ABSTRACT
Pemetrexed is an approved therapeutic in NSCLC and ovarian cancer. Our studies focused on the ability of [pemetrexed + sildenafil] exposure to alter the immunogenicity of lung and ovarian cancer cells. Treatment of lung and ovarian cancer cells with [pemetrexed + sildenafil] in vitro rapidly reduced the expression of PD-L1, PD-L2 and ornithine decarboxylase (ODC), and increased the expression of class I MHCA. In a cell-specific fashion, some cells also released the immunogenic nuclear protein HMGB1 into the extracellular environment. [Pemetrexed + sildenafil] reduced the expression of multiple histone deacetylases that was blocked by knock down of autophagy regulatory proteins. [Pemetrexed + sildenafil] lethality was enhanced by the histone deacetylase inhibitors AR42 and sodium valproate; AR42 and valproate as single agents also rapidly reduced the expression of PD-L1, PD-L2 and ODC, and increased expression of MHCA and CerS6. Nitric oxide and CerS6 signaling was required for drug-induced death receptor activation and tumor cell killing. In vivo, [pemetrexed + sildenafil] lethality against lung cancer cells was enhanced by sodium valproate. Using syngeneic mouse lung cancer cells [pemetrexed + sildenafil] enhanced the anti-tumor effects of antibodies directed to inhibit PD-1 or CTLA4. [Pemetrexed + sildenafil] interacted with the anti-PD-1 antibody to strongly enhance tumor infiltration by M1 macrophages; activated NK cells and activated T cells. Our data demonstrate that treatment of tumor cells with [pemetrexed + sildenafil] results in tumor cell killing and via autophagy-dependent downregulation of HDACs, it opsonizes the remaining tumor cells to anti-tumor immunotherapy antibodies.
Abbreviations
| ERK | = | extracellular regulated kinase |
| PI3K | = | phosphatidyl inositol 3 kinase |
| ca | = | constitutively active |
| dn | = | dominant negative |
| ER | = | endoplasmic reticulum |
| mTOR | = | mammalian target of rapamycin |
| JAK | = | Janus Kinase |
| STAT | = | Signal Transducers and Activators of Transcription |
| MAPK | = | mitogen activated protein kinase |
| PTEN | = | phosphatase and tensin homolog on chromosome 10 |
| ROS | = | reactive oxygen species |
| CMV | = | empty vector plasmid or virus |
| si | = | small interfering |
| SCR | = | scrambled |
| IP | = | immunoprecipitation |
| VEH | = | vehicle |
| PTX | = | pemetrexed |
| SIL | = | sildenafil |
| HDAC | = | histone deacetylase |
Introduction
It has recently been shown that the lung and ovarian cancer approved drug pemetrexed (Alimta®) interacts with phosphodiesterase 5 (PDE5) inhibitors such as sildenafil (Viagra) to cause endoplasmic reticulum stress PERK-eIF2a-dependent -induced downregulation of cyto-protective proteins such as c-FLIP-s, MCL-1 and BCL-XL, as well as increasing Beclin1 expression.Citation1,Citation2 One component of killing was via increasing the levels of toxic autophagosomes. Another key component of cell killing was through the activation of extant death receptors (CD95) and PERK-eIF2a-CHOP -dependent increased expression of other death receptors (DR4, DR5). Knock down of CD95, DR4 and DR5 variably prevented the drug combination from killing. Perhaps the most surprising observation from those studies was that one component of the [pemetrexed + sildenafil] killing mechanism occurred via downregulation of histone deacetylase (HDAC) 6 that was associated with increased HSP90 acetylation and reduced HSP90 ATPase activity. It is known that HDAC inhibitors such as valproate, vorinostat and AR42 can combine with multi-kinase inhibitors such as sorafenib or pazopanib to down-regulate and to inhibit the HSP90 and HSP70 chaperone proteins.Citation3–6
Immunotherapy has become a first line therapeutic regimen in non-small cell lung cancer (NSCLC). Antibodies that blockade the functions of PD-1 and PD-L1 have all been approved as lung cancer therapeutics within the last 5 y.Citation7–12 Recent studies have shown that transient in vivo treatment of melanoma tumors growing in athymic mice with the HDAC inhibitor AR42 or with [pazopanib + AR42] results in a significant increase in animal survival with surviving tumors at animal nadir expressing greater levels of MHCA and HMGB1-HSP70 co-localization, and lower levels of PD-L1.Citation6,Citation13 Additional work then demonstrated that AR42 or sodium valproate enhanced the efficacy of an anti-PD-1 or an anti-CTLA4 antibody to suppress melanoma tumor growth.Citation13
The present studies are a continuation of earlier work combining [pemetrexed + sildenafil], as well as analyses with the multi-kinase and chaperone inhibitor pazopanib with the histone deacetylase inhibitor AR42, and of this drug combination with immunotherapy approaches. In the present manuscript, we demonstrate that [pemetrexed + sildenafil], in NSCLC and ovarian cancer cells, reduces the expression of PD-L1, PD-L2 and ornithine decarboxylase (ODC) and increases the expression of the class I MHC molecule MHCA. In many tumor isolates [pemetrexed + sildenafil] also promoted the extracellular release of the immunogenic protein HMGB1.Citation14 In vivo [pemetrexed + sildenafil] and [pemetrexed + sildenafil + valproate] enhanced the anti-tumor efficacy of an anti-PD-1 antibody or of an anti-CTLA4 antibody in a mouse model of lung cancer.
Results
Previously, we have published that [pemetrexed + sildenafil] reduced the protein expression of HDAC6, as judged by immunofluorescence and immunoblotting, that correlated with increased acetylation of HSP90.Citation1 In the present studies, we discovered that [pemetrexed + sildenafil] exposure reduced the expression of multiple histone deacetylase proteins in lung and ovarian cancer cells within 6h (). The HDACs whose expression was consistently reduced in both tumor types were HDAC2, HDAC4, HDAC6 and HDAC9. The reduction in HDAC6 levels caused by [pemetrexed + sildenafil] in NSCLC cells was blocked by knock down of AMPKa, Beclin1 or ATG5 (). Similar Beclin1-dependent effects, and ATG5-dependent effects (not shown), were observed for the other downregulated HDACs in a PDX lung cancer model, and in PDX models of ovarian cancer and melanoma (; Figure S1).
Figure 1. Treatment of NSCLC cells and ovarian cancer cells with [pemetrexed + sildenafil] reduces the protein expression of multiple histone deacetylase proteins. A. NSCLC cells and ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of HDACs1–11 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells. B. NSCLC cells were transfected with a scrambled siRNA or siRNA molecules to knock down the expression of the AMPK α subunit, ATG5 or Beclin1. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunostaining performed to determine the expression of HDAC6 (n = 3 +/−SEM). *p < 0.05 lower than corresponding values in cells with knock down of AMPK, ATG5 or Beclin1; #p < 0.05 greater than corresponding vehicle control value. C. The PDX NSCLC isolate ADOR was transfected with a control siRNA or with an siRNA to knock down Beclin1 expression. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then fixed in place and the expression of the HDAC proteins determined by immunostaining. (n = 3 +/−SEM) * p < 0.05 less than corresponding intensity in siSCR cells.
![Figure 1. Treatment of NSCLC cells and ovarian cancer cells with [pemetrexed + sildenafil] reduces the protein expression of multiple histone deacetylase proteins. A. NSCLC cells and ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of HDACs1–11 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells. B. NSCLC cells were transfected with a scrambled siRNA or siRNA molecules to knock down the expression of the AMPK α subunit, ATG5 or Beclin1. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunostaining performed to determine the expression of HDAC6 (n = 3 +/−SEM). *p < 0.05 lower than corresponding values in cells with knock down of AMPK, ATG5 or Beclin1; #p < 0.05 greater than corresponding vehicle control value. C. The PDX NSCLC isolate ADOR was transfected with a control siRNA or with an siRNA to knock down Beclin1 expression. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then fixed in place and the expression of the HDAC proteins determined by immunostaining. (n = 3 +/−SEM) * p < 0.05 less than corresponding intensity in siSCR cells.](/cms/asset/0eb21e4a-3226-4d28-9bc3-d18ec0ce3b89/kcbt_a_1362511_f0001_b.gif)
The histone deacetylase inhibitors AR42 and sodium valproate both enhanced the lethality of [pemetrexed + sildenafil] against NSCLC cells as well as ovarian cancer cells (; Figures S2-S4). Knock down of individual HDAC proteins revealed that as a single knock down, only loss of HDAC3 strongly enhanced [pemetrexed + sildenafil] lethality (; Figures S5 and S6). Combined knock down of HDAC6 with HDACs 1 / 2 / 8 / 10 enhanced drug combination lethality more than either individual knock down. These events correlated with the HDAC inhibitor sodium valproate enhancing CD95 expression; previously we demonstrated that [pemetrexed + sildenafil] killed NSCLC cells in part via CD95 activation.Citation1
Figure 2. [Pemetrexed + sildenafil] lethality is enhanced by HDAC inhibitors. A. NSCLC cells were treated with vehicle control, [pemetrexed (1 μM) + sildenafil (2 μM)], sodium valproate (250 μM) or together in the indicated 3 drug combinations. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value. B. A549 cells were transfected with an siRNA control (siSCR) or siRNA molecules to knock down HDACs 1/2/3/6/8/10. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)]. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value; ## p < 0.05 greater than corresponding value in HDAC6 knock down cells.
![Figure 2. [Pemetrexed + sildenafil] lethality is enhanced by HDAC inhibitors. A. NSCLC cells were treated with vehicle control, [pemetrexed (1 μM) + sildenafil (2 μM)], sodium valproate (250 μM) or together in the indicated 3 drug combinations. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value. B. A549 cells were transfected with an siRNA control (siSCR) or siRNA molecules to knock down HDACs 1/2/3/6/8/10. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)]. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value; ## p < 0.05 greater than corresponding value in HDAC6 knock down cells.](/cms/asset/9b7403d3-9b8d-4557-bf1f-794ba1ba6ef9/kcbt_a_1362511_f0002_b.gif)
HDAC6 has been proposed to regulate the acetylation and expression of proteins involved in cell cycle control and cell viability e.g. CD95, BAX, cyclin D1, p21WAF1.Citation21–24 In lung cancer cells [pemetrexed + sildenafil] exposure increased the acetylation of HSP90 and p65 NFkB, increased the expression of BAX, and decreased the expression of cyclin D1, p21WAF1 and BCL-XL (Figure S7A). [Pemetrexed + sildenafil] exposure rapidly reduced the expression of double mutated active ERBB1 in the H1975 NSCLC line without altering the expression levels of the transcription factor proposed to regulate its expression, mutant p53 (Figure S7B).
The molecular mechanism by which pemetrexed and sildenafil interacted to kill tumor cells required death receptor signaling.Citation1,Citation2 Prior studies from our group have linked the generation of reactive oxygen species and of the lipid ceramide to drug-induced CD95 activation.Citation15–17 Treatment of cells with [pemetrexed + sildenafil] increased the levels of multiple dihydro-ceramide species (). The increase in ceramide levels was abolished by the pan-nitric oxide synthase inhibitor L-N G-Nitroarginine methyl ester; N(G)-Nitro-L-arginine methyl ester (L-NAME) whereas the reactive oxygen species detoxifying enzyme thioredoxin could partially reduce the increase only in C16 and C22 ceramide levels. The generation of C16 dihydro-ceramide requires the actions of ceramide synthase 6 (CerS6, LASS6).Citation18 Based on the data in , we determined whether molecular knock down of HDAC expression altered the protein levels of CerS6.Citation19,Citation20 Knock down of HDACs1/2/3 and HDACs8/10 enhanced CerS6 expression (). Treatment of NSCLC cells with L-NAME blocked the formation of autophagosomes and autolysosomes after [pemetrexed + sildenafil] treatment (). The [pemetrexed + sildenafil]-induced increase in CerS6 expression was blocked by L-NAME and by knock down of Beclin1 ( and ). Thus, L-NAME blocks the NO-dependent induction of autophagy, which blocks the reduction in HDAC1/HDAC2 expression, which blocks the [pemetrexed + sildenafil]-dependent induction of CerS6 expression.
Figure 3. Pemetrexed and sildenafil interact to enhance ceramide levels via CerS6 in a nitric oxide-dependent fashion. A. A549 NSCLC cells (1 × 106) were transfected with an empty vector plasmid (CMV) or with a plasmid to express thioredoxin (TRX). Twenty-four h after transfection cells were treated with vehicle control or L-NAME (10 μM). Thirty minutes later cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then harvested in 550 µl of cold PBS and 50 µl taken for lysis and protein determination using the Bradford Assay (Bio-Rad). Sphingolipid levels were normalized based on total protein levels for each sample. Cells were processed and subjected to quantitative mass spectrometry to determine the levels of sphingolipid species (n = 3 +/−SEM). #p < 0.05 greater than corresponding vehicle control; *p < 0.05 less than corresponding value in CMV transfected cells. B. NSCLC cells were transfected with a scrambled control (siSCR) or with various validated siRNA molecules to knock down the expression of the indicated histone deacetylase (HDAC) proteins. Twenty-four h after transfection, cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater fluorescence than siSCR control; * p < 0.05 less fluorescence than siSCR control. C. A549, H460, H1975 and ADOR cells were transfected with a plasmid to express LC3-GFP-RFP. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h and 12h. The mean number of intense GFP+ and RFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). The data for each bar is the mean level of GFP+/RFP+ vesicles collectively from all 4 cell lines. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of Beclin1. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. After 6h cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater than vehicle control.
![Figure 3. Pemetrexed and sildenafil interact to enhance ceramide levels via CerS6 in a nitric oxide-dependent fashion. A. A549 NSCLC cells (1 × 106) were transfected with an empty vector plasmid (CMV) or with a plasmid to express thioredoxin (TRX). Twenty-four h after transfection cells were treated with vehicle control or L-NAME (10 μM). Thirty minutes later cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then harvested in 550 µl of cold PBS and 50 µl taken for lysis and protein determination using the Bradford Assay (Bio-Rad). Sphingolipid levels were normalized based on total protein levels for each sample. Cells were processed and subjected to quantitative mass spectrometry to determine the levels of sphingolipid species (n = 3 +/−SEM). #p < 0.05 greater than corresponding vehicle control; *p < 0.05 less than corresponding value in CMV transfected cells. B. NSCLC cells were transfected with a scrambled control (siSCR) or with various validated siRNA molecules to knock down the expression of the indicated histone deacetylase (HDAC) proteins. Twenty-four h after transfection, cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater fluorescence than siSCR control; * p < 0.05 less fluorescence than siSCR control. C. A549, H460, H1975 and ADOR cells were transfected with a plasmid to express LC3-GFP-RFP. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h and 12h. The mean number of intense GFP+ and RFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). The data for each bar is the mean level of GFP+/RFP+ vesicles collectively from all 4 cell lines. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of Beclin1. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. After 6h cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater than vehicle control.](/cms/asset/4835e2d7-e8f0-4dee-af2c-5b4924a90d24/kcbt_a_1362511_f0003_b.gif)
Knock down of CerS6 prevented: i) [pemetrexed + sildenafil] from promoting CD95 cell surface clustering (); ii) prevented DISC formation (); iii) and cooperated with the nitric oxide synthase inhibitor L-NAME to prevent CD95 activatory tyrosine phosphorylation (). We did not test, in this system, whether CD95 knock down altered ceramide generation. Treatment of cells with L-NAME prevented [pemetrexed + sildenafil] from reducing the tyrosine phosphatase activity associated with CD95 (, graph). The tyrosine phosphatase proposed to regulate CD95 is PTPN13 and we determined the impact of [pemetrexed + sildenafil] on total PTPN13 expression and on its co-localization with CD95. PTPN13 co-precipitation with CD95 was reduced by [pemetrexed + sildenafil] (, blots). Treatment of cells with [pemetrexed + sildenafil] decreased expression of PTPN13 and caused CD95 to present as many intense staining punctae (Figure S8). In cells where PTEN was knocked down, the basal expression of CD95 was increased and the relative co-localization of PTPN13 with CD95 was unchanged. However, in cells lacking PTEN [pemetrexed + sildenafil] caused a profound “capping” of CD95 that co-localized with PTPN13; in the absence of PTEN, PTPN13 expression did not decline. A loss of CerS6 expression also reduced the drug-induced levels of autophagosomes, autolysosomes and of drug-induced cell killing (). Collectively, our present findings together with previously published data demonstrate that the activation of CD95 death receptor signaling by [pemetrexed + sildenafil] requires the coordinated actions of nitric oxide signaling and CerS6 ceramide signaling.
Figure 4. Nitric oxide generation and ceramide generation cooperate to cause drug-induced CD95 tyrosine phosphorylation, plasma membrane localization and DISC formation. A. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. Portions of the cells are fixed in place without permeabilization. Immunostaining is performed to detect the total membrane levels of CD95 (n = 3 +/−SEM). B. Another portion of the drug-treated cells from Panel A. is lysed and subjected to immunoprecipitation for CD95. The association of FADD and caspase 8 with CD95 is determined after SDS PAGE and immunoblotting. C. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are pre-treated for 30 min with vehicle, N-acetyl cysteine (10 μM) or L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. The cells are lysed and subjected to immunoprecipitation for CD95. The total expression and tyrosine phosphorylation of CD95 is determined after SDS PAGE and immunoblotting. The bar graph presents the mean-fold alterations in CD95 tyrosine phosphorylation from all cell lines tested under each condition (n = 3 +/− SEM). * p < 0.05 less than corresponding value in [siSCR + vehicle] cells. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of PTEN. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. CD95 was immunoprecipitated and the tyrosine phosphatase activity associated with CD95 measured as described in the Methods. Immunoprecipitates were then subjected to SDS PAGE and immunoblotting to determine the levels of PTPN13 in each precipitate (n = 3 +/−SEM).
![Figure 4. Nitric oxide generation and ceramide generation cooperate to cause drug-induced CD95 tyrosine phosphorylation, plasma membrane localization and DISC formation. A. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. Portions of the cells are fixed in place without permeabilization. Immunostaining is performed to detect the total membrane levels of CD95 (n = 3 +/−SEM). B. Another portion of the drug-treated cells from Panel A. is lysed and subjected to immunoprecipitation for CD95. The association of FADD and caspase 8 with CD95 is determined after SDS PAGE and immunoblotting. C. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are pre-treated for 30 min with vehicle, N-acetyl cysteine (10 μM) or L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. The cells are lysed and subjected to immunoprecipitation for CD95. The total expression and tyrosine phosphorylation of CD95 is determined after SDS PAGE and immunoblotting. The bar graph presents the mean-fold alterations in CD95 tyrosine phosphorylation from all cell lines tested under each condition (n = 3 +/− SEM). * p < 0.05 less than corresponding value in [siSCR + vehicle] cells. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of PTEN. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. CD95 was immunoprecipitated and the tyrosine phosphatase activity associated with CD95 measured as described in the Methods. Immunoprecipitates were then subjected to SDS PAGE and immunoblotting to determine the levels of PTPN13 in each precipitate (n = 3 +/−SEM).](/cms/asset/c6104614-63cb-4843-8853-5e27f645fe8f/kcbt_a_1362511_f0004_b.gif)
Figure 5. [Pemetrexed + sildenafil] -induced autophagosome and autolysosome formation requires CerS6 expression. A. ADOR NSCLC cells were transfected with a plasmid to express LC3-GFP and in parallel transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. The mean number of intense GFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). B. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 24h. Cell viability was determined by live/dead assay (n = 3 +/−SEM).
![Figure 5. [Pemetrexed + sildenafil] -induced autophagosome and autolysosome formation requires CerS6 expression. A. ADOR NSCLC cells were transfected with a plasmid to express LC3-GFP and in parallel transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. The mean number of intense GFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). B. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 24h. Cell viability was determined by live/dead assay (n = 3 +/−SEM).](/cms/asset/705f332c-3605-46cc-a3a8-eb82c9901af6/kcbt_a_1362511_f0005_b.gif)
Figure 6. Exposure of cells to [pemetrexed + sildenafil] reduces the expression of PD-L1, PD-L2 and ODC and increases the expression of MHCA. A: NSCLC cells; B: in vivo generated afatinib resistant H1975 clones; and C: ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC and the expression and localization of HMGB1 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells; #p < 0.05 significantly greater staining intensity than that in vehicle control treated cells.
![Figure 6. Exposure of cells to [pemetrexed + sildenafil] reduces the expression of PD-L1, PD-L2 and ODC and increases the expression of MHCA. A: NSCLC cells; B: in vivo generated afatinib resistant H1975 clones; and C: ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC and the expression and localization of HMGB1 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells; #p < 0.05 significantly greater staining intensity than that in vehicle control treated cells.](/cms/asset/3b2b404c-6484-4e98-9dbc-00aed9d4fb7b/kcbt_a_1362511_f0006_b.gif)
In a recent prior study, we demonstrated in vitro and in vivo that the HDAC inhibitors AR42 and sodium valproate increased expression of the class I MHC MHCA and decreased the expression of PD-L1 and ornithine decarboxylase (ODC), suggestive that the treated melanoma tumors had become opsonized to recently established immunotherapies.Citation13 This was then confirmed in vivo using the B16 mouse melanoma model. In multiple lung and ovarian cancer cells, including afatinib resistant H1975 clones, [pemetrexed + sildenafil] increased the expression of MHCA and decreased the levels of PD-L1, PD-L2 and ODC (). Treatment of lung and ovarian cancer cells with the pan-HDAC inhibitor sodium valproate recapitulated the effect of [pemetrexed + sildenafil] on PD-L1, PD-L2, MHCA and ODC expression (Figure S9). Using 2 PDX models, one of NSCLC and another of ovarian cancer, we attempted to define the HDACs responsible for regulating PD-L1, PD-L2, MHCA and ODC expression. Knock down of HDAC1, HDAC3, HDAC8 or HDAC10, alone or in combination, reduced PD-L1, PD-L2 and ODC expression and enhanced MHCA levels arguing the HDAC inhibitors were acting in an on-target fashion (Figures S10 and S11).
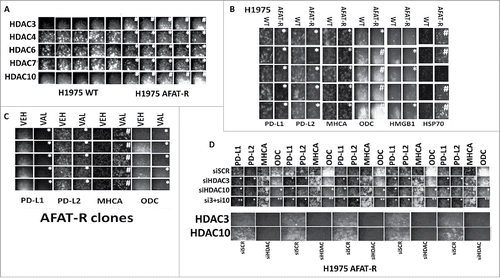
Figure 7. Afatinib-resistant H1975 cells express higher levels of HDAC3, HDAC10 and ODC and lower levels of MHCA; knock down of HDACs 3+10 increases MHCA expression and reduces ODC expression. A. H1975 cells (wild type clones and afatinib resistant clones) were fixed in place and the expression of HDACs1–11 determined by immuno-fluorescence. HDACs for whom their expression changed are presented (HDACS3/4/5/7/10). The mean fluorescence intensity from 40 cells in each wild type and afatinib resistant clone was determined and the -Fold change between wild type and afatinib resistant clones determined (n = 3 +/− SEM). # p < 0.05 greater than value in wild type clones; * p < 0.05 less than value in wild type clones. B. H1975 cells (wild type clones and afatinib resistant clones) were fixed in place and the expression of PD-L1, PD-L2, MHCA, ODC, HMGB1 and HSP70 determined by immuno-fluorescence. The mean fluorescence intensity from 40 cells in each wild type and afatinib resistant clone was determined and the -Fold change between wild type and afatinib resistant clones determined (n = 3 +/− SEM). # p < 0.05 greater than value in wild type clones; * p < 0.05 less than value in wild type clones. C. Afatinib resistant H1975 clones were treated with vehicle control or with sodium valproate (250 μM) for 6h. Cells were fixed in place and the expression of PD-L1, PD-L2, MHCA, and ODC determined by immuno-fluorescence. The mean fluorescence intensity from 40 cells in each vehicle treated and valproate treated clone was determined and the -Fold change between vehicle and valproate determined (n = 3 +/− SEM). # p < 0.05 greater than value in vehicle; * p < 0.05 less than value in vehicle. D. Afatinib resistant H1975 clones were scramble control transfected or transfected to knock down expression of HDAC3, HDAC10 or both HDACs together. Twenty-four h after transfection cells were fixed in place and immuno-fluorescence performed to determine the expression of PD-L1, PD-L2, MHCA and ODC. The mean fluorescence intensity from 40 cells in each condition was determined and the -Fold change between vehicle and valproate determined (n = 3 +/− SEM). # p < 0.05 greater than value in siSCR; * p < 0.05 less than value in siSCR; ** p < 0.05 less than value in siHDAC10 alone.
Based on the data in , we next performed additional in vitro analyses and animal studies using a syngeneic mouse Lewis Lung Carcinoma model. Treatment of LLC cells with [pemetrexed + sildenafil] significantly enhanced the levels of tumor cell death (Figure S12). The lethality of [pemetrexed + sildenafil] was enhanced by sodium valproate. Treatment of LLC cells with [pemetrexed + sildenafil] or [pemetrexed + sildenafil + valproate] significantly reduced the expression of PD-L1, PD-L2 and ODC and increased the expression of MHCA (). [Pemetrexed + sildenafil] treatment caused the extracellular release of HMGB1 and of HSP70 (Figure S13). In Lewis Lung Carcinoma tumors, valproate enhanced the anti-tumor efficacy of [pemetrexed + sildenafil] (). In LLC tumors, prior exposure to [pemetrexed + sildenafil] enhanced the efficacy of a subsequent anti-PD-1 or an anti-CTLA4 antibody administration (). Mouse weights / body mass did not significantly alter in drug-treated mice. Drug-treated mice also maintained grooming and other normal behaviors.
Figure 8. [Pemetrexed + sildenafil + valproate] kills lung cancer cells and facilitates checkpoint inhibitor immunotherapy anti-tumor effects. A. Lewis Lung Carcinoma cells were treated with vehicle control, pemetrexed (1.0 μM), sildenafil (2.0 μM), sodium valproate (250 μM), or the drugs in combination as indicated for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in pemetrexed treated cells; #p < 0.05 significantly greater staining intensity than that in pemetrexed treated cells. ¶ p < 0.05 significantly lower than in vehicle control treated cells. ** p < 0.05 less than value in [pemetrexed + sildenafil] treated cells. ## p < 0.05 greater than value in [pemetrexed + sildenafil] treated cells. B. and C. Lewis Lung Carcinoma cells were grown in C57 black mice and animals were treated with vehicle control, sodium valproate, [pemetrexed + sildenafil], [pemetrexed + sildenafil + sodium valproate] in the presence of a control IgG, and anti-PD-1 IgG or an anti-CTLA4 IgG as described in the Methods. (n = 10 per group +/−SEM). * p < 0.05 lower tumor growth than vehicle treated tumors; ** p < 0.05 lower growth than [pemetrexed + sildenafil] treated tumors; ¶ p < 0.05 less that [pemetrexed + sildenafil] + control IgG.
![Figure 8. [Pemetrexed + sildenafil + valproate] kills lung cancer cells and facilitates checkpoint inhibitor immunotherapy anti-tumor effects. A. Lewis Lung Carcinoma cells were treated with vehicle control, pemetrexed (1.0 μM), sildenafil (2.0 μM), sodium valproate (250 μM), or the drugs in combination as indicated for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in pemetrexed treated cells; #p < 0.05 significantly greater staining intensity than that in pemetrexed treated cells. ¶ p < 0.05 significantly lower than in vehicle control treated cells. ** p < 0.05 less than value in [pemetrexed + sildenafil] treated cells. ## p < 0.05 greater than value in [pemetrexed + sildenafil] treated cells. B. and C. Lewis Lung Carcinoma cells were grown in C57 black mice and animals were treated with vehicle control, sodium valproate, [pemetrexed + sildenafil], [pemetrexed + sildenafil + sodium valproate] in the presence of a control IgG, and anti-PD-1 IgG or an anti-CTLA4 IgG as described in the Methods. (n = 10 per group +/−SEM). * p < 0.05 lower tumor growth than vehicle treated tumors; ** p < 0.05 lower growth than [pemetrexed + sildenafil] treated tumors; ¶ p < 0.05 less that [pemetrexed + sildenafil] + control IgG.](/cms/asset/15ac4702-ffa8-4099-a006-e75febf46c89/kcbt_a_1362511_f0008_b.gif)
At animal nadir, tumors were isolated, fixed and paraffin embedded. Five micron sections of tumors under each condition were subjected to immuno-histochemical analyses to detect the expression of immune cell biomarkers. Prior exposure of tumors to [pemetrexed + sildenafil] increased the total number of infiltrating macrophages, with a high percentage of the macrophages belonging to the M1 subtype (Figures S14 and S15). This was further enhanced when these tumors were also exposed to an anti-PD-1 antibody. Prior exposure of tumors to either an anti-PD-1 IgG or to [pemetrexed + sorafenib] modestly enhanced the number of natural killer cells in the tumors, however, combined exposure to the agents dramatically enhanced intra-tumor NK cell levels (Figure S16). A very similar phenomenon to that observed in NK cells was also observed for neutrophils (Figure S17). Prior exposure of tumors to either [pemetrexed + sildenafil] or to an anti-PD-1 antibody enhanced total T cell levels and activated T cell levels within the LLC tumors (Figures S18 and S19). The combination of both treatments further enhanced T cell infiltration into the tumor, notably promoting the migration of T cells from blood vessel structures into the body of the tumor alongside the tumor cells themselves.
Prior studies in this manuscript linked a 6h exposure of tumor cells to [pemetrexed + sildenafil] to reduced expression of PD-L1 and HDAC6, and to increased expression of MHCA. In our tumors, approximately 2 weeks after [pemetrexed + sildenafil] treatment, the expression of PD-L1 and HDAC6 remained lower than vehicle control treated tumors and the expression of MHCA elevated (Figure S20, p < 0.05). Tumors previously exposed to either [pemetrexed + sildenafil] or to an anti-PD-1 antibody exhibited elevated expression of the immunogenic protein HMGB1 (Figures S20 and S21). Drug exposure facilitated the vesicularization of HMGB1 and extracellular release of HMGB1 (Figure S21, yellow arrows). Of further note, tumors previously exposed to both drugs exhibited very low expression levels of HMGB1.
Discussion
The present studies were initially proposed to determine whether the drug combination of pemetrexed and sildenafil, previously shown to be an efficacious way to kill NSCLC cells, could also kill ovarian cancer cells and to determine whether the drug combination opsonized tumor cells to established immunotherapy regimens. The drug combination killed multiple drug resistant PDX models of ovarian cancer and in vitro it decreased PD-L1 and ODC expression in tumor cells and enhanced the expression of MHCA and HMGB1. In vivo [pemetrexed + sildenafil] enhanced the anti-tumor efficacy of checkpoint inhibitory antibodies directed against PD-1 or CTLA4.
The molecular mechanisms by which pemetrexed and sildenafil acted to cause tumor cell death were previously shown to be complex, with cell death being mediated through multiple overlapping and congruent mechanisms. Previously we discovered that the drug combination: a) activated death receptor signaling through caspase 8 causing mitochondrial dysfunction; b) inhibited the functionality of multiple chaperones through inhibition of their ATPase activities which activated endoplasmic reticulum stress signaling that facilitated both autophagosome formation and suppressed the expression of protective proteins including c-FLIP-s, MCL-1 and BCL-XL; c) caused a DNA damage response which regulated AMPK-dependent mTOR inactivation and induction of toxic autophagosomes; d) via ATM-AMPK signaling which reduced expression of HDAC6. The present data sets demonstrated that [pemetrexed + sildenafil] reduced the expression of multiple other HDACs, notably, HDAC2; HDAC4; and HDAC9. Individual and combined knock down of HDAC protein expression enhanced the lethality of [pemetrexed + sildenafil] as did use of the pan-HDAC inhibitors AR42 and sodium valproate. Sodium valproate also enhanced the expression of CD95. Thus, collectively, our data support moving forward into the clinic combining pemetrexed, sildenafil and sodium valproate in both NSCLC and ovarian cancer.
Many cancer therapeutic studies examining the actions of HDAC inhibitors have not validated their drug-based findings with unbiased molecular approaches to define which HDACs are involved in the process being studied. Studies that have used selective knock down of different HDACs have frequently linked HDAC6 with a vital role in tumor cell growth and tumor formation in vivo.26 However, as our studies demonstrated, HDAC6 played little to no role in the regulation of immuno-regulatory protein expression. As such, clinical trials combining highly specific HDAC6 inhibitors with immunotherapy may ultimately prove unsuccessful.27
Previously, we published that knock down of CD95, of the TRAIL receptors DR4 and DR5, or overexpression of c-FLIP-s prevented sildenafil from strongly enhancing pemetrexed lethality. From several other studies, we knew that ceramide synthase 6 (CerS6) can play a key role in facilitating CD95 activation in response to stressful stimuli. Treatment of cells with [pemetrexed + sildenafil] reduced the expression of multiple HDAC proteins and knock down of these HDACs increased the expression of CerS6. Knock down of CerS6 suppressed [pemetrexed + sildenafil] -induced activation of CD95 as judged by plasma membrane localization and by DISC formation. Pre-treatment of cells with the pan-nitric oxide synthase inhibitor L-NAME suppressed the [pemetrexed + sildenafil] -induced increase in dihydro-ceramide levels whereas expression of thioredoxin exhibited only a partially inhibitory effect. Unlike the TRAIL receptors DR4 and DR5, for effective activation, the death receptor CD95 must be tyrosine phosphorylated, a modification that is primarily controlled by the phosphatase PTPN13. Phosphatases are inhibited by high levels of ROS and RNS due to chemical modification of an essential reactive cysteine residue in their active sites. L-NAME, but to a much lesser extent knock down of CerS6 or treatment with the ROS quenching agent N-acetyl cysteine, prevented [pemetrexed + sildenafil] -induced tyrosine phosphorylation of CD95. This was associated with L-NAME protecting the tyrosine phosphatase activity associated with CD95. Thus [pemetrexed + sildenafil] activates CD95 signaling through overlapping biologic events: i) the drug combination reduces the expression of multiple HDACs through autophagy, that subsequently facilitates increased protein expression of CerS6 and thus elevated ceramide levels, which permit CD95 receptor clustering and DISC formation; ii) sildenafil through activation of PKG and the generation of nitric oxide, and its reaction with tumor cell ROS results in peroxy-nitrite formation which inhibits PTPN13 inhibition that permits increased tyrosine phosphorylation of CD95, receptor trimerization and DISC formation.
Prior studies have shown that direct inhibition of HSP90 and HSP70 family chaperones with multi-kinase inhibitors such as sorafenib and pazopanib reduces the expression of a diverse array of chaperoned proteins, including growth factor receptors, e.g., ERBB1. In contrast, mutated forms of p53 can exhibit a gain of function phenotype that can act to increase the expression of growth factor receptors, e.g., ERBB1. In H1975 lung cancer cells that express a mutated inactive allele of p53, treatment of cells with [pemetrexed + sildenafil] rapidly reduced the expression of ERBB1 without altering the expression of p53. The acetylation of HSP90 was increased that is associated with reduced HDAC6 expression and this phenomenon in general is thought to correlate with reduced HSP90 chaperone functionality. Studies beyond the scope of the present manuscript will be required to understand whether this is due to less p53 transcriptional activity or reduced chaperone function.
In conclusion, we have demonstrated that [pemetrexed + sildenafil] lethality can be enhanced by the cost-effective HDAC inhibitor sodium valproate. In addition, both [pemetrexed + sildenafil] and sodium valproate opsonize tumor cells to anti-PD-1 and anti-CTLA4 checkpoint inhibitory antibodies. As pemetrexed is an approved drug for the treatment of NSCLC and ovarian cancer, our data argue for undertaking a 2-armed phase I trial in these malignancies combining pemetrexed, sildenafil, valproate and an anti-PD-1 therapeutic.
Materials and methods
Materials. Pemetrexed, sildenafil and AR42 were purchased from Selleckchem (Houston, TX). Sodium valproate was from Sigma (St. Louis, MO). Trypsin-EDTA, DMEM, RPMI, penicillin-streptomycin were purchased from GIBCOBRL (GIBCOBRL Life Technologies, Grand Island, NY). SKOV3, OVCAR, PAI cells and all “H” series NSCLC lines were purchased from the ATCC and were not further validated beyond that claimed by ATCC. Cells were re-purchased every ∼6 months. ADOR cells were a gift to the Dent laboratory from a female NSCLC patient. Spiky, CTG-1703, CTG-1677 PDX ovarian cancer cells were provided by Dr. Karen Paz (Champions Oncology, NJ). Commercially available validated short hairpin RNA molecules to knock down RNA / protein levels were from Qiagen (Valencia, CA) (Figure S22). Reagents and performance of experimental procedures were described in refs: [1–3, 6, 13].
Methods
Culture and in vitro exposure of cells to drugs. All cell lines were cultured at 37 °C (5% (v/v CO2) in vitro using RPMI supplemented with dialyzed 5% (v/v) fetal calf serum and 10% (v/v) Non-essential amino acids. For short-term cell killing assays, immune-staining studies, cells were plated at a density of 3 × 103 per cm2 and 24h after plating treated with various drugs, as indicated. In vitro drug treatments were generally from a 100 mM stock solution of each drug and the maximal concentration of Vehicle carrier (VEH; DMSO) in media was 0.02% (v/v). Cells were not cultured in reduced serum media during any study in this manuscript.
Transfection of cells with siRNA or with plasmids
For Plasmids: Cells were plated and 24h after plating, transfected. Plasmids expressing a specific mRNA (or siRNA) or appropriate vector control plasmid DNA was diluted in 50 μl serum-free and antibiotic-free medium (1 portion for each sample). Concurrently, 2 μl Lipofectamine 2000 (Invitrogen), was diluted into 50 μl of serum-free and antibiotic-free medium (1 portion for each sample). Diluted DNA was added to the diluted Lipofectamine 2000 for each sample and incubated at room temperature for 30 min. This mixture was added to each well / dish of cells containing 200 μl serum-free and antibiotic-free medium for a total volume of 300 μl, and the cells were incubated for 4 h at 37 °C. An equal volume of 2x medium was then added to each well. Cells were incubated for 24h, then treated with drugs.
Transfection for siRNA: Cells from a fresh culture growing in log phase as described above, and 24h after plating transfected. Prior to transfection, the medium was aspirated and serum-free medium was added to each plate. For transfection, 10 nM of the annealed siRNA, the positive sense control doubled stranded siRNA targeting GAPDH or the negative control (a “scrambled” sequence with no significant homology to any known gene sequences from mouse, rat or human cell lines) were used. Ten nM siRNA (scrambled or experimental) was diluted in serum-free media. Four μl Hiperfect (Qiagen) was added to this mixture and the solution was mixed by pipetting up and down several times. This solution was incubated at room temp for 10 min, then added drop-wise to each dish. The medium in each dish was swirled gently to mix, then incubated at 37 °C for 2h. Serum-containing medium was added to each plate, and cells were incubated at 37 °C for 24h before then treated with drugs (0–24h). Additional immuno-fluorescence / live-dead analyses were performed at the indicated time points.
Animal Studies. Studies were performed according to USDA regulations under VCU IACUC protocol AD20008. Male immune competent C57/BL6 mice (∼20 g) were injected with 0.5 × 106 Lewis Lung Carcinoma cancer cells into their rear flank (10 animals per treatment group). Tumors were permitted to form for 3 d with tumors at that time exhibiting a mean volume of ∼20 mm3. Mice were treated by oral gavage once every day for 3 d with vehicle control, [pemetrexed (Day 1, 25 mg/kg) + sildenafil (Days 1–3, 5.0 mg/kg)], sodium valproate (50 mg/kg) or the drugs in combination, as indicated. For antibody administrations, 2 d after cessation of drug exposure animals are injected IP with: a control IgG (100 mg); an anti-PD-1 IgG (100 mg); or an anti-CTLA4 IgG (100 mg). Before, during and after drug treatment tumors are calipered as indicated in the Figure and tumor volume was assessed up to 16 d later. The -Fold increase in tumor volume under each condition is plotted. Animals were humanely killed when the volume reached ∼300 mm3 due to ulceration, and the tumor and blood removed for further studies.
Detection of cell viability, protein expression and protein phosphorylation by immuno-fluorescence using a Hermes WiScan machine. http://www.idea-bio.com/, Cells (4 × 103) are plated into each well of a 96 well plate, and cells permitted to attach and grow for the next 18h. Based on the experiment, after 18h, cells are then either genetically manipulated, or are treated with drugs. For genetic manipulation, cells are transfected with plasmids or siRNA molecules and incubated for an additional 24h. Cells are treated with vehicle control or with drugs at the indicated final concentrations, alone or in combination. Cells are then isolated for processing at various times following drug exposure. The 96 well plate is centrifuged / cyto-spun to associate dead cells (for live-dead assays) with the base of each well. For live dead assays, after centrifugation, the media is removed and cells treated with live-dead reagent (Thermo Fisher Scientific, Waltham MA) and after 10 min this is removed and the cells in each well are visualized in the Hermes instrument at 10X magnification. Green cells = viable; yellow/red cells = dying/dead. The numbers of viable and dead cells were counted manually from 3 images taken from each well combined with data from another 2 wells of separately treated cells (i.e. the data are the mean cell dead from 9 data points from 3 separate exposures). For immuno-fluorescence studies, after centrifugation, the media is removed and cells are fixed in place and permeabilized using ice cold PBS containing 0.4% paraformaldehyde and 0.5% Triton X-100. After 30 min the cells are washed 3 times with ice cold PBS and cells are pre-blocked with rat serum for 3h. Cells are then incubated with a primary antibody to detect the expression / phosphorylation of a protein (usually at 1:100 dilution from a commercial vendor) overnight at 37°C. Cells are washed 3 times with PBS followed by application of the secondary antibody containing an associated fluorescent red or green chemical tag. After 3h of incubation the antibody is removed and the cells washed again. The cells are visualized at either 10X or 60X in the Hermes machine for imaging assessments. All immunofluorescent images for each individual protein / phospho-protein are taken using the identical machine settings so that the levels of signal in each image can be directly compared with the level of signal in the cells treated with drugs. Similarly, for presentation, the enhancement of image brightness/contrast using PhotoShop CS6 is simultaneously performed for each individual set of protein/phospho-protein to permit direct comparison of the image intensity between treatments. Antibodies used include: HSP90 (E289) (Cell Signaling); HSP90 (#2928) (Abcam); HSP90 (ab195575) Abcam; HSP90 3G3 (13495) (Abcam); GRP78 (50b12) (31772) (Cell Signaling); GRP78 (ab191023) Abcam; GRP78 (ab103336) Abcam; GRP78 (N-20) (sc-1050) Santa Cruz; HSP27 (G31) (2402P) Cell Signaling); HSP27 [EP1724Y] (ab62339) Abcam; HSP27 (H-77) (sc-9012) Santa Cruz; HSP27 (LS-C31836) Lifespan science Corp. Other antibodies were as used in prior studies by the laboratory. All immunofluorescent images were initially visualized at 75 dpi using an Odyssey infrared imager (Li-Cor, Lincoln, NE), then processed at 9999 dpi using Adobe Photoshop CS6. For presentation, immunoblots were digitally assessed using the provided Odyssey imager software. Images have their color removed and labeled figures generated in Microsoft PowerPoint.
Assessment of autophagy: Cells were transfected with a plasmid to express a green fluorescent protein (GFP) and red fluorescent protein (RFP) tagged form of LC3 (ATG8). For analysis of cells transfected with the GFP-RFP-LC3 construct, the GFP/RFP-positive vesicularized cells were examined under the × 40 objective of a Zeiss Axiovert fluorescent microscope.
Tyrosine phosphatase assay: CD95 immunoprecipitates were incubated with a PTPase kit supplied by Amsbio Inc. (Cambridge, MA).
Data analysis. Comparison of the effects of various treatments (performed in triplicate 3 times) was using one-way analysis of variance and a 2 tailed Student's t-test. Statistical examination of in vivo animal survival data used both a 2 tailed Student's t-test and log rank statistical analyses between the different treatment groups. Differences with a p-value of < 0.05 were considered statistically significant. Experiments shown are the means of multiple individual points from multiple experiments ( ± SEM).
There are no conflicts of interest to report.
Acknowledgments
Support for the present study was funded from philanthropic funding from Massey Cancer Center, the Universal Inc. Chair in Signal Transduction Research, PHS R01-CA192613. Services and products in support of the research project were generated by the VCU Massey Cancer Center Lipidomics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. Thanks to Dr. H.F. Young and the Betts family fund for support in the purchase of the Hermes Wiscan instrument. The authors have no conflicts of interest to report .
References
- Booth L, Roberts JL, Poklepovic A, Gordon S, Dent P. PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget. 2017;8:1449-68. PMID:27903966
- Booth L, Roberts JL, Poklepovic A, Dent P. PDE5 inhibitors enhance the lethality of [pemetrexed + sorafenib]. Oncotarget. 2017 Jan 9. doi:10.18632/oncotarget.14562
- Booth L, Shuch B, Albers T, Roberts JL, Tavallai M, Proniuk S, Zukiwski A, Wang D, Chen CS, Bottaro D, Ecroyd H, Lebedyeva IO, Dent P. Multi-kinase inhibitors can associate with heat shock proteins through their NH2-termini by which they suppress chaperone function. Oncotarget. 2016;7:12975-96.
- Mawatari T, Ninomiya I, Inokuchi M, Harada S, Hayashi H, Oyama K, Makino I, Nakagawara H, Miyashita T, Tajima H, Takamura H, Fushida S, Ohta T. Valproic acid inhibits proliferation of HER2-expressing breast cancer cells by inducing cell cycle arrest and apoptosis through Hsp70 acetylation. Int J Oncol. 2015;47:2073-81.
- Chen HP, Zhao YT, Zhao TC. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015; 20:35-47. doi:10.1615/CritRevOncog.2015012997. PMID:25746103
- Booth L, Roberts JL, Sander C, Lee J, Kirkwood JM, Poklepovic A, Dent P. The HDAC inhibitor AR42 interacts with pazopanib to kill trametinib/dabrafenib-resistant melanoma cells in vitro and in vivo. Oncotarget. 2017 Jan 27. doi:10.18632/oncotarget.14829
- Koller KM, Wang W, Schell TD, Cozza EM, Kokolus KM, Neves RI, Mackley HB, Pameijer C, Leung A, Anderson B, Mallon CA, Robertson G, Drabick JJ. Malignant melanoma-The cradle of anti-neoplastic immunotherapy. Crit Rev Oncol Hematol. 2016;106:25-54. doi:10.1016/j.critrevonc.2016.04.010. PMID:27637351
- Beg AA, Gray JE. HDAC inhibitors with PD-1 blockade: a promising strategy for treatment of multiple cancer types? Epigenomics. 2016;8:1015-7. doi:10.2217/epi-2016-0066. PMID:27410519
- Terranova-Barberio M, Thomas S, Munster PN. Epigenetic modifiers in immunotherapy: a focus on checkpoint inhibitors. Immunotherapy. 2016;8:705-19. doi:10.2217/imt-2016-0014. PMID:27197539
- Zheng H, Zhao W, Yan C, Watson CC, Massengill M, Xie M, Massengill C, Noyes DR, Martinez GV, Afzal R, Chen Z, Ren X, Antonia SJ, Haura EB, Ruffell B, Beg AA. HDAC Inhibitors Enhance T-Cell Chemokine Expression and Augment Response to PD-1 Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2016;22:4119-32. doi:10.1158/1078-0432.CCR-15-2584. PMID:26964571
- Shen L, Orillion A, Pili R. Histone deacetylase inhibitors as immunomodulators in cancer therapeutics. Epigenomics. 2016;8:415-28. doi:10.2217/epi.15.118. PMID:26950532
- Yang H, Lan P, Hou Z, Guan Y, Zhang J, Xu W, Tian Z, Zhang C. Histone deacetylase inhibitor SAHA epigenetically regulates miR-17-92 cluster and MCM7 to upregulate MICA expression in hepatoma.. Br J Cancer. 2015;112:112-21. doi:10.1038/bjc.2014.547. PMID:25393367
- Booth L, Roberts JL, Kirkwood J, Poklepovic A, Dent P. HDAC inhibitors regulate the immunotherapy response of melanoma cells. Oncotarget. 2017: IN PRESS. doi:10.18632/oncotarget.17950
- Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175-83. doi:10.1038/cdd.2008.143. PMID:18846108
- Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Häussinger D, Reinehr R, Larner A, Spiegel S, Fisher PB, Voelkel-Johnson C, Ogretmen B, Grant S, Dent P. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313-24.
- Park MA, Reinehr R, Häussinger D, Voelkel-Johnson C, Ogretmen B, Yacoub A, Grant S, Dent P. Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol Cancer Ther. 2010;9:2220-31.
- Tavallai M, Hamed HA, Roberts JL, Cruickshanks N, Chuckalovcak J, Poklepovic A, Booth L, Dent P. Nexavar/Stivarga and viagra interact to kill tumor cells. J Cell Physiol. 2015;230:2281-98.
- Saddoughi SA, Ogretmen B. Diverse functions of ceramide in cancer cell death and proliferation. Adv Cancer Res. 2013;117:37-58. doi:10.1016/B978-0-12-394274-6.00002-9. PMID:23290776
- Hoeferlin LA, Fekry B, Ogretmen B, Krupenko SA, Krupenko NI. Folate stress induces apoptosis via p53-dependent de novo ceramide synthesis and up-regulation of ceramide synthase 6. J Biol Chem. 2013;288:12880-90. doi:10.1074/jbc.M113.461798. PMID:23519469
- Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21:3386-97. doi:10.1096/fj.07-8621com. PMID:17548428
- Ju R, Muller MT. Histone deacetylase inhibitors activate p21(WAF1) expression via ATM. Cancer Res. 2003;63:2891-7. PMID:12782595
- Bahr JC, Robey RW, Luchenko V, Basseville A, Chakraborty AR, Kozlowski H, Pauly GT, Patel P, Schneider JP, Gottesman MM, Bates SE. Blocking downstream signaling pathways in the context of HDAC inhibition promotes apoptosis preferentially in cells harboring mutant Ras. Oncotarget. 2016;7:69804-15. PMID:27634878
- Histone Deacetylase-1-mediated Suppression of FAS in Chemoresistant Ovarian Cancer Cells. Cacan E. Anticancer Res. 2016;36:2819-26.
- Hwang JJ, Kim YS, Kim T, Kim MJ, Jeong IG, Lee JH, Choi J, Jang S, Ro S, Kim CS. A novel histone deacetylase inhibitor, CG200745, potentiates anticancer effect of docetaxel in prostate cancer via decreasing Mcl-1 and Bcl-XL. Invest New Drugs. 2012;30:1434-42. doi:10.1007/s10637-011-9718-1. PMID:21773733
- Booth L, Roberts JL, Tavallai M, Webb T, Leon D, Chen J, McGuire WP, Poklepovic A, Dent P. The afatinib resistance of in vivo generated H1975 lung cancer cell clones is mediated by SRC/ERBB3/c-KIT/c-MET compensatory survival signaling. Oncotarget. 2016;7:19620-30. doi:10.18632/oncotarget.7746. PMID:26934000
