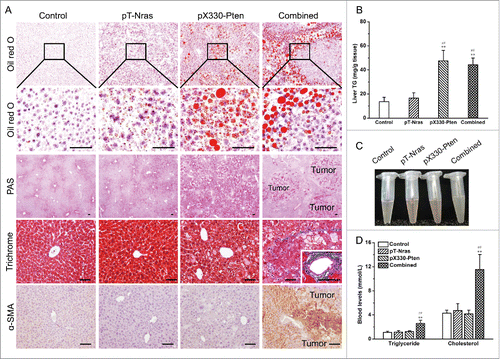
To further characterize the consequences of these genetic manipulations, we performed a series of histological examinations. Oil red O staining demonstrated that hepatic loss of Pten promoted lipid droplet formation in hepatocytes (), which was further confirmed via Nile Red staining (Fig. S1B). Biochemical examinations provided the same conclusion showing that Pten loss increased hepatic triglyceride content by ∼3.2- and ∼3.5-fold in mice with or without Nras overexpression, respectively (). Pten loss also promoted hepatic deposition of glycogen, as evidenced by the extensive red dots in the Periodic acid-Schiff (PAS) staining (). Masson's trichrome staining shows blue layers only in mice receiving the combined plasmid injection, suggesting that liver fibrosis was tightly correlated with tumor nodule development (). Consistently, α-smooth muscle actin (α-SMA) upregulation, as evidenced by the enhanced brownish color, was only observed in mice receiving the combined plasmid injection (). These pathological changes were accompanied by elevated level of AST and ALT, indicating liver tissue damage (Fig. S1C and 1D). We next studied the changes in blood lipid and glucose level. Combined injection of plasmids with Nras knock-in and Pten knockout components resulted in an increase of turbid of plasma (). The combined treatment also increased blood levels of triglyceride and cholesterol by ∼2.4- and ∼2.7-fold (), respectively, while showing no change in fasting and non-fasting glucose level (Fig. S1E). Together, these results demonstrated that Pten loss profoundly affected energy metabolism, leading to hepatic aggregation of lipid droplets and glycogen, which subsequently contributed to HCC progression.
Figure 4. Pten disruption resulted in hepatic deposition of lipid droplets and glycogen. Samples from 5 mice in each group were collected 16 weeks after plasmid injection. (A) Representative images of Oil red O staining, periodic acid-Schiff (PAS) staining, Masson's trichrome staining, and α-smooth muscle actin (α-SMA) immunohistochemistry. Scale bar = 50 μm. (B) Hepatic triglycerides content. (C) Representative images of plasma. (D) Blood levels of triglyceride and cholesterol. Values in (B) and (D) represent average ± SD (n = 5). ** P < 0.01 compared with the control; ## P < 0.01 compared with mice injected with pT-Nras.
Pten loss and Nras overexpression affect the expression of genes involved in energy metabolism
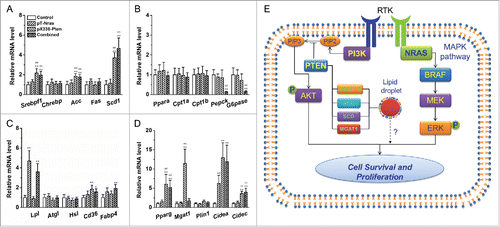
Results in suggest that Pten may play an important role in regulating energy metabolism. To explore the underlying mechanism, we examined mRNA levels of a panel of genes involved in lipid and glucose metabolism.Citation18-20 shows that Pten loss elevated mRNA levels of genes responsible for lipogenesis including Srebpf1 (∼2.0-fold), Acc (∼1.8-fold), and Scd-1 (∼4.7-fold) while Nras overexpression has no impact. Among the genes responsible for lipid oxidation and gluconeogenesis, we found significant reductions in mRNA level for Pepck (∼87%) and G6pase (∼85%) in mice receiving injection of combined plasmids (). Examination of mRNA levels for genes involved in triglyceride digestion and intracellular transport of fatty acid revealed that Nras knock-in increased Lpl mRNA level by ∼3.6 and ∼4.7-fold in mice with or without additional Pten disruption, respectively (). Of note, Pten disruption increased transcription of most of the genes responsible for fatty acid sequestration and lipid droplet formation, including Pparg and its downstream target Cidea and Cidec, while Nras overexpression generated no additional changes (). These data suggested that Pten loss and Nras overexpression regulated the expression of different set of genes in lipogenesis, gluconeogenesis, lipid oxidation, and lipid droplet formation, which may collectively contributed to HCC development.
Figure 5. Pten loss and Nras overexpression affect mRNA levels of genes involving in energy metabolism. Liver samples from 5 mice in each group were collected 16 weeks after plasmid injection. (A) Expression of genes responsible for de novo lipogenesis. (B) mRNA levels of genes responsible for fatty acid oxidation and gluconeogenesis. (C) Expression of genes for triglyceride hydrolysis and intracellular fatty acid transport. (D) Transcription levels of genes for fatty acid sequestration and lipid droplet formation. (E) Schematic diagram illustrating the possible mechanism underlying the synergistic effect between Pten loss and Nras overexpression. Pten loss activates AKT and meanwhile drives lipid droplets formation. Nras overexpression turns on the MAPK pathway leading to hepatocyte proliferation. Concurrent loss of Pten and overexpression of Nras promoted survival and proliferation of pre-malignant hepatocytes, resulting in liver cancer. Values in (A)-(D) represent average ± SD (n = 5). ** P < 0.01 compared with the control; ## P < 0.01 compared with mice injected with pT-Nras.
Discussion
Both Pten and Nras are downstream mediators of receptor tyrosine kinase activation, and mutations of these genes are linked to the pathogenesis of HCC.Citation12 Despite a long latency (∼74–78 weeks), germline disruption of hepatic Pten gave rise to steatohepatitis and HCC in mice with a C57BL/6 background.Citation9 Similarly, sustained activation of Nras induced HCC in genetically compromised mice.Citation14 Somatic manipulation of these individual genes, however, were limited in inducing HCC on wide type mice, probably due to immune clearance and other unknown reasons.Citation10,14,15 In line with these previous reports, the current investigation demonstrated that neither Pten deficiency nor Nras overexpression alone was sufficient to induce HCC in the liver of wide type immunocompetent mice in a period of 16 weeks (). Importantly, we demonstrate that combination of Pten disruption and overexpression of Nras induced HCC development in mice under the same experimental settings. The clinical relevance of our findings requires additional studies using human tissue samples. In HCC patients, the incidence of NRAS mutation is much lower than other common oncogenes and tumor suppressors such as CTNNB1 and TP53.Citation12 Despite its low incidence, we found in the current study that the oncogenic activity of Nras activation can be substantially promoted by Pten loss, suggesting a synergistic interaction between these 2 pathways in causing HCC. It is likely that, in humans, NRAS mutation alone is not sufficient to induce HCC and additional genetic defect is required for malignant transformation.
Somatic editing of mouse genome represents a new strategy in cancer research. Comparing to germline genetic manipulation, this new strategy has an advantage of not interfering with the tissue development process, and therefore better mimicking cancer pathogenesis. Somatic genome editing can be executed via various systems, such as the Sleeping Beauty Transposon and the CRISPR-Cas9 system. The Sleeping Beauty Transposon system has been long used in studying oncogenes and establishing animal cancer models.Citation13,21-24 When performed through a hydrodynamic tail vein injection, this system primarily targets the hepatocytes for transgene overexpression or knockdown, and therefore is particularly useful in studying liver cancer.Citation25 Compared with the conventional system, the newly developed CRISPR-Cas9 system is more precise in genome editing, despite a relatively low efficiency when performed in vivo without additional selection.Citation10,26 Through a combination of both systems, we achieved concurrent Nras overexpression and Pten disruption in mouse liver, as evidenced by immunofluorescence verification and Western blotting of the activation of their downstream members of the pathway (). The same strategy can also be used for investigating other oncogenes and tumor suppressors.
The tumor nodules induced by concurrent Nras and Pten manipulation displayed a series of pathological features of human HCC such as rapid proliferation, hepatic steatosis, tissue fibrosis, glycogen deposition, and hepatomegaly ( and ), suggesting that these nodules were HCC. This notion was further supported by elevated expression of HCC marker genes including Afp, Dlk1, and Cd133 (). Hepatic content of triglyceride was increased in mice with injection of pX330-Pten plasmids with and without additional Nras knock-in while hyperlipidemia was observed only in mice with tumor nodules resulting from combined plasmid injection (), indicating that the resulting hyperlipidemia was likely secondary to tumor development. Lipid droplet deposition was common in early stage HCCs,Citation27-29 while glycogen accumulation was frequently reported in clear cell HCC.Citation30 In fact, increasing evidence suggests that dysregulated glucose and lipid metabolism contributed substantially to HCC development.Citation31-33 Since the mice receiving concurrent genetic manipulation of Nras and Pten showed multiple features of HCC and hepatic lipid dysregulation, they can serve as an animal model for pathophysiological study between fatty liver and liver cancer.
The HCC resulting from concurrent Pten knockout and Nras overexpression was accompanied with formation of lipid droplets in the liver and severe hyperlipidemia in blood (). Lipid droplets as an intracellular organelle can be found in many cancer cells, particularly in HCC and renal cellular carcinoma.Citation27 One recent study by Dr. Straub and colleagues revealed that cells in about 2-thirds of HCCs patients contain abundant lipid droplets.Citation27 Despite its abundance in HCC, the exact role of the lipid droplet in HCC development remains speculative.Citation27,28 It is possible that lipid droplets serve as energy source and contribute to cancer development. It is not clear whether they play a role in providing structural support or involving signal transduction inside the rapidly proliferating cancerous cells.Citation27,34,35 At molecular level, the increased lipid droplets were primarily induced by elevated expression of genes responsible for lipogenesis and fatty acid sequestration,Citation18,19 including Srebpf1, Acc, Scd1, Pparg and its downstream targets (). Based on the gene expression data, it is conceivable that under the condition of Nras overexpression, the lipid droplets resulting from Pten knockout rendered pre-malignant hepatocytes resistant to metabolic stress, cell senescence, and immune clearance, thereby promoting cell survival and proliferation, and eventually leading to cancer development (). Considering that upregulated expression of genes for hepatic lipid accumulation was correlated with poor prognosis in patients with HCC,Citation36 additional work is warranted to further elucidate the precise role of lipid droplet in HCC initiation and progression.
In conclusion, we found in this study that somatic loss of hepatic Pten was synergized by Nras overexpression in giving rise to HCC and extensive formation of lipid droplets in hepatocytes. These findings may have significant implications for liver cancer prevention, diagnosis, and treatment in patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary_Materials.zip
Download Zip (333.5 KB)Acknowledgments
The study was supported in part by a grant from NIH (RO1HL098295). We would like to thank Dr. Megan Morgan for English editing.
References
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer 2011; 11:289-301; PMID:21430697; https://doi.org/10.1038/nrc3037
- Peyrou M, Bourgoin L, Foti M. PTEN in liver diseases and cancer. World J Gastroenterol 2010; 16:4627-33; PMID:20872961; https://doi.org/10.3748/wjg.v16.i37.4627
- Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest 2002; 110:815-25; PMID:12235113; https://doi.org/10.1172/JCI0213829
- Saga Y, Mizukami H, Takei Y, Ozawa K, Suzuki M. Suppression of cell migration in ovarian cancer cells mediated by PTEN overexpression. Int J Oncol 2003; 23:1109-13; PMID:12963992; https://doi.org/10.3892/ijo.23.4.1109
- Li MF, Guan H, Zhang DD. Effect of overexpression of PTEN on apoptosis of liver cancer cells. Genet Mol Res 2016; 15:1-9; PMID:27173355; https://doi.org/10.4238/gmr.15028120
- Zhu X, Qin X, Fei M, Hou W, Greshock J, Bachman KE, Wooster R, Kang J, Qin CY. Combined phosphatase and tensin homolog (PTEN) loss and fatty acid synthase (FAS) overexpression worsens the prognosis of Chinese patients with hepatocellular carcinoma. Int J Mol Sci 2012; 13:9980-91; PMID:22949843; https://doi.org/10.3390/ijms13089980
- Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci U S A 1999; 96:1563-8; PMID:9990064; https://doi.org/10.1073/pnas.96.4.1563
- Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc Natl Acad Sci U S A 2004; 101:2082-7; PMID:14769918; https://doi.org/10.1073/pnas.0308617100
- Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest 2004; 113:1774-83; PMID:15199412; https://doi.org/10.1172/JCI20513
- Xue W, Chen S, Yin H, Tammela T, Papagiannakopoulos T, Joshi NS, Cai W, Yang G, Bronson R, Crowley DG, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 2014; 514:380-4; PMID:25119044; https://doi.org/10.1038/nature13589
- Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochim Biophys Acta 2007; 1773:1177-95; PMID:17428555; https://doi.org/10.1016/j.bbamcr.2007.01.012
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers 2016; 2:16018; PMID:27158749; https://doi.org/10.1038/nrdp.2016.18
- Carlson CM, Frandsen JL, Kirchhof N, McIvor RS, Largaespada DA. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc Natl Acad Sci U S A 2005; 102:17059-64; PMID:16286660; https://doi.org/10.1073/pnas.0502974102
- Rudalska R, Dauch D, Longerich T, McJunkin K, Wuestefeld T, Kang TW, Hohmeyer A, Pesic M, Leibold J, von Thun A, et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med 2014; 20:1138-46; PMID:25216638; https://doi.org/10.1038/nm.3679
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547-51; PMID:22080947; https://doi.org/10.1038/nature10599
- Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999; 6:1258-66; PMID:10455434; https://doi.org/10.1038/sj.gt.3300947
- Gao M, Bu L, Ma Y, Liu D. Concurrent activation of liver X receptor and peroxisome proliferator-activated receptor alpha exacerbates hepatic steatosis in high fat diet-induced obese mice. PLoS One 2013; 8:e65641; PMID:23762402; https://doi.org/10.1371/journal.pone.0065641
- Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 2010; 45:199-214; PMID:20218765; https://doi.org/10.3109/10409231003667500
- Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011; 1812:1007-22; PMID:21382489; https://doi.org/10.1016/j.bbadis.2011.02.014
- Yabaluri N, Bashyam MD. Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci 2010; 35:473-84; PMID:20826956; https://doi.org/10.1007/s12038-010-0052-0
- Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, Kay MA, Wang R, Bishop JM. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A 2007; 104:14771-6; PMID:17785413; https://doi.org/10.1073/pnas.0706578104
- Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, Long C, Demorest ZL, Zamora EA, Low WC, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res 2009; 69:431-9; PMID:19147555; https://doi.org/10.1158/0008-5472.CAN-08-1800
- Ho C, Wang C, Mattu S, Destefanis G, Ladu S, Delogu S, Armbruster J, Fan L, Lee SA, Jiang L, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology 2012; 55:833-45; PMID:21993994; https://doi.org/10.1002/hep.24736
- Ivics Z, Li MA, Mates L, Boeke JD, Nagy A, Bradley A, Izsvák Z. Transposon-mediated genome manipulation in vertebrates. Nat Methods 2009; 6:415-22; PMID:19478801; https://doi.org/10.1038/nmeth.1332
- Chen X, Calvisi DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am J Pathol 2014; 184:912-23; PMID:24480331; https://doi.org/10.1016/j.ajpath.2013.12.002
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014; 157:1262-78; PMID:24906146; https://doi.org/10.1016/j.cell.2014.05.010
- Straub BK, Herpel E, Singer S, Zimbelmann R, Breuhahn K, Macher-Goeppinger S, Warth A, Lehmann-Koch J, Longerich T, Heid H, et al. Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Mod Pathol 2010; 23:480-92; PMID:20081801; https://doi.org/10.1038/modpathol.2009.191
- Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol 2000; 33:282-9; PMID:10952246; https://doi.org/10.1016/S0168-8278(00)80369-4
- Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009; 49:658-64; PMID:19177576; https://doi.org/10.1002/hep.22709
- Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int 1996; 46:503-9; PMID:8870006; https://doi.org/10.1111/j.1440-1827.1996.tb03645.x
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012; 56:952-64; PMID:22173168; https://doi.org/10.1016/j.jhep.2011.08.025
- Mello T, Materozzi M, Galli A. PPARs and mitochondrial metabolism: from NAFLD to HCC. PPAR Res 2016; 2016:7403230; PMID:28115925; https://doi.org/10.1155/2016/7403230
- Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012; 56:1384-91; PMID:22326465; https://doi.org/10.1016/j.jhep.2011.10.027
- Koizume S, Miyagi Y. Lipid Droplets: A key cellular organelle associated with cancer cell survival under normoxia and hypoxia. Int J Mol Sci 2016; 17:1430; PMID:27589734; https://doi.org/10.3390/ijms17091430
- Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids 2010; 82:243-50; PMID:20206487; https://doi.org/10.1016/j.plefa.2010.02.005
- Yamashita T, Honda M, Takatori H, Nishino R, Minato H, Takamura H, Ohta T, Kaneko S. Activation of lipogenic pathway correlates with cell proliferation and poor prognosis in hepatocellular carcinoma. J Hepatol 2009; 50:100-10; PMID:19008011; https://doi.org/10.1016/j.jhep.2008.07.036
